-
PDF
- Split View
-
Views
-
Cite
Cite
Sofie A Gevaert, Sigrun Halvorsen, Peter R Sinnaeve, Antonia Sambola, Geeta Gulati, Patrizio Lancellotti, Peter Van Der Meer, Alexander R Lyon, Dimitrios Farmakis, Geraldine Lee, Giuseppe Boriani, Ashutosh Wechalekar, Alicia Okines, Riccardo Asteggiano, Evaluation and management of cancer patients presenting with acute cardiovascular disease: a Consensus Document of the Acute CardioVascular Care (ACVC) association and the ESC council of Cardio-Oncology—Part 1: acute coronary syndromes and acute pericardial diseases, European Heart Journal. Acute Cardiovascular Care, Volume 10, Issue 8, October 2021, Pages 947–959, https://doi.org/10.1093/ehjacc/zuab056
Close - Share Icon Share
Abstract
Advances in treatment, common cardiovascular (CV) risk factors and the ageing of the population have led to an increasing number of cancer patients presenting with acute CV diseases. These events may be related to the cancer itself or the cancer treatment. Acute cardiac care specialists must be aware of these acute CV complications and be able to manage them. This may require an individualized and multidisciplinary approach. We summarize the most common acute CV complications of cytotoxic, targeted, and immune-based therapies. This is followed by a proposal for a multidisciplinary approach where acute cardiologists work close together with the treating oncologists, haematologists, and radiation specialists, especially in situations where immediate therapeutic decisions are needed. In this first part, we further focus on the management of acute coronary syndromes and acute pericardial diseases in patients with cancer.
Introduction
The incidence of acute cardiovascular (CV) disease in patients with active cancer has increased due to epidemiologic factors and shared risk factors.1 Cancer treatment,2,3 as well as the cancer disease itself,4 may trigger an acute CV event (Figure 1). Rarely, an acute cardiac event may be the first presentation of cancer and mandate further diagnostic work-up.
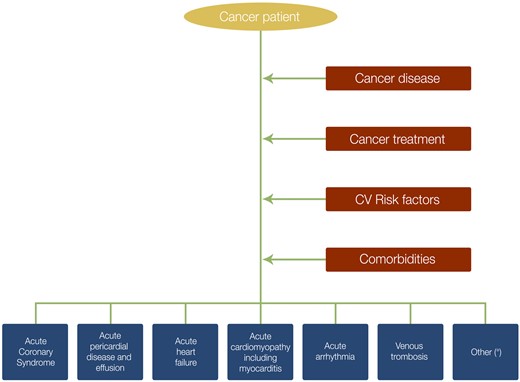
Acute cardiovascular disease and contributing factors in the cancer patient. aHypertensive crisis, acute pulmonary hypertension, and acute peripheral vascular event.
The management of acute CV disease in cancer patients often requires a multidisciplinary and patient-centred approach, taking into account proven therapeutic benefits and cancer prognosis. Therefore, in case of acute CV complications of cancer treatment, a careful evaluation of treatment cessation vs. continuation is needed.
Training of acute cardiac care physicians should therefore include basic knowledge of acute CV complications of cancer therapy as well as diagnostic and therapeutic management of acute CV disease in cancer patients.
This consensus paper was prepared by a Task Force representing the Association of Acute CardioVascular Care (ACVC) and the Council of Cardio-Oncology (CO-council) of the European Society of Cardiology (ESC). The goal was to review the acute CV conditions most frequently occurring in cancer patients, including those with a primary palliative context, and propose a consensus-based management. Consensus statements are derived primarily from published, mostly observational, data in addition to existing guidelines in non-cancer patients. For controversial areas, a consensus was achieved by the authors.
The consensus paper consists of two parts. In this first part, a general overview will be given of the most common acute CV complications related to cancer therapy followed by sections dealing with multidisciplinary approach, diagnosis and management of acute coronary syndromes (ACS) and acute pericardial diseases in cancer patients. A second part will deal with acute heart failure, acute myocarditis, acute arrhythmia, and acute venous thromboembolic disease.
Methods
We performed a PUBMED search for papers describing acute CV complications of different cancer treatment as well as diagnostic and therapeutic management of the aforementioned acute CV diseases in cancer patients. Following search terms were used: cancer, cardio-oncology, CV toxicity, cardiotoxicity, cytotoxic therapy, targeted therapy, immune based therapy, ACS, acute pericarditis, pericardial tamponade, pericardial effusion. Selection involved screening of titles and abstracts followed by full-text evaluation if relevant.
Acute cardiovascular complications associated with cancer therapy
Several cancer therapies may trigger acute CV complications. In most cases, cancer therapies associated with CV toxicity should be interrupted until the acute event has been stabilized and a risk-benefit analysis of restarting therapy vs. switching to an alternative cancer therapy has been performed and discussed with the patient.2,5 Complications can be class-specific or drug specific. Table 1 (references: see Supplementary material online, web addendum) provides an overview of cancer treatments and their reported acute CV complications.
Cancer treatments and acute cardiovascular complications (for references, please see the Supplementary material online)
| Cancer treatment . | Indications . | Cardiovascular complications . | ||
|---|---|---|---|---|
| Cytotoxic cancer therapies | ||||
| Anti-tumour antibiotics | ||||
| Anthracyclines | Doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxantrone | Breast cancer, haematological malignancies, sarcoma |
| |
| Other | Bleomycin | Lymphomas, head and neck tumours, testicular cancer |
| |
| Alkylating agents | ||||
| Nitrogen mustards | Cyclophosphamide, ifosfamide, busulfan, melphalan | Haematological malignancies, breast, ovarian cancer, retinoblastoma |
| |
| Platinum compounds | Cisplatin, carboplatin, oxaliplatin | Gastro-intestinal tumours, bladder cancer, testicular cancer, cervical and ovarian cancer, non-small-cell lung cancer, mesothelioma |
| |
| Antimetabolites | ||||
| 5-FU, capecitabine, gemcitabine, cytarabine, fludarabine | Gastro-intestinal tumours, breast cancer, head and neck cancer, haematologic malignancies |
| ||
| Antimicrotubule agents | ||||
| Taxanes | Docetaxel, paclitaxel | Breast cancer, ovarian cancer, prostate cancer, oesophago-gastric cancer, non-small-cell lung cancer |
| |
| Vinca alkaloids | Vincristine, vinblastine | Testicular cancer, lymphoma’s, breast cancer, choriocarcinoma |
| |
| Radiation | ||||
| Chest radiation | Breast cancer, lung cancer, lymphoma, oesophageal cancer |
| ||
| Targeted therapies | ||||
| Anti-oestrogen | ||||
| Oestrogen receptor modulator | Tamoxifen | Breast cancer |
| |
| Differentiation agents | ||||
| ATRA | Acute promyelocytic leukaemia |
| ||
| Arsenic trioxide | Acute promyelocytic leukaemia |
| ||
| Monoclonal antibodies | ||||
| HER2 | Trastuzumab, pertuzumab, trastuzumab-emtansine (T-DM1), trastuzumab-deruxtecan (T-Dxd) | HER2+ breast cancer, gastric cancer |
| |
| VEGF | Bevacizumab | Renal cell carcinoma, colorectal carcinoma, cervical carcinoma, non-small-cell lung cancer, glioblastoma |
| |
| CD 20 | Rituximab | Non-Hodgkin’s lymphoma, chronic lymphocytic leukaemia |
| |
| EGFR | Cetuximab, panitumumab | KRAS wild-type colorectal cancer, head and neck tumours |
| |
| Small molecule tyrosine kinase inhibitors | ||||
| VEGF | Sorafenib, sunitinib, pazopanib, and vandetanib, axitinib, lenvatinib, regorafenib, tivozanib | Renal cell carcinoma, gastro-intestinal tumours, thyroid cancer, hepatocellular cancer |
| |
| HER2 | Lapatinib, neratinib, tucatinib | HER2+ breast carcinoma |
| |
| EGFR | Osimertinib | EGFR mutated non-small-cell lung cancer |
| |
| Multitarget, Bcr-Abl | Ponatinib, nilotinib, dasatinib, imatinib, bosutinib | Chronic myeloid leukaemia, acute lymphocytic leukaemia, stromal tumours |
| |
| ALK | Crizotinib, ceritinib, alectinib, brigatinib | Non-small-cell lung cancer |
| |
| Bruton’s kinase | Ibrutinib, zanbrutinib | Chronic lymphatic leukaemia, mantle cell lymphoma, Waldenstrom’s macroglobulinemia,graft vs. host disease |
| |
| BRAF and MEK | BRAF: dabrafenib, vermurafenib, encorafenib MEK: trametinib, cobimetinib, binimetinib | Melanoma, non-small-cell lung cancer, colorectal cancer |
| |
| CDK 4-6 | Ribociclib, abemaciclib | Advanced breast cancer |
| |
| Proteasome inhibitors | ||||
| Bortezomib, carfilzomib, ixazomib | Multiple myeloma, B-cell malignancies |
| ||
| Histone deacetylase inhibitors | ||||
| Vorinostat, romidepsin | T-cell lymphoma, haematologic malignancies |
| ||
| Immune-based therapies | ||||
| Immunomodulatory drugs | ||||
| Lenalidomide, thalidomide, pomalidomide | Multiple myeloma |
| ||
| Biologic response modifiers | ||||
| IL-2 | Metastatic melanoma, renal cell carcinoma |
| ||
| Interferon-α | Melanoma, chronic myeloid leukaemia |
| ||
| Immune checkpoint inhibitors | ||||
| Ipilimumab, nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab | Melanoma, lung cancer, renal cell carcinoma, urothelial carcinoma, hepatocellular carcinoma, breast cancer, cervical cancer, Merkel cell carcinoma, head and neck cancer, Hodgkin’s lymphoma, gastro-intestinal cancer |
| ||
| CAR-T-cell therapy | ||||
| B-cell malignancies |
| |||
| Cancer treatment . | Indications . | Cardiovascular complications . | ||
|---|---|---|---|---|
| Cytotoxic cancer therapies | ||||
| Anti-tumour antibiotics | ||||
| Anthracyclines | Doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxantrone | Breast cancer, haematological malignancies, sarcoma |
| |
| Other | Bleomycin | Lymphomas, head and neck tumours, testicular cancer |
| |
| Alkylating agents | ||||
| Nitrogen mustards | Cyclophosphamide, ifosfamide, busulfan, melphalan | Haematological malignancies, breast, ovarian cancer, retinoblastoma |
| |
| Platinum compounds | Cisplatin, carboplatin, oxaliplatin | Gastro-intestinal tumours, bladder cancer, testicular cancer, cervical and ovarian cancer, non-small-cell lung cancer, mesothelioma |
| |
| Antimetabolites | ||||
| 5-FU, capecitabine, gemcitabine, cytarabine, fludarabine | Gastro-intestinal tumours, breast cancer, head and neck cancer, haematologic malignancies |
| ||
| Antimicrotubule agents | ||||
| Taxanes | Docetaxel, paclitaxel | Breast cancer, ovarian cancer, prostate cancer, oesophago-gastric cancer, non-small-cell lung cancer |
| |
| Vinca alkaloids | Vincristine, vinblastine | Testicular cancer, lymphoma’s, breast cancer, choriocarcinoma |
| |
| Radiation | ||||
| Chest radiation | Breast cancer, lung cancer, lymphoma, oesophageal cancer |
| ||
| Targeted therapies | ||||
| Anti-oestrogen | ||||
| Oestrogen receptor modulator | Tamoxifen | Breast cancer |
| |
| Differentiation agents | ||||
| ATRA | Acute promyelocytic leukaemia |
| ||
| Arsenic trioxide | Acute promyelocytic leukaemia |
| ||
| Monoclonal antibodies | ||||
| HER2 | Trastuzumab, pertuzumab, trastuzumab-emtansine (T-DM1), trastuzumab-deruxtecan (T-Dxd) | HER2+ breast cancer, gastric cancer |
| |
| VEGF | Bevacizumab | Renal cell carcinoma, colorectal carcinoma, cervical carcinoma, non-small-cell lung cancer, glioblastoma |
| |
| CD 20 | Rituximab | Non-Hodgkin’s lymphoma, chronic lymphocytic leukaemia |
| |
| EGFR | Cetuximab, panitumumab | KRAS wild-type colorectal cancer, head and neck tumours |
| |
| Small molecule tyrosine kinase inhibitors | ||||
| VEGF | Sorafenib, sunitinib, pazopanib, and vandetanib, axitinib, lenvatinib, regorafenib, tivozanib | Renal cell carcinoma, gastro-intestinal tumours, thyroid cancer, hepatocellular cancer |
| |
| HER2 | Lapatinib, neratinib, tucatinib | HER2+ breast carcinoma |
| |
| EGFR | Osimertinib | EGFR mutated non-small-cell lung cancer |
| |
| Multitarget, Bcr-Abl | Ponatinib, nilotinib, dasatinib, imatinib, bosutinib | Chronic myeloid leukaemia, acute lymphocytic leukaemia, stromal tumours |
| |
| ALK | Crizotinib, ceritinib, alectinib, brigatinib | Non-small-cell lung cancer |
| |
| Bruton’s kinase | Ibrutinib, zanbrutinib | Chronic lymphatic leukaemia, mantle cell lymphoma, Waldenstrom’s macroglobulinemia,graft vs. host disease |
| |
| BRAF and MEK | BRAF: dabrafenib, vermurafenib, encorafenib MEK: trametinib, cobimetinib, binimetinib | Melanoma, non-small-cell lung cancer, colorectal cancer |
| |
| CDK 4-6 | Ribociclib, abemaciclib | Advanced breast cancer |
| |
| Proteasome inhibitors | ||||
| Bortezomib, carfilzomib, ixazomib | Multiple myeloma, B-cell malignancies |
| ||
| Histone deacetylase inhibitors | ||||
| Vorinostat, romidepsin | T-cell lymphoma, haematologic malignancies |
| ||
| Immune-based therapies | ||||
| Immunomodulatory drugs | ||||
| Lenalidomide, thalidomide, pomalidomide | Multiple myeloma |
| ||
| Biologic response modifiers | ||||
| IL-2 | Metastatic melanoma, renal cell carcinoma |
| ||
| Interferon-α | Melanoma, chronic myeloid leukaemia |
| ||
| Immune checkpoint inhibitors | ||||
| Ipilimumab, nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab | Melanoma, lung cancer, renal cell carcinoma, urothelial carcinoma, hepatocellular carcinoma, breast cancer, cervical cancer, Merkel cell carcinoma, head and neck cancer, Hodgkin’s lymphoma, gastro-intestinal cancer |
| ||
| CAR-T-cell therapy | ||||
| B-cell malignancies |
| |||
5-FU, 5-fluoro-uracil; ACS, acute coronary syndromes; AHF, acute heart failure; CAR-T-cell, Chimere antigen receptor T-cell; ICDs, implantable cardioverter-defibrillators; IL-2, interleukin 2; Mg, magnesium.
References can be found in the Supplementary material online, web addendum.
Cancer treatments and acute cardiovascular complications (for references, please see the Supplementary material online)
| Cancer treatment . | Indications . | Cardiovascular complications . | ||
|---|---|---|---|---|
| Cytotoxic cancer therapies | ||||
| Anti-tumour antibiotics | ||||
| Anthracyclines | Doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxantrone | Breast cancer, haematological malignancies, sarcoma |
| |
| Other | Bleomycin | Lymphomas, head and neck tumours, testicular cancer |
| |
| Alkylating agents | ||||
| Nitrogen mustards | Cyclophosphamide, ifosfamide, busulfan, melphalan | Haematological malignancies, breast, ovarian cancer, retinoblastoma |
| |
| Platinum compounds | Cisplatin, carboplatin, oxaliplatin | Gastro-intestinal tumours, bladder cancer, testicular cancer, cervical and ovarian cancer, non-small-cell lung cancer, mesothelioma |
| |
| Antimetabolites | ||||
| 5-FU, capecitabine, gemcitabine, cytarabine, fludarabine | Gastro-intestinal tumours, breast cancer, head and neck cancer, haematologic malignancies |
| ||
| Antimicrotubule agents | ||||
| Taxanes | Docetaxel, paclitaxel | Breast cancer, ovarian cancer, prostate cancer, oesophago-gastric cancer, non-small-cell lung cancer |
| |
| Vinca alkaloids | Vincristine, vinblastine | Testicular cancer, lymphoma’s, breast cancer, choriocarcinoma |
| |
| Radiation | ||||
| Chest radiation | Breast cancer, lung cancer, lymphoma, oesophageal cancer |
| ||
| Targeted therapies | ||||
| Anti-oestrogen | ||||
| Oestrogen receptor modulator | Tamoxifen | Breast cancer |
| |
| Differentiation agents | ||||
| ATRA | Acute promyelocytic leukaemia |
| ||
| Arsenic trioxide | Acute promyelocytic leukaemia |
| ||
| Monoclonal antibodies | ||||
| HER2 | Trastuzumab, pertuzumab, trastuzumab-emtansine (T-DM1), trastuzumab-deruxtecan (T-Dxd) | HER2+ breast cancer, gastric cancer |
| |
| VEGF | Bevacizumab | Renal cell carcinoma, colorectal carcinoma, cervical carcinoma, non-small-cell lung cancer, glioblastoma |
| |
| CD 20 | Rituximab | Non-Hodgkin’s lymphoma, chronic lymphocytic leukaemia |
| |
| EGFR | Cetuximab, panitumumab | KRAS wild-type colorectal cancer, head and neck tumours |
| |
| Small molecule tyrosine kinase inhibitors | ||||
| VEGF | Sorafenib, sunitinib, pazopanib, and vandetanib, axitinib, lenvatinib, regorafenib, tivozanib | Renal cell carcinoma, gastro-intestinal tumours, thyroid cancer, hepatocellular cancer |
| |
| HER2 | Lapatinib, neratinib, tucatinib | HER2+ breast carcinoma |
| |
| EGFR | Osimertinib | EGFR mutated non-small-cell lung cancer |
| |
| Multitarget, Bcr-Abl | Ponatinib, nilotinib, dasatinib, imatinib, bosutinib | Chronic myeloid leukaemia, acute lymphocytic leukaemia, stromal tumours |
| |
| ALK | Crizotinib, ceritinib, alectinib, brigatinib | Non-small-cell lung cancer |
| |
| Bruton’s kinase | Ibrutinib, zanbrutinib | Chronic lymphatic leukaemia, mantle cell lymphoma, Waldenstrom’s macroglobulinemia,graft vs. host disease |
| |
| BRAF and MEK | BRAF: dabrafenib, vermurafenib, encorafenib MEK: trametinib, cobimetinib, binimetinib | Melanoma, non-small-cell lung cancer, colorectal cancer |
| |
| CDK 4-6 | Ribociclib, abemaciclib | Advanced breast cancer |
| |
| Proteasome inhibitors | ||||
| Bortezomib, carfilzomib, ixazomib | Multiple myeloma, B-cell malignancies |
| ||
| Histone deacetylase inhibitors | ||||
| Vorinostat, romidepsin | T-cell lymphoma, haematologic malignancies |
| ||
| Immune-based therapies | ||||
| Immunomodulatory drugs | ||||
| Lenalidomide, thalidomide, pomalidomide | Multiple myeloma |
| ||
| Biologic response modifiers | ||||
| IL-2 | Metastatic melanoma, renal cell carcinoma |
| ||
| Interferon-α | Melanoma, chronic myeloid leukaemia |
| ||
| Immune checkpoint inhibitors | ||||
| Ipilimumab, nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab | Melanoma, lung cancer, renal cell carcinoma, urothelial carcinoma, hepatocellular carcinoma, breast cancer, cervical cancer, Merkel cell carcinoma, head and neck cancer, Hodgkin’s lymphoma, gastro-intestinal cancer |
| ||
| CAR-T-cell therapy | ||||
| B-cell malignancies |
| |||
| Cancer treatment . | Indications . | Cardiovascular complications . | ||
|---|---|---|---|---|
| Cytotoxic cancer therapies | ||||
| Anti-tumour antibiotics | ||||
| Anthracyclines | Doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxantrone | Breast cancer, haematological malignancies, sarcoma |
| |
| Other | Bleomycin | Lymphomas, head and neck tumours, testicular cancer |
| |
| Alkylating agents | ||||
| Nitrogen mustards | Cyclophosphamide, ifosfamide, busulfan, melphalan | Haematological malignancies, breast, ovarian cancer, retinoblastoma |
| |
| Platinum compounds | Cisplatin, carboplatin, oxaliplatin | Gastro-intestinal tumours, bladder cancer, testicular cancer, cervical and ovarian cancer, non-small-cell lung cancer, mesothelioma |
| |
| Antimetabolites | ||||
| 5-FU, capecitabine, gemcitabine, cytarabine, fludarabine | Gastro-intestinal tumours, breast cancer, head and neck cancer, haematologic malignancies |
| ||
| Antimicrotubule agents | ||||
| Taxanes | Docetaxel, paclitaxel | Breast cancer, ovarian cancer, prostate cancer, oesophago-gastric cancer, non-small-cell lung cancer |
| |
| Vinca alkaloids | Vincristine, vinblastine | Testicular cancer, lymphoma’s, breast cancer, choriocarcinoma |
| |
| Radiation | ||||
| Chest radiation | Breast cancer, lung cancer, lymphoma, oesophageal cancer |
| ||
| Targeted therapies | ||||
| Anti-oestrogen | ||||
| Oestrogen receptor modulator | Tamoxifen | Breast cancer |
| |
| Differentiation agents | ||||
| ATRA | Acute promyelocytic leukaemia |
| ||
| Arsenic trioxide | Acute promyelocytic leukaemia |
| ||
| Monoclonal antibodies | ||||
| HER2 | Trastuzumab, pertuzumab, trastuzumab-emtansine (T-DM1), trastuzumab-deruxtecan (T-Dxd) | HER2+ breast cancer, gastric cancer |
| |
| VEGF | Bevacizumab | Renal cell carcinoma, colorectal carcinoma, cervical carcinoma, non-small-cell lung cancer, glioblastoma |
| |
| CD 20 | Rituximab | Non-Hodgkin’s lymphoma, chronic lymphocytic leukaemia |
| |
| EGFR | Cetuximab, panitumumab | KRAS wild-type colorectal cancer, head and neck tumours |
| |
| Small molecule tyrosine kinase inhibitors | ||||
| VEGF | Sorafenib, sunitinib, pazopanib, and vandetanib, axitinib, lenvatinib, regorafenib, tivozanib | Renal cell carcinoma, gastro-intestinal tumours, thyroid cancer, hepatocellular cancer |
| |
| HER2 | Lapatinib, neratinib, tucatinib | HER2+ breast carcinoma |
| |
| EGFR | Osimertinib | EGFR mutated non-small-cell lung cancer |
| |
| Multitarget, Bcr-Abl | Ponatinib, nilotinib, dasatinib, imatinib, bosutinib | Chronic myeloid leukaemia, acute lymphocytic leukaemia, stromal tumours |
| |
| ALK | Crizotinib, ceritinib, alectinib, brigatinib | Non-small-cell lung cancer |
| |
| Bruton’s kinase | Ibrutinib, zanbrutinib | Chronic lymphatic leukaemia, mantle cell lymphoma, Waldenstrom’s macroglobulinemia,graft vs. host disease |
| |
| BRAF and MEK | BRAF: dabrafenib, vermurafenib, encorafenib MEK: trametinib, cobimetinib, binimetinib | Melanoma, non-small-cell lung cancer, colorectal cancer |
| |
| CDK 4-6 | Ribociclib, abemaciclib | Advanced breast cancer |
| |
| Proteasome inhibitors | ||||
| Bortezomib, carfilzomib, ixazomib | Multiple myeloma, B-cell malignancies |
| ||
| Histone deacetylase inhibitors | ||||
| Vorinostat, romidepsin | T-cell lymphoma, haematologic malignancies |
| ||
| Immune-based therapies | ||||
| Immunomodulatory drugs | ||||
| Lenalidomide, thalidomide, pomalidomide | Multiple myeloma |
| ||
| Biologic response modifiers | ||||
| IL-2 | Metastatic melanoma, renal cell carcinoma |
| ||
| Interferon-α | Melanoma, chronic myeloid leukaemia |
| ||
| Immune checkpoint inhibitors | ||||
| Ipilimumab, nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab | Melanoma, lung cancer, renal cell carcinoma, urothelial carcinoma, hepatocellular carcinoma, breast cancer, cervical cancer, Merkel cell carcinoma, head and neck cancer, Hodgkin’s lymphoma, gastro-intestinal cancer |
| ||
| CAR-T-cell therapy | ||||
| B-cell malignancies |
| |||
5-FU, 5-fluoro-uracil; ACS, acute coronary syndromes; AHF, acute heart failure; CAR-T-cell, Chimere antigen receptor T-cell; ICDs, implantable cardioverter-defibrillators; IL-2, interleukin 2; Mg, magnesium.
References can be found in the Supplementary material online, web addendum.
With respect to cytotoxic therapies, anthracyclines, alkylating agents, platinum containing drugs, and fluoropyrimidines are the drugs that are most often associated with acute CV complications.
Radiation can cause acute pericarditis usually occurring during or early after radiation therapy, whereas other CV complications such as conduction abnormalities, coronary artery disease (CAD), pericardial constriction, and valvular disease may manifest several years later.
Targeted therapies, including anti-oestrogens, arsenic compounds, and all-trans retinoic acid (ATRA) are associated with a variety of acute complications. Monoclonal antibodies and small molecule tyrosine kinase inhibitors (TKIs) are more recent targeted therapies; those targeting the vascular endothelial growth factor (VEGF) pathway are typically associated with hypertension including hypertensive crises, but many other acute events can occur with different types of TKIs.
Immunomodulatory drugs are known for their arterial (coronary and peripheral) and venous thromboembolic complications. Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment especially in patients with advanced cancer. ICI-induced myocarditis is the most serious acute complication requiring immediate interruption and high doses of steroids, which will be discussed further in the second part of the consensus paper. Many other ICI-related acute CV complications may occur. Chimeric antigen receptor T-cell therapy can also induce myocarditis and heart failure as part of the cytokine release syndrome (Table 1).
Cancer patients who are prescribed therapies that are potentially associated with CV complications are recommended to have a baseline CV risk assessment using baseline risk stratification proformas and appropriate surveillance based upon their baseline risk using cardiac imaging and biomarkers.6
Acute multidisciplinary approach
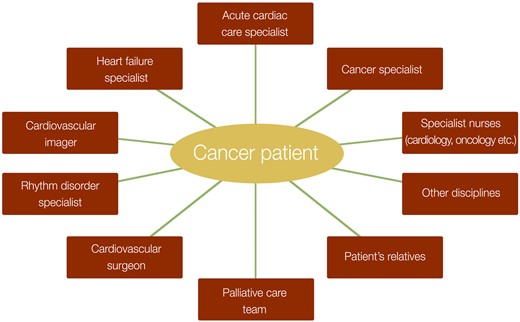
Cancer patients presenting with acute CV complications may be unstable and require multidisciplinary care with dedicated healthcare professionals care in a specialized acute cardiology department with level 2 care and monitoring.7 A rapid diagnosis and the differentiation between a primary cardiac problem and a cardiac problem secondary to cancer or cancer treatment, if possible, are important to direct appropriate therapy in a timely manner and avoid any inappropriate escalation or de-escalation of the cancer treatment. An interdisciplinary discussion, preferably based on a local protocol, should be initiated as soon as possible after admission with precise documentation of the statements of the different specialists consulted.8 Urgent discussions may be necessary when immediate interruption of cancer treatment and/or specific treatments are indicated (e.g. ICI-induced myocarditis, hypertensive crises, life-threatening arrhythmia). At the centre is the patient, who should be involved in the decision-making especially regarding interruption or change of cancer therapy, cardiac interventions as well as the decision regarding continuation vs. cessation of further cancer therapy. Close relatives of the patient can be involved in the decision-making process at explicit patient’s wish (Figure 2).2,8
Acute coronary syndromes in cancer patients
Consensus statements for acute coronary syndromes in cancer patients
The proportion of ACS patients with active cancer currently constitutes about 3%.9
| Diagnosis |
| The same diagnostic algorithms for diagnosis and monitoring of ACS as in non-cancer patients apply. |
| Early echocardiography is advised to evaluate regional and global left ventricular function and to exclude other cancer or cancer therapy-related complications. |
| An invasive strategy is suggested in patients with STEMI and high-risk NSTE-ACS. |
| A conservative non-invasive approach can be attempted in patients with stable NSTE-ACS with poor cancer prognosis and/or high bleeding risk. |
| Management |
| A temporary interruption of cancer therapy is suggested after interdisciplinary discussion if a causal role of cancer therapy is suspected. |
| Aspirin and clopidogrel are first choice antiplatelet drugs in cancer patients with a recent cancer diagnosis (<12 months) or other risk factors for bleeding. |
| A shorter DAPT duration is advised in patients at high bleeding risk. |
| Single antiplatelet therapy with clopidogrel is suggested in patients on oral anticoagulation. |
| Invasive treatment |
| In case of stenting, DES is preferred over balloon angioplasty or BMS unless need for urgent surgery or very high bleeding risk. |
| FFR or iFR can be used to avoid unnecessary stenting. |
| IVUS or OCT can be used to ensure optimal stent apposition. |
| Thrombocytopenia and invasive management |
| Platelet transfusion before catheterization is suggested if platelets are <10 000/µL, or <20 000/µL for patients with colorectal, gynaecological, bladder, or necrotizing tumours. |
| An adjusted lower unfractionated heparin dose (30–50 U/kg) is suggested. |
| Aspirin and clopidogrel can be administered if platelets are >10 000/μL and >30 000/μL, respectively. |
| A minimum platelet count of respectively 30 000/μL and 50 000/μL is required for PCI and CABG. |
| Diagnosis |
| The same diagnostic algorithms for diagnosis and monitoring of ACS as in non-cancer patients apply. |
| Early echocardiography is advised to evaluate regional and global left ventricular function and to exclude other cancer or cancer therapy-related complications. |
| An invasive strategy is suggested in patients with STEMI and high-risk NSTE-ACS. |
| A conservative non-invasive approach can be attempted in patients with stable NSTE-ACS with poor cancer prognosis and/or high bleeding risk. |
| Management |
| A temporary interruption of cancer therapy is suggested after interdisciplinary discussion if a causal role of cancer therapy is suspected. |
| Aspirin and clopidogrel are first choice antiplatelet drugs in cancer patients with a recent cancer diagnosis (<12 months) or other risk factors for bleeding. |
| A shorter DAPT duration is advised in patients at high bleeding risk. |
| Single antiplatelet therapy with clopidogrel is suggested in patients on oral anticoagulation. |
| Invasive treatment |
| In case of stenting, DES is preferred over balloon angioplasty or BMS unless need for urgent surgery or very high bleeding risk. |
| FFR or iFR can be used to avoid unnecessary stenting. |
| IVUS or OCT can be used to ensure optimal stent apposition. |
| Thrombocytopenia and invasive management |
| Platelet transfusion before catheterization is suggested if platelets are <10 000/µL, or <20 000/µL for patients with colorectal, gynaecological, bladder, or necrotizing tumours. |
| An adjusted lower unfractionated heparin dose (30–50 U/kg) is suggested. |
| Aspirin and clopidogrel can be administered if platelets are >10 000/μL and >30 000/μL, respectively. |
| A minimum platelet count of respectively 30 000/μL and 50 000/μL is required for PCI and CABG. |
ACS, acute coronary syndromes; BMS, bare metal stent; CABG, coronary artery bypass grafting; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; FFR, fractional flow reserve; iFR, instantaneous free wave ratio; IVUS, intravascular ultrasound; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
| Diagnosis |
| The same diagnostic algorithms for diagnosis and monitoring of ACS as in non-cancer patients apply. |
| Early echocardiography is advised to evaluate regional and global left ventricular function and to exclude other cancer or cancer therapy-related complications. |
| An invasive strategy is suggested in patients with STEMI and high-risk NSTE-ACS. |
| A conservative non-invasive approach can be attempted in patients with stable NSTE-ACS with poor cancer prognosis and/or high bleeding risk. |
| Management |
| A temporary interruption of cancer therapy is suggested after interdisciplinary discussion if a causal role of cancer therapy is suspected. |
| Aspirin and clopidogrel are first choice antiplatelet drugs in cancer patients with a recent cancer diagnosis (<12 months) or other risk factors for bleeding. |
| A shorter DAPT duration is advised in patients at high bleeding risk. |
| Single antiplatelet therapy with clopidogrel is suggested in patients on oral anticoagulation. |
| Invasive treatment |
| In case of stenting, DES is preferred over balloon angioplasty or BMS unless need for urgent surgery or very high bleeding risk. |
| FFR or iFR can be used to avoid unnecessary stenting. |
| IVUS or OCT can be used to ensure optimal stent apposition. |
| Thrombocytopenia and invasive management |
| Platelet transfusion before catheterization is suggested if platelets are <10 000/µL, or <20 000/µL for patients with colorectal, gynaecological, bladder, or necrotizing tumours. |
| An adjusted lower unfractionated heparin dose (30–50 U/kg) is suggested. |
| Aspirin and clopidogrel can be administered if platelets are >10 000/μL and >30 000/μL, respectively. |
| A minimum platelet count of respectively 30 000/μL and 50 000/μL is required for PCI and CABG. |
| Diagnosis |
| The same diagnostic algorithms for diagnosis and monitoring of ACS as in non-cancer patients apply. |
| Early echocardiography is advised to evaluate regional and global left ventricular function and to exclude other cancer or cancer therapy-related complications. |
| An invasive strategy is suggested in patients with STEMI and high-risk NSTE-ACS. |
| A conservative non-invasive approach can be attempted in patients with stable NSTE-ACS with poor cancer prognosis and/or high bleeding risk. |
| Management |
| A temporary interruption of cancer therapy is suggested after interdisciplinary discussion if a causal role of cancer therapy is suspected. |
| Aspirin and clopidogrel are first choice antiplatelet drugs in cancer patients with a recent cancer diagnosis (<12 months) or other risk factors for bleeding. |
| A shorter DAPT duration is advised in patients at high bleeding risk. |
| Single antiplatelet therapy with clopidogrel is suggested in patients on oral anticoagulation. |
| Invasive treatment |
| In case of stenting, DES is preferred over balloon angioplasty or BMS unless need for urgent surgery or very high bleeding risk. |
| FFR or iFR can be used to avoid unnecessary stenting. |
| IVUS or OCT can be used to ensure optimal stent apposition. |
| Thrombocytopenia and invasive management |
| Platelet transfusion before catheterization is suggested if platelets are <10 000/µL, or <20 000/µL for patients with colorectal, gynaecological, bladder, or necrotizing tumours. |
| An adjusted lower unfractionated heparin dose (30–50 U/kg) is suggested. |
| Aspirin and clopidogrel can be administered if platelets are >10 000/μL and >30 000/μL, respectively. |
| A minimum platelet count of respectively 30 000/μL and 50 000/μL is required for PCI and CABG. |
ACS, acute coronary syndromes; BMS, bare metal stent; CABG, coronary artery bypass grafting; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; FFR, fractional flow reserve; iFR, instantaneous free wave ratio; IVUS, intravascular ultrasound; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Pathophysiology
Cancer patients are at risk for ACS for several reasons. First, cancer patients are often older and may have traditional CV risk factors like smoking, obesity, diabetes, hypertension, hyperlipidaemia, and physical inactivity.9 Second, many cancer therapies may provoke ACS through different pathophysiological mechanisms. Coronary vasospasm is one of the most reported acute CV side-effects and is typically caused by the administration of 5-fluoro-uracil (5-FU) or its prodrug capecitabine but has also been associated with other agents. Predisposing factors for vasospasm are pre-existing CAD, high doses of 5-FU, genetic slow metabolizers, continuous infusion, prior radiation, and co-administration of other chemotherapeutic agents.10 Plaque rupture can occur with various cancer therapies including platinum-based chemotherapies, VEGF TKIs, some BCR-ABL TKIs, fluoropyrimidines and ICIs. Acute coronary thrombosis caused by endothelial damage is typically associated with the platinum containing anti-cancer drug cisplatin but has also been described with the Bcr-Abl TKIs nilotinib and ponatinib (Table 1). Radiotherapy causes direct endothelial injury and is also associated with accelerated CAD, typically affecting the ostia of the left main and right coronary artery11 but rarely provoking ACS during cancer treatment.12 The cancer therapy-induced problems are compounded by a direct cancer-induced pro-inflammatory and prothrombotic state that is associated with increased platelet activity and aggregability.13 Primary cardiac neoplasms complicated by coronary embolism,14 and external coronary compression by a tumourous mass like lymphoma15 are other rare causes of ACS in cancer patients (Figure 3).
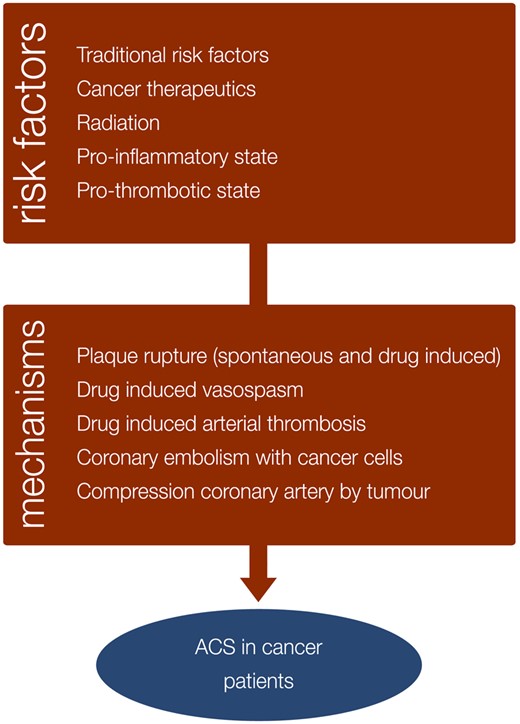
Risk factors and mechanisms of acute coronary syndromes in cancer patients.
Clinical presentation and diagnosis
The clinical presentation and the diagnostic algorithms in cancer patients are relatively similar to those for ACS patients without cancer.16 However, symptoms of ischaemia can be atypical17 or masked by other side-effects of the cancer treatment or confused with cancer symptoms. Therefore, when patients develop chest pain or dyspnoea, the threshold for performing 12-lead electrocardiogram (ECG) and serial measurements of high-sensitive cardiac troponin should be low. Correct information on previous and current cancer treatment is mandatory. Early echocardiography is recommended to demonstrate regional wall motion abnormalities or identify other cancer-related cardiac complications. Cancer patients with vasospasm due to fluoropyrimidines can be especially challenging because they can present with chest pain without ECG changes or rise in cardiac troponin levels. Invasive coronary angiography or computed tomography angiography should be considered to exclude underlying CAD when coronary vasospasm is suspected.18
Initial management
Besides prompt recognition, individualized guideline-based management is recommended to improve prognosis, both with respect to the CV disease and to the cancer.16 Cancer therapy should be temporarily interrupted, especially if a causal relation is suspected8 (Table 1).
Invasive strategy
Whether the patient has an active or a historical cancer can have a significant impact on the decision whether or not an ACS patient should be managed invasively. In addition, comorbidities including thrombocytopenia, coagulopathy, anaemia, organ failure, cachexia, recent or planned surgery directly influence this decision. The decision to manage the oncological patient with an ACS invasively vs. conservatively should always be tailored to the individual patient and based on a shared decision-making with the patient. Retrospective studies from the USA showed that concomitant cancer diagnosis was more often associated with a conservative medical management strategy for ACS and worse clinical outcomes, compared to a more frequent invasive approach in patients without cancer.9,19
Although cancer patients in general have a higher risk of death and bleeding following a percutaneous coronary intervention (PCI) than those without,20 in-hospital mortality and the risk of procedural complications in these patients are largely driven by the type and stage of the tumour.21 Lung cancer patients have an increased in-hospital mortality risk, while patients with active colon cancer have an increased risk of major bleeding. Complications are less common in prostate or breast cancer patients.20–22
A conservative approach can be considered in cancer patients with a lower risk non-ST-segment elevation acute coronary syndrome (NSTE-ACS), especially when responding well to initial non-invasive therapies. A large retrospective analysis suggested that in patients with metastatic cancer and ACS, contemporary medical management resulted in similar in-hospital outcomes as PCI for the non-ST-segment elevation myocardial infarction patients, while PCI was associated with lower mortality in the ST-segment elevation myocardial infarction (STEMI) patients.23 Therefore, in case of STEMI, an invasive management should always be considered in the absence of major contraindications, even if the oncological prognosis is limited. The need for an invasive approach in the case of late presentation in a stable patient should be evaluated on a case-by-case basis.
Cancer is associated with a higher risk of stent thrombosis24 therefore experts advise the use of fractional flow reserve or instantaneous free wave ratio to avoid unnecessary stenting.25 Balloon angioplasty only was associated with a trend towards worse outcome in a retrospective analysis26 and should be avoided unless severe thrombocytopenia or need for urgent surgery. In a large registry of cancer patients undergoing PCI and stenting [>85% drug-eluting stent (DES)], a similar rate of target vessel revascularization, reinfarction, and stent thrombosis at 1 year was observed as in a propensity score-matched cohort of patients without cancer.20 Newer generation DES with shortened dual antiplatelet therapy (DAPT) duration together with bleeding prevention strategies are probably the safest choice in cancer patients undergoing PCI who are not in need of short-term surgery.16 Advanced imaging techniques like intravascular ultrasound and optical coherence tomography can be helpful to ensure optimal stent placement and expansion to allow early DAPT interruption especially in patients at high bleeding risk.
Antithrombotic treatment
Antiplatelets
The choice and duration of antiplatelet drugs should be individualized depending on the type and stage of cancer, the management of the ACS, and the need for chemotherapy and/or cancer surgery after ACS. For patients who underwent cancer therapy many years previously, standard guidelines should be applied.16,27 Patients with ACS within a few months after cancer diagnosis and/or undergoing active cancer treatment, need individualized case-based treatment, considering their procoagulant state, anaemia and thrombocytopenia, among other factors.
The European NSTE-ACS guidelines promote the use of the Academic Research Consortium for high bleeding risk (ARC-HBR) criteria to evaluate bleeding risk. These consensus-based criteria classify patients with an active cancer diagnosed within the last 12 months as high bleeding risk.28 Therefore, the first-choice antiplatelet drugs in ACS patients with a recent cancer diagnosis are aspirin (300/75–100 mg) and clopidogrel (300–600/75 mg) provided that platelet counts are >10 000/μL29 and >30 000/μL, respectively.16,25 Ticagrelor and prasugrel should usually not be used because of the high bleeding risk and limited data regarding both efficacy and safety in patients with active cancer.30,31 However, in very selected cases with previous stent thrombosis during treatment with clopidogrel, ticagrelor, or prasugrel may be considered, under strict surveillance of the bleeding risk. The standard duration of DAPT after ACS is 12 months but can be safely shortened to 3 months in case of high bleeding risk.16,27,32 In case of an urgent surgery for cancer in patients with ACS, recommendations for interrupting clopidogrel are the same as in non-cancer patients.27
Anticoagulation
In cancer patients with STEMI, the standard parenteral anticoagulation during PCI is unfractionated heparin which allows close therapeutic monitoring (activated clotting time) in order to avoid bleeding complications.25
The management of ACS patients with cancer who have indications for chronic anticoagulation (e.g. atrial fibrillation, mechanical heart valves) is particularly challenging due to their increased risk of bleeding. Cancer-related risk factors (type, liver metastases, cerebral metastases, coagulopathy, renal/hepatic function etc.) and treatment-related risk factors (thrombocytopenia, surgery, radiation, central lines etc.) need to be taken into consideration. After stenting, single antiplatelet therapy with clopidogrel as short as possible is suggested in addition to oral anticoagulation in patients with stable cancer.16 Direct oral anticoagulants are increasingly used in cancer patients with atrial fibrillation and venous thromboembolism and are at least neutrally safe compared to low molecular weight heparin in both prevention and treatment of thrombosis in cancer patients.33,34
Thrombocytopenia
Thrombocytopenia (platelet count <100 000/µL) due to cancer or cancer therapy is encountered in about 10% of cancer patients and makes ACS management challenging because of increased risk of bleeding and thrombotic complications.35A low platelet count does not necessarily preclude an invasive approach to the cancer patient. Based on a small series,36 coronary angiography and PCI can be safely performed in patients with thrombocytopenia, taking into account preventive measures including radial access, careful haemostasis, and the use of lower heparin doses (30–50 U/kg).25 Importantly, antiplatelet therapy should not be withheld from these patients. A retrospective single-centre study found an association between aspirin use and improved short-term outcome in thrombocytopenic ACS patients with cancer.29 A more recent retrospective analysis demonstrated a trend for better outcome in those treated with aspirin plus clopidogrel.36 Experts advise a minimum platelet count of 10 000 (or 20 000/µL for patients with active colorectal, gynaecological or bladder cancer or necrotizing tumours) 30 000 and 50 000/μL respectively, for coronary angiography, PCI, and coronary artery bypass grafting.25
Implications for cancer treatment
When ACS is not directly related to the cancer treatment, the cancer therapy can usually be resumed early after stabilization and treatment. The same secondary preventive measures apply as in non-cancer patients.16 When the ACS is directly provoked by the cancer treatment switching to an alternative treatment should be discussed interdisciplinary. In case of therapy-related vasospasm without a reasonable alternative, a re-challenge with the same treatment under close monitoring, although controversial, can be attempted provided that underlying CAD has been excluded or treated. In that case, pre-treatment with a non-dihydropyridine calcium blocker together with a long acting nitrate and aspirin for at least 48 h together with switching to a bolus 5-FU injection, instead of infusion, and lowering the dose have been suggested with variable outcomes.37–40 There is no evidence that hormone treatment with tamoxifen should be discontinued in patients developing ACS41; in contrast to aromatase inhibitors tamoxifen has a positive effect on the lipid profile and is associated with a better outcome.42
Acute pericardial diseases in cancer patients
Consensus statements for acute pericardial disease in cancer patients
| Pericardial effusions in malignancy |
| In haemodynamically unstable patients presenting with cardiac tamponade, immediate echo-guided pericardiocentesis should be performed. |
| Prolonged drainage (2–5 days) together with instillation of sclerosing agents are suggested to reduce the risk of recurrences. |
| When safe percutaneous approach is not possible or in haemodynamically stable patients with recurrent large effusions, a thoracoscopic or surgical pericardial window is suggested. |
| A percutaneous balloon pericardiotomy is suggested for relapsing pericardial effusions if surgical expertise is not available. |
| Pericardial fluid samples should be sent for cytological, biochemical and microbiological analysis. |
| Pericarditis in malignancy |
| Acute pericarditis in cancer patients should be treated with NSAIDs and colchicine in the absence of contraindications. |
| Corticosteroids (methylprednisolone 1 mg/kg/day) and interruption are indicated for ICI-related pericarditis. |
| First presentation of malignancy |
| When malignancy is suspected further exams (fluid analysis, contrast-enhanced computed tomography, mammography) are suggested according to patient profile and clinical presentation. |
| Pericardial effusions in malignancy |
| In haemodynamically unstable patients presenting with cardiac tamponade, immediate echo-guided pericardiocentesis should be performed. |
| Prolonged drainage (2–5 days) together with instillation of sclerosing agents are suggested to reduce the risk of recurrences. |
| When safe percutaneous approach is not possible or in haemodynamically stable patients with recurrent large effusions, a thoracoscopic or surgical pericardial window is suggested. |
| A percutaneous balloon pericardiotomy is suggested for relapsing pericardial effusions if surgical expertise is not available. |
| Pericardial fluid samples should be sent for cytological, biochemical and microbiological analysis. |
| Pericarditis in malignancy |
| Acute pericarditis in cancer patients should be treated with NSAIDs and colchicine in the absence of contraindications. |
| Corticosteroids (methylprednisolone 1 mg/kg/day) and interruption are indicated for ICI-related pericarditis. |
| First presentation of malignancy |
| When malignancy is suspected further exams (fluid analysis, contrast-enhanced computed tomography, mammography) are suggested according to patient profile and clinical presentation. |
ICI, immune checkpoint inhibitor; NSAIDs, non-steroidal anti-inflammatory drugs.
| Pericardial effusions in malignancy |
| In haemodynamically unstable patients presenting with cardiac tamponade, immediate echo-guided pericardiocentesis should be performed. |
| Prolonged drainage (2–5 days) together with instillation of sclerosing agents are suggested to reduce the risk of recurrences. |
| When safe percutaneous approach is not possible or in haemodynamically stable patients with recurrent large effusions, a thoracoscopic or surgical pericardial window is suggested. |
| A percutaneous balloon pericardiotomy is suggested for relapsing pericardial effusions if surgical expertise is not available. |
| Pericardial fluid samples should be sent for cytological, biochemical and microbiological analysis. |
| Pericarditis in malignancy |
| Acute pericarditis in cancer patients should be treated with NSAIDs and colchicine in the absence of contraindications. |
| Corticosteroids (methylprednisolone 1 mg/kg/day) and interruption are indicated for ICI-related pericarditis. |
| First presentation of malignancy |
| When malignancy is suspected further exams (fluid analysis, contrast-enhanced computed tomography, mammography) are suggested according to patient profile and clinical presentation. |
| Pericardial effusions in malignancy |
| In haemodynamically unstable patients presenting with cardiac tamponade, immediate echo-guided pericardiocentesis should be performed. |
| Prolonged drainage (2–5 days) together with instillation of sclerosing agents are suggested to reduce the risk of recurrences. |
| When safe percutaneous approach is not possible or in haemodynamically stable patients with recurrent large effusions, a thoracoscopic or surgical pericardial window is suggested. |
| A percutaneous balloon pericardiotomy is suggested for relapsing pericardial effusions if surgical expertise is not available. |
| Pericardial fluid samples should be sent for cytological, biochemical and microbiological analysis. |
| Pericarditis in malignancy |
| Acute pericarditis in cancer patients should be treated with NSAIDs and colchicine in the absence of contraindications. |
| Corticosteroids (methylprednisolone 1 mg/kg/day) and interruption are indicated for ICI-related pericarditis. |
| First presentation of malignancy |
| When malignancy is suspected further exams (fluid analysis, contrast-enhanced computed tomography, mammography) are suggested according to patient profile and clinical presentation. |
ICI, immune checkpoint inhibitor; NSAIDs, non-steroidal anti-inflammatory drugs.
Pericardial effusion and tamponade
Causes
In the majority of patients, malignancy-related pericardial effusions are caused by direct or metastatic invasion of a non-cardiac tumour, or secondary to mediastinal lymph node involvement, and are often associated with a poor prognosis.43,44 Primary solid tumours, such as malignant pericardial mesotheliomas or cardiac synovial sarcomas, are very rare.45 Effusions can also be paraneoplastic or caused by specific cancer treatments, infections in immunocompromised patients, as well as post-operatively after mediastinal surgery.46,47 Acute pericardial effusions may also follow radiation therapy especially after chemoradiotherapy for lung and oesophageal cancers.48,49 Pericardial effusions have been reported with different cancer therapies (Table 1). Causes of effusions unrelated to a malignancy are not rare in cancer patients; their diagnosis depends on the clinical context (Table 2).
Malignancy
|
Cancer treatment
|
Infection
|
Malignancy
|
Cancer treatment
|
Infection
|
Malignancy
|
Cancer treatment
|
Infection
|
Malignancy
|
Cancer treatment
|
Infection
|
Clinical presentation
In cancer patients, pericardial effusion is often diagnosed as an incidental finding. As in non-cancer patients, the clinical presentation of the effusion depends on the velocity of fluid accumulation.43,45 Overall, a subacute tamponade insidiously developing over days to even weeks appears to be more common in malignant pericardial effusions, causing unspecific symptoms such as fatigue and dyspnoea.46
Management
Initial management
Small to medium-sized effusions can be monitored with an interval reassessment, e.g. repeat echocardiography 7–14 days after initial diagnosis and then 4–6 weekly intervals. In unstable patients, immediate pericardiocentesis is indicated to reverse the haemodynamic effects50 Meanwhile, rapid intravenous volume resuscitation is recommended if the patient is hypovolaemic, while intubation and mechanical ventilation should be avoided if possible. In clinically stable patients appropriate cross-sectional imaging can be helpful to differentiate acute pericarditis from other cancer-related causes.51
Pericardial drainage
Pericardial effusions in malignancy often progress to tamponade requiring pericardial drainage. An echocardiography-guided pericardiocentesis and placement of a pericardial catheter with prolonged drainage (2–5 days) is the preferred approach for most patients.50,52 A surgical pericardiotomy is less effective and associated with more complications in cancer patients,50,53,54 but can be considered when the location of the effusion precludes a safe percutaneous approach or, rarely, when a pericardial biopsy is considered.55 In case of recurrent effusions, the creation of pericardial window (surgically or via percutaneous balloon pericardiotomy) can reduce the risk of repeat interventions by allowing drainage into an adjacent space, usually the pleura.50,56–58 The risks and benefits associated with such a procedure should always be carefully balanced. The outcome of a pericardial effusion requiring a pericardiocentesis in cancer patients is poor regardless of the presence of malignant cells in the pericardial fluid.52,55 Therefore, in the absence of tamponade, a pericardiocentesis should only be considered if this will have therapeutic impact. A pericardiocentesis is rarely required in patients on ICIs because the effusions are not often associated with haemodynamic compromise.59 However, a high mortality up to 21% has been reported in ICI-related pericarditis in the acute phase.60
Pericardial fluid analysis
Cancer-related pericardial involvement is established by pericardial cytology, although the sensitivity of pericardial fluid cytology varies considerably.61,62 In a contemporary cohort, a positive cytology was detected in about half of the known cancer patients.52 The sensitivity increases with the examined volume of pericardial fluid (preferably >60 mL).63 In sanguineous samples with negative cytology, tumour marker assessment can help distinguishing malignant from non-malignant effusions, although clinically meaningful cut-off values of these markers remain unknown.64 In ICI-related pericarditis, lymphocytes are predominant.60 Flow cytometry is useful when a haematological malignancy is suspected. Pericardial fluid samples should also be sent for culture, PCR, and biochemical tests.
Further diagnostics if first presentation of malignancy
Pericardial effusions or tamponade may be the first presentation of advanced cancer,44 especially lung and breast cancers which may spread via local infiltration through the pericardium. Also, lymphoma can present with a pericardial effusion. Besides fluid analysis, a search for underlying malignancy is recommended with additional investigations depending on the clinical findings and patient profile (contrast-enhanced computed tomography of the thorax65 and the abdomen, mammography). In stable patients, a pericardial biopsy for histological analysis is less sensitive than fluid cytology but it can increase the diagnostic yield, especially in low-volume effusions.61
Intrapericardial treatment
After percutaneous drainage of a proven malignant pericardial effusion, intrapericardial treatment can be considered to prevent recurrences. Multiple cytotoxic and sclerosing agents have been evaluated in small series, but the effect on recurrences is at best modest.66 In a systematic review, pericardial sclerosis was associated with a reduced pooled recurrence rate of 10.8%67 compared to 12.1% in case of extended drainage and 38.3% in case of an isolated pericardiocentesis. Possible complications are chest pain, mild transient fever and atrial fibrillation. There are no large randomized controlled trials comparing the different agents although bleomycin was as effective as tetracyclines but associated with fewer side effects.68 Local intrapericardial chemotherapy as an adjunctive to systemic cancer treatment rather than as a tool to prevent effusion recurrences can be considered although evidence is lacking.69
Implications for cancer treatment
Pericardial effusions can be indicative of advanced cancer, treatment resistance or failure or can be caused by the therapy itself. A change in systemic therapy may thus be required.
Recurrent malignancy should be excluded by cytological examination of the fluids accompanied by appropriate cross-sectional imaging. However, sometimes cancer therapy-related effusions occur months-years after completing treatment. ‘Differentiation syndrome’ (previously retinoic acid syndrome), characterized by fevers, oedema, respiratory distress, pericardial and pleural effusions, hypotension and renal failure, occurs in approximately 15% of ATRA-treated patients and usually peaks during the initial 2 weeks of treatment. Untreated, this treatment complication is potentially fatal, and, prompt treatment with corticosteroids together with temporary discontinuation of ATRA are indicated.70
During ICI therapy, pericardial effusions may represent ‘pseudo-progression’ secondary to immune-mediated pericarditis. Despite the high mortality in the acute phase (up to 21%),60 successful treatment with high dose corticosteroids and temporary interruption of immunotherapy in addition to drainage of the effusion has been reported.71
Pericarditis
Causes
In cancer patients, pericarditis or myopericarditis is often caused by therapies, especially radiotherapy to the chest, but also by different cancer treatments (Table 1). Acute pericarditis may also be paraneoplastic, while infectious pericarditis should also be suspected in immune-compromised cancer patients. Acute pericarditis in a patient without known malignancy can be the marker of an occult cancer.72
Clinical presentation
The clinical diagnosis of a pericarditis is made with at least two of the following criteria: (i) pericardial chest pain, (ii) a pericardial friction rub, (iii) typical ECG-changes, and (iv) pericardial effusion.50 Additional findings are elevated inflammatory markers and evidence of pericardial inflammation on cardiac magnetic resonance imaging.73 Elevated troponins are indicative for concomitant myocarditis and are associated with a poor prognosis.74 Acute radiation pericarditis occurs early after treatment (days, weeks), while a chronic effusive-constrictive pericarditis may develop years after radiation.
Management and implications for cancer treatment
In general, acute pericarditis is treated in the same way as in non-cancer patients with non-steroidal anti-inflammatory drugs and colchicine in the absence of contraindications, to relieve symptoms and reduce the risk of relapse, as well as to avoid the development of constrictive pericarditis.50 Pericarditis caused by conventional cancer therapies often resolves after discontinuing these treatments. In pericarditis secondary to ICIs, additional treatment with methylprednisolone treatment (1 mg/kg/day) is indicated while temporarily discontinuing the ICI.50,75 After recovery, a re-challenge of the ICI can be considered. In steroid-refractory pericarditis, immunosuppressive drugs present an alternative.76 The care for constrictive pericarditis includes diuretics for symptomatic relieve, or pericardiectomy in selected cases although outcome is poor.50
Conclusion and future perspective
Cancer patients are at increased risk of acute CV disease. Health care workers treating these patients as well as acute cardiac care specialists need to be aware of the potential acute CV complications of cancer therapies. The management of cancer patients with acute cardiac disease should be discussed in a multidisciplinary way. Despite the increasing incidence of acute CV disease in cancer patients, evidence on optimal management is still limited making acute cardio-oncology a field in development with many unmet needs and important knowledge gaps:
data on true prevalence of clinically relevant acute CV complications;
tools for balancing the need between necessary cancer treatment and risk of acute CV complications;
optimal treatment strategy for ACS patients with active cancer, including both invasive and pharmacological management; and
tools for differentiating malignant pericardial effusions from cancer therapy-related effusions.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Conflict of interest: D.F.: speaker’s honoraria or advisory board fees from Abbott Laboratories, Bayer, Boehringer Ingelheim, Leo Pharmaceuticals, Menarini, Novartis, Orion Pharma, Roche Diagnostics.
S.A.G.: Speaker’s and advisory fees (all institutional) from AstraZeneca, Daiichi-Sankyo, Boehringer.
P.R.S.: speaker’s and advisory fees (all institutional) from AstraZeneca, Daiichi-Sankyo, Bayer, Boehringer, Pfizer, BMS, Amgen, Sanofi. Co-recipient of a Chair at the University of Leuven funded by Bayer.
P.V.D.M.: consultancy and/or research grants from Vifor Pharma, AstraZeneca, Servier, Novartis, Pfizer, Pharmacosmos, Ionis. Supported by the ERC (StG 715732).
A.R.L.: speaker, advisory board or consultancy fees and/or research grants from Pfizer, Novartis, Servier, Amgen, Takeda, Roche, Janssens-Cilag Ltd, Clinigen, Eli Lily, Eisai, Bristol Myers Squibb, Ferring Pharmaceuticals, Boehringer Ingelheim, Myocardial Solutions, iOWNA and Heartfelt Technologies Ltd. Supported by the Fondation Leducq Network of Excellence in Cardio-Oncology.
A.O.: speaker’s and advisory fees from Seagen, Roche, Diaichi-Sankyo and Astra Zeneca. Research funding from Pfizer and Roche. Travel support from Leo Pharmaceuticals.
A.W.: honorarium, advisory board orconsultancy fees: Janssen Cilag, GSK, Alexion, Caelum, Takeda, Celgene. All other authors have declared no conflict of interest.
Review coordinator
Alain Combes, Medical-Surgical ICU, Hôpital Pitié–Salpêtrière, France
Sorbonne University, Institute of Cardiometabolism and Nutrition, France
Reviewers
Roman Pfister, Oberarzt der Klinik III für Innere Medizin, Facharzt für Innere Medizin—Kardiologie—Pneumologie—internistische Intensivmedizin, Herzzentrum der Universität zu Köln, Germany
Jutta Bergler-Klein, MD, Professor of Medicine and Cardiology, Head of Cardiac Outpatients and Acute first care unit, Vice head Day Clinic, Senior Consultant Cardiology, Department of Cardiology, Univ. Clinic of Internal Med. II, Medical University of Vienna, AKH General Hospital Vienna, Austria
Maddalena Lettino, Cardiovascular Department, Humanitas Research Hospital, Italy




Comments