-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher F Barnett, Connor O’Brien, Teresa De Marco, Critical care management of the patient with pulmonary hypertension, European Heart Journal. Acute Cardiovascular Care, Volume 11, Issue 1, January 2022, Pages 77–83, https://doi.org/10.1093/ehjacc/zuab113
Close - Share Icon Share
Abstract
Pulmonary hypertension (PH) is a common diagnosis in patients admitted to the cardiac intensive care unit with a wide range of underlying causes. A detailed evaluation to identify all factors contributing to the elevated pulmonary artery pressure and provide an assessment of right ventricular haemodynamics and function is needed to guide treatment and identify patients at highest risk for poor outcomes. While in many patients management of underlying and triggering medical problems with careful monitoring is appropriate, a subset of patients may benefit from specialized treatments targeting the pulmonary circulation and support of the right ventricle. In such cases, collaboration with or transfer to a centre with special expertise in the management of PH may be warranted.
Introduction
Pulmonary hypertension (PH) refers to a mean pulmonary artery pressure (mPAP) >20 mmHg, which is a common finding in patients admitted to the cardiac intensive care unit (CICU). The current classification of PH (Table 1) is a framework for the diagnostic evaluation of PH. Most commonly, PH is from left heart disease (LHD; Group 2 PH). Often multiple causes of PH co-exist and identifying potentially multiple factors that are contributing is necessary for optimal management. Haemodynamic findings of mPAP >20 mmHg, pulmonary artery wedge pressure (PAWP) ≤15 mmHg and pulmonary vascular resistance (PVR) ≥3 Wood units defines pre-capillary PH (Table 2) and in the correct clinical context suggest a diagnosis of Group 1 pulmonary arterial hypertension (PAH), Group 3 PH from lung disease (without comorbid LHD) or Group 4 PH from pulmonary artery obstruction from chronic thrombo-embolic PH (CTEPH) or other obstruction (Figure 1).
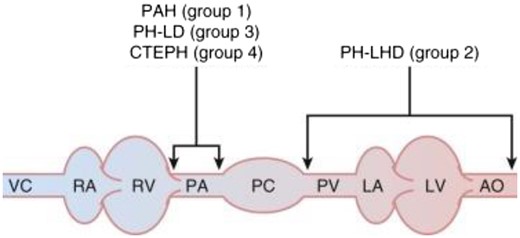
Localization of abnormalities in pulmonary hypertension. Pathologic changes that result in Group 1 pulmonary arterial hypertension (PAH), Group 3 pulmonary hypertension (PH) from lung disease (PH-LD), and Group 4 pulmonary hypertension from chronic thrombo-embolic pulmonary hypertension (CTEPH) primarily affect pulmonary arterioles, abnormalities that result in Group 2 PH left heart disease (PH-LHD) are found mostly on the venous side of the pulmonary circulation. AO, aorta; LA, left atrium; LV, left ventricle; PA, pulmonary arteries; PC, pulmonary capillaries; PV, pulmonary veins; RA, right atrium; RV, right ventricle; VC, vena cavae. Adapted from Barnett and De Marco.34
| 1. PAH |
|
| 2. PH due to left heart disease |
|
| 3. PH due to lung disease and/or hypoxia |
|
| 4. PH due to pulmonary artery obstructions |
|
| 5. PH with unclear and/or multifactorial mechanisms |
|
| 1. PAH |
|
| 2. PH due to left heart disease |
|
| 3. PH due to lung disease and/or hypoxia |
|
| 4. PH due to pulmonary artery obstructions |
|
| 5. PH with unclear and/or multifactorial mechanisms |
|
Adapted from Simonneau et al.14
CHD, congenital heart disease; CTD, connective tissue disease; CV, cardiovascular; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PAH, pulmonary arterial hypertension; PCH, pulmonary capillary hemangiomatosis; PH, pulmonary hypertension; PVOD, pulmonary veno-occlusive disease.
| 1. PAH |
|
| 2. PH due to left heart disease |
|
| 3. PH due to lung disease and/or hypoxia |
|
| 4. PH due to pulmonary artery obstructions |
|
| 5. PH with unclear and/or multifactorial mechanisms |
|
| 1. PAH |
|
| 2. PH due to left heart disease |
|
| 3. PH due to lung disease and/or hypoxia |
|
| 4. PH due to pulmonary artery obstructions |
|
| 5. PH with unclear and/or multifactorial mechanisms |
|
Adapted from Simonneau et al.14
CHD, congenital heart disease; CTD, connective tissue disease; CV, cardiovascular; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PAH, pulmonary arterial hypertension; PCH, pulmonary capillary hemangiomatosis; PH, pulmonary hypertension; PVOD, pulmonary veno-occlusive disease.
| Definitions . | Characteristics . | Clinical groupsa . |
|---|---|---|
| Pre-capillary PH | mPAP >20 mmHg | 1, 3, 4, and 5 |
| PAWP ≤15 mmHg | ||
| PVR ≥3 WU | ||
| Isolated post-capillary PH (IpcPH) | mPAP >20 mmHg | 2 and 5 |
| PAWP >15 mmHg | ||
| PVR <3 WU | ||
| Combined pre- and post-capillary PH (CpcPHI) | mPAP >20 mmHg | 2 and 5 |
| PAWP >15 mmHg | ||
| PVR ≥3 WU |
| Definitions . | Characteristics . | Clinical groupsa . |
|---|---|---|
| Pre-capillary PH | mPAP >20 mmHg | 1, 3, 4, and 5 |
| PAWP ≤15 mmHg | ||
| PVR ≥3 WU | ||
| Isolated post-capillary PH (IpcPH) | mPAP >20 mmHg | 2 and 5 |
| PAWP >15 mmHg | ||
| PVR <3 WU | ||
| Combined pre- and post-capillary PH (CpcPHI) | mPAP >20 mmHg | 2 and 5 |
| PAWP >15 mmHg | ||
| PVR ≥3 WU |
Adapted from Simonneau et al.14
mPAP, mean pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; WU, Wood units.
Group 1: PAH; Group 2: PH due to left heart disease; Group 3: PH due to lung diseases and/or hypoxia; Group 4: PH due to pulmonary artery obstructions; Group 5: PH with unclear and/or multifactorial mechanisms.
| Definitions . | Characteristics . | Clinical groupsa . |
|---|---|---|
| Pre-capillary PH | mPAP >20 mmHg | 1, 3, 4, and 5 |
| PAWP ≤15 mmHg | ||
| PVR ≥3 WU | ||
| Isolated post-capillary PH (IpcPH) | mPAP >20 mmHg | 2 and 5 |
| PAWP >15 mmHg | ||
| PVR <3 WU | ||
| Combined pre- and post-capillary PH (CpcPHI) | mPAP >20 mmHg | 2 and 5 |
| PAWP >15 mmHg | ||
| PVR ≥3 WU |
| Definitions . | Characteristics . | Clinical groupsa . |
|---|---|---|
| Pre-capillary PH | mPAP >20 mmHg | 1, 3, 4, and 5 |
| PAWP ≤15 mmHg | ||
| PVR ≥3 WU | ||
| Isolated post-capillary PH (IpcPH) | mPAP >20 mmHg | 2 and 5 |
| PAWP >15 mmHg | ||
| PVR <3 WU | ||
| Combined pre- and post-capillary PH (CpcPHI) | mPAP >20 mmHg | 2 and 5 |
| PAWP >15 mmHg | ||
| PVR ≥3 WU |
Adapted from Simonneau et al.14
mPAP, mean pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; WU, Wood units.
Group 1: PAH; Group 2: PH due to left heart disease; Group 3: PH due to lung diseases and/or hypoxia; Group 4: PH due to pulmonary artery obstructions; Group 5: PH with unclear and/or multifactorial mechanisms.
| Drug . | Cardiac output . | PVR . | SVR . | Tachycardia/arrhythmia . |
|---|---|---|---|---|
| Inotropes | ||||
| Dobutamine | ||||
| <5 µg·kg−1·min−1 |  |  |  or or  | ++ |
| 5–15 µg·kg−1·min−1 |  |  |  | +++ |
| Milrinone |  |  |   | ++ |
| Epinephrine |  |  |  | +++ |
| Vasopressors | ||||
| Norepinephrine |  |  or or  |  | ++ |
| Vasopressin (low doses) |  or or  |  |  | – |
| Drug . | Cardiac output . | PVR . | SVR . | Tachycardia/arrhythmia . |
|---|---|---|---|---|
| Inotropes | ||||
| Dobutamine | ||||
| <5 µg·kg−1·min−1 |  |  |  or or  | ++ |
| 5–15 µg·kg−1·min−1 |  |  |  | +++ |
| Milrinone |  |  |   | ++ |
| Epinephrine |  |  |  | +++ |
| Vasopressors | ||||
| Norepinephrine |  |  or or  |  | ++ |
| Vasopressin (low doses) |  or or  |  |  | – |
Adapted from Hoeper et al.16
PH, pulmonary hypertension; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance.
| Drug . | Cardiac output . | PVR . | SVR . | Tachycardia/arrhythmia . |
|---|---|---|---|---|
| Inotropes | ||||
| Dobutamine | ||||
| <5 µg·kg−1·min−1 |  |  |  or or  | ++ |
| 5–15 µg·kg−1·min−1 |  |  |  | +++ |
| Milrinone |  |  |   | ++ |
| Epinephrine |  |  |  | +++ |
| Vasopressors | ||||
| Norepinephrine |  |  or or  |  | ++ |
| Vasopressin (low doses) |  or or  |  |  | – |
| Drug . | Cardiac output . | PVR . | SVR . | Tachycardia/arrhythmia . |
|---|---|---|---|---|
| Inotropes | ||||
| Dobutamine | ||||
| <5 µg·kg−1·min−1 |  |  |  or or  | ++ |
| 5–15 µg·kg−1·min−1 |  |  |  | +++ |
| Milrinone |  |  |   | ++ |
| Epinephrine |  |  |  | +++ |
| Vasopressors | ||||
| Norepinephrine |  |  or or  |  | ++ |
| Vasopressin (low doses) |  or or  |  |  | – |
Adapted from Hoeper et al.16
PH, pulmonary hypertension; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance.
Patients may be admitted to the CICU with worsening PH and right ventricular (RV) failure, other resultant end-organ dysfunction, comorbid medical and surgical problems, or an initial presentation with PH. This review will describe the approach to diagnosis and evaluation of PH in the critically ill patient as well as to managing PH causing RV failure. The approach to RV failure from other causes is not addressed.
Epidemiology and outcomes
The frequency of PH in unselected intensive care unit (ICU) patients is high. In a retrospective study of ICU patients, 42% with an echocardiogram had PH. Patients with PH had a mortality of 37% vs. 25% in controls. Moreover, PH was associated with low ejection fraction and pulmonary embolism.1 Therefore, careful evaluation to identify treatable causes of PH may be useful; however, a high rate of LHD (Group 2 PH) reduces the likelihood that agents targeting the pulmonary circulation would impact most patient outcomes.
Survival among patients with PAH admitted with critical illness is especially poor. A report of 119 patients with PAH hospitalized for acute RV failure described death or urgent transplantation in 38% by 90 days. Higher respiratory rate, renal dysfunction, hyponatraemia, and worse tricuspid regurgitation were associated with a poor outcome.2 In the ASPIRE registry, hospital survival was 59.3%, 94%, and 92% among PAH patients admitted for medical, surgical, or obstetric reasons. Risk factors that correlated with higher mortality included: oxygen (SpO2/FiO2) ≤185; platelets ≤196 × 109·L−1; age ≥37.5 years; lactate ≥2.45 mmol·L−1; and sodium ≤130.5 mmol·L−1. Importantly, only 11% of patients treated with mechanical ventilation and 50% of patients treated with renal replacement therapy survived to hospital discharge.3
The outcomes of cardiac arrest are particularly grim and most attempts at resuscitation are unsuccessful. In an international study of PH expert centres, 6% of patients survived beyond 90 days after in-hospital arrest.4 These poor outcomes support a need for early and aggressive management, early consideration for transfer to a PH specialty centre and, if appropriate, early consideration for mechanical circulatory support (MCS) and transplantation.5
The right ventricle
The healthy RV is a thin-walled, crescent-shaped structure that ejects blood into the low resistance, high compliance, and low impedance pulmonary circulation. Contraction of the left ventricle augments RV output. Coronary blood flow to the RV myocardium occurs throughout the cardiac cycle.6 Any cause of PH may ultimately progress to RV failure. As PH develops and afterload increases, the RV hypertrophies and eventually dilates taking on a spherical shape. Right ventricular wall stress increases, and heart rate rises thereby increasing myocardial oxygen consumption while the coronary perfusion gradient across the RV is diminished due to reduced diastolic blood pressure and high RV end-diastolic pressure, leading to an imbalance between supply and demand, contributing to RV ischaemia. Progressive impairment of myocardial contractility and worsening tricuspid regurgitation reduce cardiac output. As a result of ventricular interdependence, RV dilation causes a shift of the interventricular septum impairing left ventricular (LV) filling and systemic cardiac output (Figure 2).
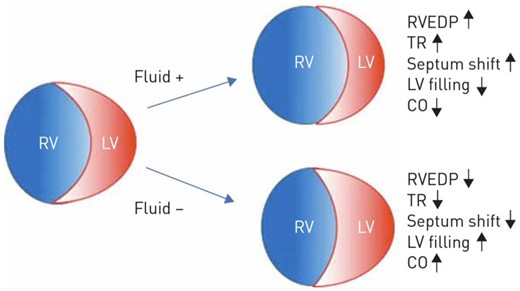
Effects of volume changes on the right ventricular geometry and haemodynamics. Reproduced from Olsson KM, Halank M, Egenlauf B, et al. Decompensated right heart failure, intensive care and perioperative management in patients with pulmonary hypertension: updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018; 272S: 46–52.16
Furthermore, as the right atrial (RA) pressure rises, the intrapericardial pressure also rises, reducing the transmural LV distending pressure, the effective preload to the LV. The rise of the RA pressure and coronary sinus pressure can also lead to LV myocardial congestion and stiffness, impairing LV diastolic function which, combined with diastolic interventricular dependence, can further reduce LV compliance and coronary flow. Reduced coronary blood flow to the RV myocardium and acute increases in RV wall stress can lead to worsening RV ischaemia, reduced RV filling, and haemodynamic collapse.7
Diagnostic testing to detect pulmonary hypertension in the intensive care unit
Echocardiography facilitates expeditious identification of PH and RV dysfunction in the CICU. The diagnostic approach is similar in established and de novo presentations of PH. Worsening of underlying PH and RV failure is common. Additionally, intercurrent comorbid medical and surgical problems often trigger episodes of RV failure and must be rapidly identified and treated.3 A diagnostic evaluation for all possible causes of PH is recommended to identify causes previously missed and because co-existence of multiple causes of PH is common. In patients with Group 3 PH from lung disease or Group 4 PH from CTEPH, evaluation for lung transplantation and surgical pulmonary endarterectomy/balloon angioplasty, respectively, are often indicated and, in our opinion, such patients should be considered for early transfer to specialty centres with these capabilities.
A medical history can identify factors that cause or worsen PH. Risk factors for PAH or Group 4 CTEPH should be elicited, including family history of PAH, connective tissue disease (CTD), recreational drug and medication use, HIV infection, liver disease, congenital heart disease (CHD), deep vein thrombosis, pulmonary embolism, or thrombophilia.
The physical exam may identify findings that suggest an underlying cause of PH: stigmata of liver disease, parenchymal lung disease, or skin changes consistent with scleroderma. Findings for RV failure are generally insensitive, non-specific, and in the CICU environment may be difficult to detect. Exam findings of RV failure include an elevated jugular venous pressure with a large v wave, a loud P2 component of the second heart sound, and palpable RV heave. Hepatomegaly, ascites, and peripheral oedema may also be present. Low pulse pressure and cool extremities may indicate hypoperfusion and impending cardiogenic shock.
Recommended laboratory testing includes HIV, thyroid function tests, antinuclear antibody testing, additional serologies for specific CTD, and urine toxicology. Elevated troponin, B-type natriuretic peptide and lactic acid, markers of renal and hepatic function as well as hyponatraemia are indicative of a worse prognosis and can be used to guide management.2 Liver transaminase elevation, low albumin, elevated INR, and hepatitis serologies are useful to identify patients at risk of cirrhosis and portal hypertension.
The electrocardiogram is insensitive in the assessment of the RV and may only become abnormal in the presence of severe abnormalities. Findings of an abnormal RV include right axis deviation, R/S wave >1 in V1 with R wave >0.5 mV, and P wave was peaked and >2.5 mm in height in leads II, III, or aVF.8 The chest radiograph is similarly insensitive, though enlarged pulmonary arteries may be appreciated. Abnormalities consistent with parenchymal lung disease should prompt further diagnostic imaging.9
Echocardiography is among the most useful tools to assess haemodynamics, evaluate the RV, and identify LHD causing PH.10 An enlarged and hypertrophied RV, a small left heart and mid-systolic notching of the pulse wave Doppler signal in the RV outflow tract all suggest a pre-capillary cause of PH. Qualitative echocardiography findings that indicate RV dysfunction include a tricuspid annular plane systolic excursion (TAPSE) <1.8 cm, a fractional area change <35%, a systolic excursion velocity (RV S’) <10 cm • s−1, and an RV mid-cavitary diameter >35 mm.10 A saline contrast (‘bubble’) study is useful to identify some forms of CHD. Interrogation of the inferior vena cava is used to estimate the right atrial pressure.
Additional diagnostic testing should be performed to exclude other causes of PH. A liver ultrasound with portal blood flow assessment is performed to exclude cirrhosis and portal hypertension. A computed tomography (CT) scan excludes parenchymal lung disease and adenopathy that could suggest sarcoidosis. Ventilation-perfusion (V/Q) scanning is the test of choice to initially screen for CTEPH. Computed tomography pulmonary angiography is less sensitive and cannot exclude CTEPH but is useful when a V/Q is not possible. A CT angiogram is also useful to identify extracardiac shunting and varices caused by portal hypertension. Cardiac magnetic resonance imaging (MRI) is excellent to assess the RV, detect CHD, infiltrative cardiomyopathies, and cardiomyopathies affecting the RV. Long scanning times, contraindications, and medical devices that are incompatible with the MRI scanner limit the use of MRI in CICU patients.11
Invasive haemodynamic assessment and monitoring with a pulmonary artery catheter (PAC) should be considered early in CICU patients with PH to define haemodynamics (Table 2), optimize cardiac filling pressures, and titrate vasoactive drug. While use of the PAC in routine management of heart failure is discouraged, using the PAC to guide management in PH is an accepted practice12 and may improve outcomes.13 Invasive haemodynamics are mandatory to make a diagnosis of group 1 PAH prior to the initiation of PAH therapies.14 Trained and experienced ICU team members are needed to ensure that PAC use is safe and that data collection, interpretation, and translation into management are executed correctly.12
Overview of patient management
Rapid decompensation with cardiogenic shock from RV failure is common in PH patients, may be irreversible and may lead to death.4,15 Rapid initiation of therapies, frequent reassessment, and careful monitoring of the response to those therapies is important to optimize outcomes (Figure 3). End-organ dysfunction secondary to low cardiac output and venous congestion is common and must be aggressively managed. Management of PH patients in a PH expert centre that can provide access to all treatment modalities including PAH medications, MCS, and transplantation has been strongly recommended.5,16 Transfer to a PH centre should be considered early for all hospitalized severe PH patients.5
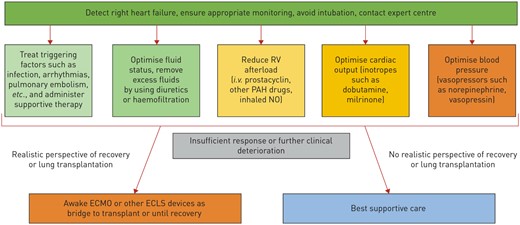
Approach to management of right ventricular failure. RV, right ventricle. Reproduced from Olsson KM, Halank M, Egenlauf B, et al. Decompensated right heart failure, intensive care and perioperative management in patients with pulmonary hypertension: updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018; 272S: 46–52.
While many patients with PH present with progressive disease, decompensation may also be precipitated by other common conditions, including infection, respiratory failure, bleeding, arrhythmia, myocardial infarction, pulmonary embolism and thyroid disease.2,15,17 A rapid assessment to identify and determine appropriate management for these problems, if present, is necessary. Hypoxaemia is common and should be treated with supplemental oxygen. Because hypoxaemia can cause pulmonary vasoconstriction and target oxygen saturation over 90% is recommended.16 Supraventricular arrhythmias are poorly tolerated. Rapid intervention to restore sinus rhythm is recommended, including early cardioversion.18 Medications, including non-dihydropyridine calcium channel blockers and beta-blockers, are often contraindicated and amiodarone is not well tolerated. Digoxin has favourable haemodynamic effects and may be especially useful in patients with PH.
Management of pulmonary hypertension medications in the intensive care unit
Decisions to continue or initiate PAH medications in critically ill patients are complex and should be made by an experienced PH team that includes pharmacists.19 In most patients admitted to the CICU, home PAH medications should be continued. Because most PAH therapies preferentially lower PVR, systemic hypotension may not necessitate cessation. Abrupt cessation or dose-reduction of PAH therapy can lead to rapid decompensation and death. Some therapies, such as intravenous prostacyclins, require supplies, equipment, protocols, and training that are not available outside of PH expert centres. Harm, including death, due to PAH medication administration errors has been reported.5,19 Initiation of a new PAH medication should be in collaboration with the patient and patient’s caregivers as well as PAH team members to ensure that the patient will be able to continue the medication after discharge.19
There are few data to guide the use of PAH medications in critically ill patients. Decisions about initiation and titrations must be made by extrapolating data from other populations, knowledge of the haemodynamic effects of each drug, and local expertise. With few exceptions, PAH medications are indicated for the treatment of Group 1 PAH and administration to patients with non-Group 1 PAH may cause worsening symptoms and increase mortality.20–22 Group 2 PH treated with PAH medications can rapidly precipitate pulmonary oedema.20
There are three available classes of PAH medications.16 Epoprostenol is a potent pulmonary vasodilator that improves outcomes in Group 1 PAH and is frequently the drug of choice in PAH with RV failure. It is administered as a continuous intravenous infusion and is typically started at 1–2 µg•kg−1•min−1 then increased in 0.5–1 µg•kg−1•min−1 increments as often as every 30 min as tolerated. Patients should frequently be reassessed to ensure that cardiac output increases, PAP is reduced and that a high cardiac output state is not precipitated. Slow titration is required to avoid common side effects such as systemic hypotension, nausea, vomiting, and jaw pain. Continuous inhaled epoprostenol has less haemodynamic effect than the intravenous route but does not cause systemic hypotension. Inhaled epoprostenol is frequently used when inhaled nitric oxide is not available or based on consideration of cost. Inhaled epoprostenol is typically initiated at a dose of 50 µg•kg−1•min−1 and when no longer needed is reduced by 10 µg•kg−1•min−1 increments.
Treprostinil is an analogue of epoprostenol with intravenous, subcutaneous, inhaled, and oral formulations. It is less commonly initiated in the ICU setting due to its longer half-life. Selexipag is an oral prostacyclin receptor antagonist and may be part of a PAH patient’s outpatient regimen.
Sildenafil and tadalafil are phosphodiesterase type 5 antagonists that augment the effects of nitric oxide. Sildenafil has a short half-life and few side effects making it a useful for critically ill patients. It is typically initiated at 20 mg three times daily and increased daily until a target dose of 100 mg three times daily is achieved.23 Riociguat acts in the same pathway but directly stimulates soluble guanylate cyclase. Due to risk of hypotension, riociguat is typically titrated to target over weeks and is not useful in the ICU.
Bosentan, macitentan, and ambrisentan are the final class of commonly used PAH medication and act by blocking the action of endothelin, a potent vasoconstrictor.10 Common side effects of this class of medications include anaemia and fluid retention and they are highly teratogenic. They are commonly used in outpatient PAH management but less commonly initiated in the ICU.
Inhaled nitric oxide (NO) is administered only in the ICU while triggers of RV failure are treated or other long-term PAH therapy is implemented. Due to high cost, it is not widely available. It is a selective pulmonary vasodilator that reduces the PVR without reducing systemic vascular resistance (SVR) and causing systemic hypotension. NO also does not lead to V/Q mismatch and worsening hypoxaemia. NO is typically started at a maximal dose of 40 ppm. It is slowly reduced to avoid a rebound in pulmonary artery pressure. Monitoring for methemoglobinemia is needed during NO therapy.24
Fluid management
Meticulous volume management to optimize cardiac filling pressures is crucial in the management of PH and RV failure. Volume overload leads to worsening of RV geometry, septal shift into the LV, and tricuspid regurgitation, worsening cardiac output and RV myocardial ischaemia (Figure 2). Elevated RA/RV pressures lead to systemic venous congestion and causes malperfusion and injury to other organs especially the liver, kidney, and intestines.16 Intravenous diuretics or haemofiltration may markedly improve haemodynamics. Invasive haemodynamic monitoring with frequent reassessment of cardiac filling may be optimal to guide volume management.
Haemodynamic support with vasopressors and inotropes
The goals of haemodynamic support in PH are restoring cardiac output and maintaining adequate systemic blood pressure while reducing PVR. There are limited data to inform haemodynamic goals. Expert opinion favours maintaining SVR>PVR, mean arterial pressure>mPAP, and systolic blood pressure >systolic PAP. Our practice is to increase vasopressor and inotropic medications until examination and laboratory testing suggests adequate clinical perfusion with a cardiac index typically higher than 2 L•min−1•m−2.
Dobutamine and milrinone, in particular, can be used to lower SVR and PVR and increase cardiac output in RV failure (Table 3).16 Milrinone may reduce the SVR more than the PVR especially in patients with pre-capillary PH and must be used with caution as it can lead to prolonged systemic hypotension given its long half-life. Systemic hypotension necessitates the addition of vasopressors. Norepinephrine does not lower PVR but may have the beneficial effect of improving RV–PA coupling.25 Data from ex vivo vascular tissue preparation studies has informed the current understanding that vasopressin reduces PVR and increases systemic blood pressure making it a desirable agent for use in PAH. However, at very high doses of 1.16 units • kg−1 • h−1 (much higher than typical doses used in the ICU), it causes pulmonary vasoconstriction.24 Phenylephrine is a potent alpha agonist that is known to increase PVR and should generally be avoided in the setting of PH and RV dysfunction.24
Mechanical circulatory support
Use of extracorporeal membrane oxygenation (ECMO) as a bridge to recovery and bridge to transplantation has been described for use in patients with PAH.16,26 A report from Columbia University describes a multidisciplinary approach to identifying candidates suitable for lung transplant or with a reversible cause of decompensation. Selected patients were placed on either veno-arterial ECMO or a double lumen ECMO cannula was placed across a large atrial septal defect (ASD) creating an oxygenated right to left shunt. In this series, two patients were bridged to lung transplantation, three patients recovered, and one patient died.26 Extracorporeal membrane oxygenation should only be considered in patients with a realistic chance of lung transplant or recovery.16
Respiratory support
Some patients with respiratory failure may require non-invasive positive pressure ventilation or mechanical ventilation. Effects of positive pressure ventilation include a reduction in venous return and reduction of RV preload, and increased RV afterload from compression of the pulmonary vasculature (Figure 4). High-positive end-expiratory pressure can worsen hypoxaemia and hypercapnia if compression of extra alveolar vessels causes shunting of blood to poorly ventilated areas.27
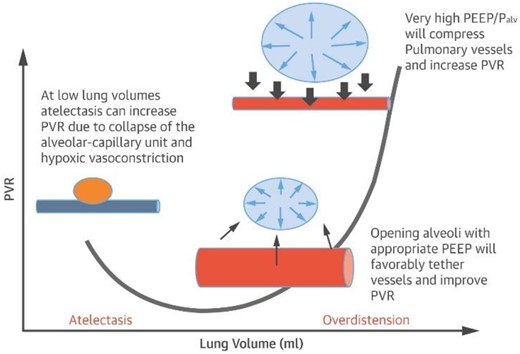
Relationship between alveolar volume/pressure and pulmonary vascular resistance. Adapted from Alviar et al.27 PVR, Pulmonary vascular resistance; PEEP, positive end expiratory pressure; Palv, alveolar pressure. Blue vessel represents deoxygenated blood.
Intubation and mechanical ventilation in patients with PH and RV failure can cause acute changes in RV preload and afterload along with systemic vasodilation and hypotension that lead to rapid decompensation and death. A technique of awake, spontaneously breathing, semi-recumbent positioned intubation over a bronchoscope has been described.28 Vasopressors should be available at the time of intubation to treat hypotension. Sedation and analgesia should be selected to minimize effects on systemic blood pressure and cardiac output.27
Pregnancy and elective surgery
Pregnancy and surgery are high risk for PAH patients with historical mortality rates as high as 56% with pregnancy29 and 18% for elective surgery.30 If possible, management of pregnancy and surgical procedures should be done at a PH specialty centre. Pregnancy is considered generally contraindicated in PAH due to the high related risk of death. Pregnancy termination should be considered.31 Contemporary reported maternal mortality is 12% with 62% of pregnancies successful.32 Collaborative care by a multidisciplinary expert team, including PH, maternal-foetal medicine, critical care medicine, anaesthesia, and CT surgery specialists may improve outcomes.31
Advanced care planning and end-of-life care
Despite the poor outcomes, few CICU patients with PAH have advanced directives.13 Early consultation of a palliative care team in the ICU facilitates psychological and spiritual support to the patient and family, and assists with goal setting, advanced care planning, symptom management, and in the transition to comfort care or hospice.33
Conclusion
Pulmonary hypertension is common in patients admitted to the CICU. Patients with known PAH have a particularly poor prognosis after ICU admission. A rapid assessment is necessary to evaluate right heart structure and function and identify medical problems contributing to PH and RV failure so that appropriate management can be quickly initiated. Given the poor prognosis of critically ill patients with PH, early referral to a PH expert centre should be considered.
Conflict of interest: C.F.B. received funding from AbbVie Inc., BeiGene, Ltd., United Therapeutics Corporation; Acceleron Pharma. Owns stock in Biogen Inc. T.D.M. research grant from Acceleron. Consultant- Actelion, Janssen, United Therapeutics, Aerovate, Bial, Trio-Health. Advisory Board: Liquidia, Altevant.




Comments