-
PDF
- Split View
-
Views
-
Cite
Cite
Uwe Zeymer, Peter Ludman, Nicolas Danchin, Petr Kala, Cécile Laroche, Chris P Gale, Aldo P Maggioni, Soraya Siabani, Masoumeh Sadeghi, Ahmed Wafa, Stanislaw Bartus, Franz Weidinger, on behalf of the ACS STEMI investigators group, Reperfusion therapy for ST-elevation myocardial infarction complicated by cardiogenic shock: the European Society of Cardiology EurObservational programme acute cardiovascular care-European association of PCI ST-elevation myocardial infarction registry, European Heart Journal. Acute Cardiovascular Care, Volume 11, Issue 6, June 2022, Pages 481–490, https://doi.org/10.1093/ehjacc/zuac049
Close - Share Icon Share
Abstract
To determine the current state of the use of reperfusion and adjunctive therapies and in-hospital outcomes in European Society of Cardiology (ESC) member and affiliated countries for patients with ST-segment elevation myocardial infarction (STEMI) complicated by cardiogenic shock (CS).
ESC EurObservational Research Programme prospective international cohort study of admissions with STEMI within 24 h of symptom onset (196 centres; 26 ESC member and 3 affiliated countries). Of 11 462 patients enrolled, 448 (3.9%) had CS. Patients with compared to patients without CS, less frequently received primary percutaneous coronary intervention (PCI) (65.5% vs. 72.2%) and fibrinolysis (15.9% vs. 19.0), and more often had no reperfusion therapy (19.0% vs. 8.5%). Mechanical support devices (intraaortic ballon pump 11.2%, extracoporeal membrane oxygenation 0.7%, other 1.1%) were used infrequently in CS. Bleeding definition academic research consortium 2–5 bleeding complications (10.1% vs. 3.0%, P < 0.01) and stroke (4.2% vs. 0.9%, P < 0.01) occurred more frequently in patients with CS. In-hospital mortality was 10-fold higher (35.5% vs. 3.1%) in patients with CS. Mortality in patients with CS in the groups with PCI, fibrinolysis, and no reperfusion therapy were 27.4%, 36.6%, and 62.4%, respectively.
In this multi-national registry, patients with STEMI complicated by CS less frequently receive reperfusion therapy than patients with STEMI without CS. Early mortality in patients with CS not treated with primary PCI is very high. Therefore, strategies to improve clinical outcome in STEMI with CS are needed.
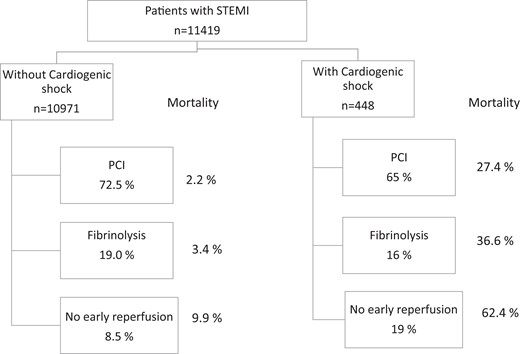
Acute reperfusion therapies and in-hospital mortality in patients with and without cardiogenic shock.
In line with the Journal’s conflict of interest policy, this paper was handled by Borja Ibanez.
Introduction
The highest mortality in patients with acute ST-elevation myocardial infarction (STEMI) is observed in the subgroup of patients with cardiogenic shock (CS). Despite improvements in management, early mortality in patients with STEMI and CS approach 40%.1 The European Society of Cardiology (ESC) has issued practice guidelines for patients with STEMI, the two latest versions published in 2012 and 2017,2,3 which recommend primary percutaneous coronary intervention (PCI) as preferred reperfusion therapy in CS. It has been shown that adherence to these guidelines improves outcomes.4,5 The ‘Stent for life’ initiative of the ESC has been created to increase the rate of patients treated with primary PCI within Europe and the Mediterranean basin.6 However, previous ACS-Surveys within the Euro Heart Survey Program performed in 2000, 2004, and 2008 and the snapshot registry in 2009 revealed gaps between recommendations by guidelines and their implementation into clinical practice.7–10 These gaps may have the greatest impact in the sickest patients, such as those with CS.1 Here, we report the current status of reperfusion therapy and outcomes in patients with STEMI complicated by CS in an international prospective registry.
Methods
The design and methods of the registry have been published.11,12 This study describes the demographic, clinical, and biological characteristics of patients with STEMI admitted to cardiology centres in ESC-member and affiliated countries. Information on reperfusion therapies and the reasons why reperfusion therapy was not used were also evaluated. Details on technical aspects of PCI and adjunctive antithrombotic therapies as well as hospital events were collected.
Study organization
This registry is a Joint initiative of the association of acute cardiovascular care and the European Association of PCI (EAPCI) under the umbrella of the EurObservational Research Programme (EORP). Centres with and without PCI facilities were invited to participate.
Patients
Between 1 January 2015 and 31 March 2018, patients aged > 18 years with an initial diagnosis of STEMI according to the ESC 2012 STEMI guidelines admitted within 24 h after symptom onset were identified on admission to the hospital, in the emergency room or directly in the catherization laboratory and given a unique study number. For this analysis, replicate counts of cases for STEMI occurring in the same patient were removed and only the earliest presentation included.
Data
Baseline data included demographic data, patient history, risk factors, and time intervals. Invasive coronary angiographic data and details of the revascularization procedures were collected. Medications given in the pre-hospital phase, during hospitalization and discharge were documented, as well as clinical events.
Definitions
CS was defined according to the ESC STEMI guidelines2 and included hypotension <90 mmHg and/or the need of catecholamines, pulmonary congestion, and signs of end organ failure. Bleeding complications were classified according to the bleeding definition academic research consortium (BARC) definition.13
Statistics
Descriptive statistics are used to summarize frequency tabulations (n, %) and distributions (mean, SD). All the results are summarized with and without CS. For categorical data, frequency tabulations are presented (without missing values if applicable).
Results
Patients
A total of 11 462 patients from 196 centres in 29 countries were enrolled into the registry. From these, 448 patients (4.4%) presented with CS. The baseline demographics of the patients with and without CS are given in Table 1. Patients with CS were older and more often female. They had more co-morbidity, with more prior MI, prior stroke, history of atrial fibrillation, and also more frequently diabetic and cancer. While the rate of anterior AMI was around 50% in patients with and without CS, heart rate was higher and systolic blood pressure was lower in patients with CS (Table 2). The time-intervals between symptom onset, first medical contact and the start of primary PCI were not significantly different between patients with and without CS and about 60% of patients had primary PCI within 120 min after first medical contact in both groups (Table 3).
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Patients (N) | 10 971 | 448 | |
| Age, years, mean ± SD | 60.9 ±12.7 | 65.2 ± 13.6 | <0.001 |
| Women | 2485 (22.7%) | 151 (33.7%) | <0.001 |
| Prior myocardial infarction | 1190/10 008 | 74/398 | <0.001 |
| (11.9%) | (18.6%) | ||
| Previous percutaneous coronary intervention | 1093/10 910 | 50/422 | 0.221 |
| (10.0%) | (11.9%) | ||
| Previous CABG surgery | 188/10 937 | 5/426 | 0.393 |
| (1.7%) | (1.2%) | ||
| Previous stroke/transient ischemic attack | 560/10 915 | 38/417 | <0.001 |
| (5.1%) | (9.1%) | ||
| Peripheral artery disease | 445/10 443 | 23/397 | 0.140 |
| (4.3%) | (5.8%) | ||
| History of atrial fibrillation | 414/10 872 | 35/434 | <0.001 |
| (3.8%) | (8.1%) | ||
| Diabetes mellitus | 2855/10 741 | 135/420 | 0.012 |
| (26.6%) | (32.1%) | ||
| Current smoker | 4923/10 746 | 182/415 | 0.432 |
| (45.8%) | (43. 9%) | ||
| Hypercholesterolemia | 3611/9410 | 133/390 | 0.749 |
| (38.4%) | (39.2%) | ||
| Cancer or other life-limiting diseases | 972/10 787 | 54/408 | 0.020 |
| (9.0%) | (13.2%) |
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Patients (N) | 10 971 | 448 | |
| Age, years, mean ± SD | 60.9 ±12.7 | 65.2 ± 13.6 | <0.001 |
| Women | 2485 (22.7%) | 151 (33.7%) | <0.001 |
| Prior myocardial infarction | 1190/10 008 | 74/398 | <0.001 |
| (11.9%) | (18.6%) | ||
| Previous percutaneous coronary intervention | 1093/10 910 | 50/422 | 0.221 |
| (10.0%) | (11.9%) | ||
| Previous CABG surgery | 188/10 937 | 5/426 | 0.393 |
| (1.7%) | (1.2%) | ||
| Previous stroke/transient ischemic attack | 560/10 915 | 38/417 | <0.001 |
| (5.1%) | (9.1%) | ||
| Peripheral artery disease | 445/10 443 | 23/397 | 0.140 |
| (4.3%) | (5.8%) | ||
| History of atrial fibrillation | 414/10 872 | 35/434 | <0.001 |
| (3.8%) | (8.1%) | ||
| Diabetes mellitus | 2855/10 741 | 135/420 | 0.012 |
| (26.6%) | (32.1%) | ||
| Current smoker | 4923/10 746 | 182/415 | 0.432 |
| (45.8%) | (43. 9%) | ||
| Hypercholesterolemia | 3611/9410 | 133/390 | 0.749 |
| (38.4%) | (39.2%) | ||
| Cancer or other life-limiting diseases | 972/10 787 | 54/408 | 0.020 |
| (9.0%) | (13.2%) |
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Patients (N) | 10 971 | 448 | |
| Age, years, mean ± SD | 60.9 ±12.7 | 65.2 ± 13.6 | <0.001 |
| Women | 2485 (22.7%) | 151 (33.7%) | <0.001 |
| Prior myocardial infarction | 1190/10 008 | 74/398 | <0.001 |
| (11.9%) | (18.6%) | ||
| Previous percutaneous coronary intervention | 1093/10 910 | 50/422 | 0.221 |
| (10.0%) | (11.9%) | ||
| Previous CABG surgery | 188/10 937 | 5/426 | 0.393 |
| (1.7%) | (1.2%) | ||
| Previous stroke/transient ischemic attack | 560/10 915 | 38/417 | <0.001 |
| (5.1%) | (9.1%) | ||
| Peripheral artery disease | 445/10 443 | 23/397 | 0.140 |
| (4.3%) | (5.8%) | ||
| History of atrial fibrillation | 414/10 872 | 35/434 | <0.001 |
| (3.8%) | (8.1%) | ||
| Diabetes mellitus | 2855/10 741 | 135/420 | 0.012 |
| (26.6%) | (32.1%) | ||
| Current smoker | 4923/10 746 | 182/415 | 0.432 |
| (45.8%) | (43. 9%) | ||
| Hypercholesterolemia | 3611/9410 | 133/390 | 0.749 |
| (38.4%) | (39.2%) | ||
| Cancer or other life-limiting diseases | 972/10 787 | 54/408 | 0.020 |
| (9.0%) | (13.2%) |
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Patients (N) | 10 971 | 448 | |
| Age, years, mean ± SD | 60.9 ±12.7 | 65.2 ± 13.6 | <0.001 |
| Women | 2485 (22.7%) | 151 (33.7%) | <0.001 |
| Prior myocardial infarction | 1190/10 008 | 74/398 | <0.001 |
| (11.9%) | (18.6%) | ||
| Previous percutaneous coronary intervention | 1093/10 910 | 50/422 | 0.221 |
| (10.0%) | (11.9%) | ||
| Previous CABG surgery | 188/10 937 | 5/426 | 0.393 |
| (1.7%) | (1.2%) | ||
| Previous stroke/transient ischemic attack | 560/10 915 | 38/417 | <0.001 |
| (5.1%) | (9.1%) | ||
| Peripheral artery disease | 445/10 443 | 23/397 | 0.140 |
| (4.3%) | (5.8%) | ||
| History of atrial fibrillation | 414/10 872 | 35/434 | <0.001 |
| (3.8%) | (8.1%) | ||
| Diabetes mellitus | 2855/10 741 | 135/420 | 0.012 |
| (26.6%) | (32.1%) | ||
| Current smoker | 4923/10 746 | 182/415 | 0.432 |
| (45.8%) | (43. 9%) | ||
| Hypercholesterolemia | 3611/9410 | 133/390 | 0.749 |
| (38.4%) | (39.2%) | ||
| Cancer or other life-limiting diseases | 972/10 787 | 54/408 | 0.020 |
| (9.0%) | (13.2%) |
ECG and clinical findings on admission in patients without and with cardiogenic shock
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Anterior STEMI | 5047/10 246 (49.2%) | 209/431 (48.5%) | 0.263 |
| Other STEMI | 5163/10 264 (50.3%) | 217/431 (50.4%) | |
| Left bundle branch block | 51/10 264 (0.5%) | 5/431 (1.2%) | |
| Atrial fibrillation on qualifying ECG | 511/10 966 | 59/448 | <0.001 |
| (4.7%) | (13.2%) | ||
| Heart rate (beats per minute) mean ± SD | 79.6 ± 19.2 | 84.9 ± 34.8 | 0.006 |
| Systolic blood pressure (mmHg) mean ± SD | 134.8 ± 26.7 | 93.1 ± 30.6 | <0.001 |
| Out of hospital cardiac arrest | 376/10 501 (3.5%) | 112/442 (25.3%) | <0.001 |
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Anterior STEMI | 5047/10 246 (49.2%) | 209/431 (48.5%) | 0.263 |
| Other STEMI | 5163/10 264 (50.3%) | 217/431 (50.4%) | |
| Left bundle branch block | 51/10 264 (0.5%) | 5/431 (1.2%) | |
| Atrial fibrillation on qualifying ECG | 511/10 966 | 59/448 | <0.001 |
| (4.7%) | (13.2%) | ||
| Heart rate (beats per minute) mean ± SD | 79.6 ± 19.2 | 84.9 ± 34.8 | 0.006 |
| Systolic blood pressure (mmHg) mean ± SD | 134.8 ± 26.7 | 93.1 ± 30.6 | <0.001 |
| Out of hospital cardiac arrest | 376/10 501 (3.5%) | 112/442 (25.3%) | <0.001 |
ECG and clinical findings on admission in patients without and with cardiogenic shock
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Anterior STEMI | 5047/10 246 (49.2%) | 209/431 (48.5%) | 0.263 |
| Other STEMI | 5163/10 264 (50.3%) | 217/431 (50.4%) | |
| Left bundle branch block | 51/10 264 (0.5%) | 5/431 (1.2%) | |
| Atrial fibrillation on qualifying ECG | 511/10 966 | 59/448 | <0.001 |
| (4.7%) | (13.2%) | ||
| Heart rate (beats per minute) mean ± SD | 79.6 ± 19.2 | 84.9 ± 34.8 | 0.006 |
| Systolic blood pressure (mmHg) mean ± SD | 134.8 ± 26.7 | 93.1 ± 30.6 | <0.001 |
| Out of hospital cardiac arrest | 376/10 501 (3.5%) | 112/442 (25.3%) | <0.001 |
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Anterior STEMI | 5047/10 246 (49.2%) | 209/431 (48.5%) | 0.263 |
| Other STEMI | 5163/10 264 (50.3%) | 217/431 (50.4%) | |
| Left bundle branch block | 51/10 264 (0.5%) | 5/431 (1.2%) | |
| Atrial fibrillation on qualifying ECG | 511/10 966 | 59/448 | <0.001 |
| (4.7%) | (13.2%) | ||
| Heart rate (beats per minute) mean ± SD | 79.6 ± 19.2 | 84.9 ± 34.8 | 0.006 |
| Systolic blood pressure (mmHg) mean ± SD | 134.8 ± 26.7 | 93.1 ± 30.6 | <0.001 |
| Out of hospital cardiac arrest | 376/10 501 (3.5%) | 112/442 (25.3%) | <0.001 |
Mean time intervals (± SD) between symptom-onset, first medical contact (FMC) and percutaneous coronary intervention (PCI)
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Symptom-onset to FMC (min) | (n = 10756) | (n = 440) | 0.568 |
| 220.6 ± 461.4 | 228.5 ± 318.6 | ||
| FMC to PCI (min) | (n = 7854) | (n = 289) | |
| 195.3 ± 1119.3 | 152.6 ± 173.0 | ||
| FMC to PCI <30 min | 283/7854 (3.6%) | 7/289 (2.4%) | |
| FMC to PCI <60 min | 1646/7854 (21.0%) | 40/289 (13.8%) | |
| FMC to PCI <120 min | 4869/7854 (62.0%) | 168/289 (58.1%) |
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Symptom-onset to FMC (min) | (n = 10756) | (n = 440) | 0.568 |
| 220.6 ± 461.4 | 228.5 ± 318.6 | ||
| FMC to PCI (min) | (n = 7854) | (n = 289) | |
| 195.3 ± 1119.3 | 152.6 ± 173.0 | ||
| FMC to PCI <30 min | 283/7854 (3.6%) | 7/289 (2.4%) | |
| FMC to PCI <60 min | 1646/7854 (21.0%) | 40/289 (13.8%) | |
| FMC to PCI <120 min | 4869/7854 (62.0%) | 168/289 (58.1%) |
Mean time intervals (± SD) between symptom-onset, first medical contact (FMC) and percutaneous coronary intervention (PCI)
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Symptom-onset to FMC (min) | (n = 10756) | (n = 440) | 0.568 |
| 220.6 ± 461.4 | 228.5 ± 318.6 | ||
| FMC to PCI (min) | (n = 7854) | (n = 289) | |
| 195.3 ± 1119.3 | 152.6 ± 173.0 | ||
| FMC to PCI <30 min | 283/7854 (3.6%) | 7/289 (2.4%) | |
| FMC to PCI <60 min | 1646/7854 (21.0%) | 40/289 (13.8%) | |
| FMC to PCI <120 min | 4869/7854 (62.0%) | 168/289 (58.1%) |
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| Symptom-onset to FMC (min) | (n = 10756) | (n = 440) | 0.568 |
| 220.6 ± 461.4 | 228.5 ± 318.6 | ||
| FMC to PCI (min) | (n = 7854) | (n = 289) | |
| 195.3 ± 1119.3 | 152.6 ± 173.0 | ||
| FMC to PCI <30 min | 283/7854 (3.6%) | 7/289 (2.4%) | |
| FMC to PCI <60 min | 1646/7854 (21.0%) | 40/289 (13.8%) | |
| FMC to PCI <120 min | 4869/7854 (62.0%) | 168/289 (58.1%) |
Reperfusion therapy
The intended treatment in patients with and without CS was PCI in the centre in 70.9% vs. 72.6%, transfer out for PCI at another hospital in 2.1% vs. 3.2%, fibrinolysis in 14.8% vs. 18.7%, no acute reperfusion therapy in 12.2% vs. 5.5%, and not determined in 3.5% vs. 5.8%, respectively Treatment received with and without CS occurred for primary PCI in 65.2% vs. 72.5%, fibrinolysis in 15.9% vs. 19.0%, and without acute reperfusion therapy in 19.0 vs. 8.5%, respectively (Figure 1). Acute reperfusion therapies in seven predefined regions13 are shown in Supplementary material online, Table S1. The reasons for not performing acute reperfusion therapy in the patients with and without CS were as follows: deemed clinically inappropriate (45.3% vs. 17.2%), contraindication to anticoagulation/antiplatelet therapy (13.2% vs. 4.6%), late presentation (28.3% vs. 40.4%), spontaneous reperfusion (0% vs. 16.9%), wrong diagnosis (0% vs. 4.0%), patient refusal (0% vs. 5.6%), and other (13.2% vs. 11.2%), respectively.
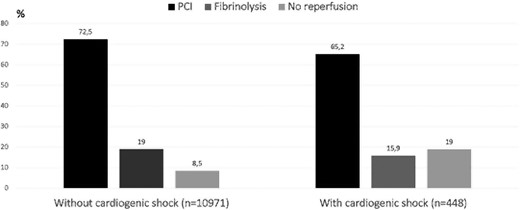
Rate of early reperfusion therapies performed in patients with and without cardiogenic shock. PCI , primary percutaneous coronary intervention.
Invasive coronary findings and interventional features
Patients with CS more often had three-vessel disease and unprotected left main stem disease (Table 4). Before PCI, the culprit lesion was more often occluded in patients with CS vs. those without CS. Restoration of normal flow [thrombolysis in myocardial infarction (TIMI) 3 patency] in the infarct-related artery after PCI was observed less often in CS (81.1% vs. 89.9%, P < 0.001). Thrombectomy was used infrequently in both groups. Mechanical support devices [intraaortic ballon pump (IABP) 11.2%, extracoporeal membrane oxygenation (ECMO) 0.7%, other 1.1%] were used in about 14% in CS.
Angiographic findings and procedural features of PCI in patients with and without cardiogenic shock
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| N | 8885 | 313 | |
| Single vessel disease | 4079 (45.7%) | 122 (39.0%) | <0.001 |
| Two-vessel disease | 2755 (30.8%) | 83 (26.5%) | |
| Three-vessel disease | 1886 (21.1%) | 103 (32.9%) | |
| Unprotected left main disease | 745/8885 (8.4%) | 56/317 (17.7%) | <0.001 |
| Arterial access | |||
| Femoral | 3740/8993 (41.6%) | 188/319 (58.9%) | <0.001 |
| Radial | 5147/8993 (57.2%) | 122/319 (38.2%) | |
| Both | 99/8993 (1.1%) | 7/31 (2.2%) | |
| TIMI flow culprit vessel before PCI | N = 8401 | N = 296 | |
| 0/1 | 6582 (78.5%) | 258 (87.2%) | <0.001 |
| 2 | 936 (11.1%) | 27 (9.1%) | |
| 3 | 873 (10.4%) | 11 (3.7%) | |
| TIMI flow culprit vessel after PCI | N = 8633 | N = 302 | |
| 0/1 | 311 (3.6%) | 28 (9.3%) | <0.001 |
| 2 | 558 (6.5%) | 29 (9.6%) | |
| 3 | 7764 (89.9%) | 245 (81.1%) | |
| Thrombectomy | 1686/8395 (20.1%) | 72/303 (23.8%) | 0.117 |
| Non-culprit PCI during index PCI procedure | 628/8911 (7.1%) | 43/318 (13.5%) | <0.001 |
| Non-culprit PCI during primary PCI procedure | 557/7950 (7.0%) | 40/292 (13.8%) | <0.001 |
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| N | 8885 | 313 | |
| Single vessel disease | 4079 (45.7%) | 122 (39.0%) | <0.001 |
| Two-vessel disease | 2755 (30.8%) | 83 (26.5%) | |
| Three-vessel disease | 1886 (21.1%) | 103 (32.9%) | |
| Unprotected left main disease | 745/8885 (8.4%) | 56/317 (17.7%) | <0.001 |
| Arterial access | |||
| Femoral | 3740/8993 (41.6%) | 188/319 (58.9%) | <0.001 |
| Radial | 5147/8993 (57.2%) | 122/319 (38.2%) | |
| Both | 99/8993 (1.1%) | 7/31 (2.2%) | |
| TIMI flow culprit vessel before PCI | N = 8401 | N = 296 | |
| 0/1 | 6582 (78.5%) | 258 (87.2%) | <0.001 |
| 2 | 936 (11.1%) | 27 (9.1%) | |
| 3 | 873 (10.4%) | 11 (3.7%) | |
| TIMI flow culprit vessel after PCI | N = 8633 | N = 302 | |
| 0/1 | 311 (3.6%) | 28 (9.3%) | <0.001 |
| 2 | 558 (6.5%) | 29 (9.6%) | |
| 3 | 7764 (89.9%) | 245 (81.1%) | |
| Thrombectomy | 1686/8395 (20.1%) | 72/303 (23.8%) | 0.117 |
| Non-culprit PCI during index PCI procedure | 628/8911 (7.1%) | 43/318 (13.5%) | <0.001 |
| Non-culprit PCI during primary PCI procedure | 557/7950 (7.0%) | 40/292 (13.8%) | <0.001 |
Angiographic findings and procedural features of PCI in patients with and without cardiogenic shock
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| N | 8885 | 313 | |
| Single vessel disease | 4079 (45.7%) | 122 (39.0%) | <0.001 |
| Two-vessel disease | 2755 (30.8%) | 83 (26.5%) | |
| Three-vessel disease | 1886 (21.1%) | 103 (32.9%) | |
| Unprotected left main disease | 745/8885 (8.4%) | 56/317 (17.7%) | <0.001 |
| Arterial access | |||
| Femoral | 3740/8993 (41.6%) | 188/319 (58.9%) | <0.001 |
| Radial | 5147/8993 (57.2%) | 122/319 (38.2%) | |
| Both | 99/8993 (1.1%) | 7/31 (2.2%) | |
| TIMI flow culprit vessel before PCI | N = 8401 | N = 296 | |
| 0/1 | 6582 (78.5%) | 258 (87.2%) | <0.001 |
| 2 | 936 (11.1%) | 27 (9.1%) | |
| 3 | 873 (10.4%) | 11 (3.7%) | |
| TIMI flow culprit vessel after PCI | N = 8633 | N = 302 | |
| 0/1 | 311 (3.6%) | 28 (9.3%) | <0.001 |
| 2 | 558 (6.5%) | 29 (9.6%) | |
| 3 | 7764 (89.9%) | 245 (81.1%) | |
| Thrombectomy | 1686/8395 (20.1%) | 72/303 (23.8%) | 0.117 |
| Non-culprit PCI during index PCI procedure | 628/8911 (7.1%) | 43/318 (13.5%) | <0.001 |
| Non-culprit PCI during primary PCI procedure | 557/7950 (7.0%) | 40/292 (13.8%) | <0.001 |
| . | Without CS . | With CS . | P-value . |
|---|---|---|---|
| N | 8885 | 313 | |
| Single vessel disease | 4079 (45.7%) | 122 (39.0%) | <0.001 |
| Two-vessel disease | 2755 (30.8%) | 83 (26.5%) | |
| Three-vessel disease | 1886 (21.1%) | 103 (32.9%) | |
| Unprotected left main disease | 745/8885 (8.4%) | 56/317 (17.7%) | <0.001 |
| Arterial access | |||
| Femoral | 3740/8993 (41.6%) | 188/319 (58.9%) | <0.001 |
| Radial | 5147/8993 (57.2%) | 122/319 (38.2%) | |
| Both | 99/8993 (1.1%) | 7/31 (2.2%) | |
| TIMI flow culprit vessel before PCI | N = 8401 | N = 296 | |
| 0/1 | 6582 (78.5%) | 258 (87.2%) | <0.001 |
| 2 | 936 (11.1%) | 27 (9.1%) | |
| 3 | 873 (10.4%) | 11 (3.7%) | |
| TIMI flow culprit vessel after PCI | N = 8633 | N = 302 | |
| 0/1 | 311 (3.6%) | 28 (9.3%) | <0.001 |
| 2 | 558 (6.5%) | 29 (9.6%) | |
| 3 | 7764 (89.9%) | 245 (81.1%) | |
| Thrombectomy | 1686/8395 (20.1%) | 72/303 (23.8%) | 0.117 |
| Non-culprit PCI during index PCI procedure | 628/8911 (7.1%) | 43/318 (13.5%) | <0.001 |
| Non-culprit PCI during primary PCI procedure | 557/7950 (7.0%) | 40/292 (13.8%) | <0.001 |
Pharmacotherapies
The acute antithrombotic therapies used according to reperfusion therapies are summarized in Table 5. The use of aspirin was over 91%, and the most widely used P2Y12 inhibitor was clopidogrel. Intravenous antiplatelet agents were given somewhat more often in patients with CS (24.8% vs. 19.2%), with glycoprotein (GP) IIb/IIIa inhibitors as preferred choice. With respect to anticoagulation unfractionated heparin was most commonly used followed by low molecular weight heparins, while bivalirudin and fondaparinux were administered only rarely in both groups.
Antithrombotic therapy during the first 24 h according to the presence of cardiogenic shock (CS)
| . | Without CS . | With CS . |
|---|---|---|
| Aspirin | 10701/10 959 (97.7%) | 410/447 (91.7%) |
| Clopidogrel | 7192/10 960 (65.6%) | 287/448 (64.1%) |
| Prasugrel | 1207/10 959 (11.0%) | 28/448 (6.3%) |
| Ticagrelor | 2582/10 960 (23.6%) | 99/448 (22.1%) |
| Dual antiplatelet therapy | 10559/10 958 (96.4%) | 397/447 (88.8%) |
| GP IIb/IIIa inhibitors | 1971/10 254 (19.2%) | 107/431 (24.8%) |
| Cangrelor | 5/10 254 (0.1%) | 3/431 (0.7%) |
| Unfractionated heparin | 6991/10 952 (63.8%) | 300/447 (67.1%) |
| Low-molecular-weight heparin | 4092/10 953 (37.4%) | 146/448 (32.6%) |
| Bivalirudin | 129/10 862 (1.2%) | 4/446 (0.9%) |
| Fondaparinux | 217/10 856 (2.0%) | 5 (446) 1.1% |
| . | Without CS . | With CS . |
|---|---|---|
| Aspirin | 10701/10 959 (97.7%) | 410/447 (91.7%) |
| Clopidogrel | 7192/10 960 (65.6%) | 287/448 (64.1%) |
| Prasugrel | 1207/10 959 (11.0%) | 28/448 (6.3%) |
| Ticagrelor | 2582/10 960 (23.6%) | 99/448 (22.1%) |
| Dual antiplatelet therapy | 10559/10 958 (96.4%) | 397/447 (88.8%) |
| GP IIb/IIIa inhibitors | 1971/10 254 (19.2%) | 107/431 (24.8%) |
| Cangrelor | 5/10 254 (0.1%) | 3/431 (0.7%) |
| Unfractionated heparin | 6991/10 952 (63.8%) | 300/447 (67.1%) |
| Low-molecular-weight heparin | 4092/10 953 (37.4%) | 146/448 (32.6%) |
| Bivalirudin | 129/10 862 (1.2%) | 4/446 (0.9%) |
| Fondaparinux | 217/10 856 (2.0%) | 5 (446) 1.1% |
Antithrombotic therapy during the first 24 h according to the presence of cardiogenic shock (CS)
| . | Without CS . | With CS . |
|---|---|---|
| Aspirin | 10701/10 959 (97.7%) | 410/447 (91.7%) |
| Clopidogrel | 7192/10 960 (65.6%) | 287/448 (64.1%) |
| Prasugrel | 1207/10 959 (11.0%) | 28/448 (6.3%) |
| Ticagrelor | 2582/10 960 (23.6%) | 99/448 (22.1%) |
| Dual antiplatelet therapy | 10559/10 958 (96.4%) | 397/447 (88.8%) |
| GP IIb/IIIa inhibitors | 1971/10 254 (19.2%) | 107/431 (24.8%) |
| Cangrelor | 5/10 254 (0.1%) | 3/431 (0.7%) |
| Unfractionated heparin | 6991/10 952 (63.8%) | 300/447 (67.1%) |
| Low-molecular-weight heparin | 4092/10 953 (37.4%) | 146/448 (32.6%) |
| Bivalirudin | 129/10 862 (1.2%) | 4/446 (0.9%) |
| Fondaparinux | 217/10 856 (2.0%) | 5 (446) 1.1% |
| . | Without CS . | With CS . |
|---|---|---|
| Aspirin | 10701/10 959 (97.7%) | 410/447 (91.7%) |
| Clopidogrel | 7192/10 960 (65.6%) | 287/448 (64.1%) |
| Prasugrel | 1207/10 959 (11.0%) | 28/448 (6.3%) |
| Ticagrelor | 2582/10 960 (23.6%) | 99/448 (22.1%) |
| Dual antiplatelet therapy | 10559/10 958 (96.4%) | 397/447 (88.8%) |
| GP IIb/IIIa inhibitors | 1971/10 254 (19.2%) | 107/431 (24.8%) |
| Cangrelor | 5/10 254 (0.1%) | 3/431 (0.7%) |
| Unfractionated heparin | 6991/10 952 (63.8%) | 300/447 (67.1%) |
| Low-molecular-weight heparin | 4092/10 953 (37.4%) | 146/448 (32.6%) |
| Bivalirudin | 129/10 862 (1.2%) | 4/446 (0.9%) |
| Fondaparinux | 217/10 856 (2.0%) | 5 (446) 1.1% |
In-hospital procedures
Less than half of the patients with CS (44.2%) were mechanically ventilated during the hospital stay and 6.0% received therapeutic hypothermia. Emergency coronary artery bypass graft (CABG) surgery was performed in only 5 (1.1%) of the patients with CS. Additional revascularization procedures after Day 1 were performed in 11.9% of the patients with CS and are listed in Figure 2.
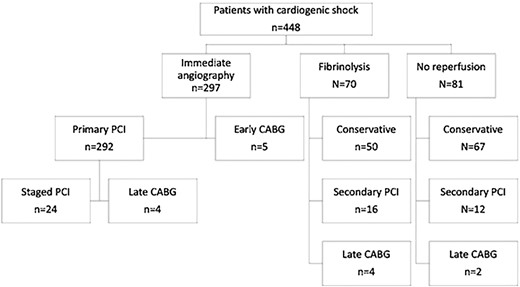
Flow diagram with the rate of initial reperfusion strategy and subsequent revascularization procedures in patients with cardiogenic shock. CABG, coronary artery bypass graft surgery; PCI, percutaneous coronary intervention.
In-hospital outcomes
Median length of stay was 6.2 vs. 8.6 days in patients without and with CS. The in-hospital mortality was 3.1% without and 35.5% with CS. The association between different reperfusion strategies and the in-hospital mortality in patients with and without CS is given in Figure 3. There were 40 of 292 patients (13.8%) with primary PCI and immediate treatment of non-culprit lesions during the index PCI procedure. However, we do not know for sure if this was complete revascularization. The mortality of patients with immediate non-culprit PCI was 27.5% vs. 27.0% of those without immediate non-culprit PCI. Mechanical complications to myocardial infarction were reported in 19 (4.2%) patients with CS. Three had a ventricular septal defect, nine mitral insufficiency, and seven a cardiac tamponade. Definite or probable stent thrombosis and stroke occurred significantly more often patients with CS (Table 6). In addition, BARC bleeding complications were reported more often in patients with CS (Table 6). Left ventricular ejection fraction before discharge in patients with shock (n = 386) was 40 + 12% vs. 46.4 + 11% without shock (n = 10312).
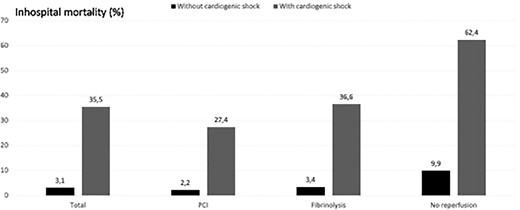
In-hospital mortality according to the initial reperfusion therapy and the presence of cardiogenic shock on admission.
| . | Without CS . | With CS . |
|---|---|---|
| Re-infarction | 113/10 969 (1.0%) | 7/448 (1.6%) |
| Stent thrombosis | ||
| All | 138/10 927 (1.3%) | 17/446 (3.8%) |
| Definite | 97/10 927 (0.9%) | 9/446 (2.0%) |
| Probable | 28/10 927 (0.3%) | 6/446 (1.4%) |
| Stroke | 96/10 969 (0.9%) | 19/448 (4.2%) |
| Total BARC bleeding complications | 597/10 966 (5.4%) | 70/447 (15.6%) |
| BARC 2–5 bleeding | 327 (3.0%) | 45/447 (10.0%) |
| . | Without CS . | With CS . |
|---|---|---|
| Re-infarction | 113/10 969 (1.0%) | 7/448 (1.6%) |
| Stent thrombosis | ||
| All | 138/10 927 (1.3%) | 17/446 (3.8%) |
| Definite | 97/10 927 (0.9%) | 9/446 (2.0%) |
| Probable | 28/10 927 (0.3%) | 6/446 (1.4%) |
| Stroke | 96/10 969 (0.9%) | 19/448 (4.2%) |
| Total BARC bleeding complications | 597/10 966 (5.4%) | 70/447 (15.6%) |
| BARC 2–5 bleeding | 327 (3.0%) | 45/447 (10.0%) |
| . | Without CS . | With CS . |
|---|---|---|
| Re-infarction | 113/10 969 (1.0%) | 7/448 (1.6%) |
| Stent thrombosis | ||
| All | 138/10 927 (1.3%) | 17/446 (3.8%) |
| Definite | 97/10 927 (0.9%) | 9/446 (2.0%) |
| Probable | 28/10 927 (0.3%) | 6/446 (1.4%) |
| Stroke | 96/10 969 (0.9%) | 19/448 (4.2%) |
| Total BARC bleeding complications | 597/10 966 (5.4%) | 70/447 (15.6%) |
| BARC 2–5 bleeding | 327 (3.0%) | 45/447 (10.0%) |
| . | Without CS . | With CS . |
|---|---|---|
| Re-infarction | 113/10 969 (1.0%) | 7/448 (1.6%) |
| Stent thrombosis | ||
| All | 138/10 927 (1.3%) | 17/446 (3.8%) |
| Definite | 97/10 927 (0.9%) | 9/446 (2.0%) |
| Probable | 28/10 927 (0.3%) | 6/446 (1.4%) |
| Stroke | 96/10 969 (0.9%) | 19/448 (4.2%) |
| Total BARC bleeding complications | 597/10 966 (5.4%) | 70/447 (15.6%) |
| BARC 2–5 bleeding | 327 (3.0%) | 45/447 (10.0%) |
Discussion
In this large international registry, including over 11 000 patients with STEMI from 29 countries, the incidence of recorded CS was about 5%.13 The main finding was that patients with CS less often received primary PCI than patients without CS. CS is still the most important factor associated with mortality in patients with STEMI.1 Early reperfusion therapy with PCI has been shown to improve outcome and is therefore recommended in ESC guidelines and position statements.1–3
We found that less than two-third of patients with CS were treated with primary PCI. The in-hospital mortality of these patients was 27.4%, which is somewhat lower compared with the mortality of patients in the two largest randomized trials in CS IABP-shock 215 and culprit-shock.16 This might be due to a selection bias, where for example patients dying very early might have been less likely to be included in our registry. However, our results underscore the clinical benefit of primary PCI in CS.
The data about the efficacy of fibrinolysis in CS are limited. In our analysis, 70 patients (16%) were given fibrinolysis and only 20 of the latter received subsequent revascularization with either PCI (n = 16) or CABG (n = 4). Despite this low rate of revascularization therapy in-hospital mortality was only 36% compared to 62% without reperfusion therapy suggesting the possibility of a beneficial effect of fibrinolysis on outcome in STEMI with CS. However, it is not possible to use these observational data to infer cause and effect, so whether fibrinolysis was responsible for reduced mortality or simply associated, cannot be determined. In the STREAM study in patients with STEMI without CS fibrinolysis followed by early PCI within 6–24 h was not inferior to immediate primary PCI,17 thus it might be speculated that a higher rate of secondary revascularization procedures after fibrinolysis might have led to improved outcomes.
As reported before18,19, the mortality in patients with STEMI and CS not receiving early reperfusion therapy is extremely high, only one-third of these patients survived until discharge. There were multiple reasons given for not performing early reperfusion therapy, however, given the very high mortality these reasons should be evaluated in more depth. Attempts to reduce the high mortality in CS might include an increased rate of primary PCI in these patients and also the use of sophisticated left ventricular support. Such strategies need to be tested in randomized clinical trials.
In this cohort, the use of CABG surgery in CS was rare with only 5 patients receiving emergency CABG surgery, and 10 patients operated on a later timepoint during the initial hospital stay. Because of these low numbers the mortality of this approach cannot be properly evaluated in our data set.20
The culprit-shock trial16 has shown that immediate multivessel PCI with PCI of non-culprit lesions is associated with an impaired prognosis in CS. In our registry, the overall rate of immediate non-culprit PCI was low, but performed two times more often in patients with CS. This might be due to the fact that the 2012 ESC STEMI Guidelines2 (current at the time of recruitment into this registry) were more in favour of immediate multivessel PCI in CS, as these guidelines were published before the culprit-shock trial has been published. Procedural success defined as TIMI 3 flow of the culprit lesion was around 10% lower in patients with CS. However, the TIMI flow rate in CS was above 80%, suggesting that these patients can be treated with a high success rate in clinical practice in different countries and centres. These results are in line with earlier reports of the procedural success rates in CS.21
Mechanical support devices were used infrequently in CS. Despite the fact that the IABP is not recommended in the ESC guidelines for routine use in CS about 11% were treated with an IABP, while only less than 2% received other mechanical support devices. While the IABP has been studied in a large-randomized trial, the evidence for the use of ECMO and Impella is limited,1 which could be the reason for low usage in our registry.
Limitations
Despite the large number of patients included the representativeness of the patient population for the participating countries and Europe was limited. The rate of patients with CS was lower than 5%, which suggests selection bias, and limits the ability to extrapolate our findings to the wider population. Data about left ventricular ejection fraction were not available on admission and for the acute phase but only at discharge.
Conclusion
In our observational study, we observed a low use of primary PCI in patients with STEMI complicated by CS. Mortality in CS without reperfusion therapy is high and efforts should be made to try to understand ways to improve this, which might include an increase in the rate of early revascularization.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care.
Acknowledgements
EORP Oversight Committee, The Registry Executive Committee of the EURObservational Research Programme (EORP). Data collection was conducted by the EORP department from the ESC by Marème Konte and Florian Larras as Data Managers, Elin Folkesson Lefrancq as Project Officer, Souad Mekhaldi as Clinical Project Manager. Statistical analyses were performed by Cécile Laroche. Overall activities are coordinated by Aldo P. Maggioni (Scientific Coordinator EORP). Saudi Heart Association. The Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia (Research group number: RG -1436-013).
Funding
Since the start of EORP, the following companies have supported the programme: Abbott Vascular Int. (2011–2021), Amgen Cardiovascular (2009–2018), AstraZeneca (2014–2021), Bayer AG (2009–2018), Boehringer Ingelheim (2009–2019), Boston Scientific (2009–2012), The Bristol Myers Squibb and Pfizer Alliance (2011–2019), Daiichi Sankyo Europe GmbH (2011–2020), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–2017), Edwards (2016–2019), Gedeon Richter Plc. (2014–2016), Menarini Int. Op. (2009–2012), MSD-Merck & Co. (2011–2014), Novartis Pharma AG (2014–2020), ResMed (2014–2016), Sanofi 2009–2011), SERVIER (2009–2021), and Vifor (2019–2022).
Conflicts of interest: U.Z. reports personal fees from Amgen, grants and personal fees from Astra Zeneca, Abiomed, Bayer, BMS, personal fees from Novartis, Sanofi, Boehringer Ingelheim, grants and personal fees from Tommsdorf, personal fees from Pfizer, The Medicines Company, outside the submitted work. P.L. Stanislaw Bartus, Cécile Laroche, Masoumeh Sadeghi, and Ahmed Wafa have nothing to disclose. N.D. reports grants, personal fees and non-financial support from Amgen, grants, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from Bayer, grants, personal fees and non-financial support from BMS/Pfizer, personal fees from Boehringer Ingelheim, personal fees from Intercept, personal fees from MSD, personal fees from Sanofi, personal fees from Servier, personal fees from UCB, personal fees from Vifor, outside the submitted work. P.K. reports personal fees from Boston Scientific, Novartis, Edwards Lifesciences, Astra Zeneca, Medtronic, outside the submitted work. C.P.G. reports personal fees from AstraZeneca, personal fees from Amgen, personal fees from Bayer, personal fees from Daiichi Sankyo, grants from Abbott, grants from BMS, other from WondrMedical, outside the submitted work. A.P.M. reports personal fees from Bayer, personal fees from Fresenius, personal fees from Novartis, outside the submitted work. S.S. would like to acknowledge Kermanshah University Of Medical Sciences (KUMS) for their support in data gathering enabled us to make this work. F.W. reports personal fees from Boehringer Ingelheim, outside the submitted work.
Data availability
The data belong to the EORP of the ESC and are not available.
References
Author notes
Listed in the Supplementary material online, Appendix 1.




Comments