-
PDF
- Split View
-
Views
-
Cite
Cite
Katarina Mars, John Wallert, Claes Held, Sophia Humphries, Ronnie Pingel, Tomas Jernberg, Erik M G Olsson, Robin Hofmann, Association between β-blocker dose and cardiovascular outcomes after myocardial infarction: insights from the SWEDEHEART registry, European Heart Journal. Acute Cardiovascular Care, Volume 10, Issue 4, April 2021, Pages 372–379, https://doi.org/10.1093/ehjacc/zuaa002
Close - Share Icon Share
Abstract
Dose-dependent effects of β-blockers on survival and cardiovascular outcomes after myocardial infarction (MI) are not well understood. We investigated the long-term risk of cardiovascular events in patients with different doses of β-blockers after MI.
This was a nationwide observational study linking morbidity, mortality, socioeconomic, and medication data from Swedish national registries. Between 2006 and 2015, 97 575 unique patients with first-time MI were included. In total, 33 126 (33.9%) patients were discharged with ≥50% of the target β-blocker dose and 64 449 (66.1%) patients with <50% of the target β-blocker dose used in previous randomized trials. The primary composite endpoint was re-infarction or all-cause death within 1 year from discharge. Multivariable adjusted 1-year follow-up estimates using mixed effects Cox regression [HR (95% CI)] showed that patients treated with ≥50% of the target dose had a similar risk of the composite endpoint [1.03 (0.99–1.08)] and a somewhat higher risk when stroke, atrial fibrillation, or heart failure hospitalization were added to the composite endpoint [1.08 (1.04–1.12)], compared with patients on <50% of the target β-blocker dose. Results remained similar up to 5 years of follow-up and consistent across relevant patient subgroups, including patients who developed heart failure during the index hospitalization.
In contrast to doses of β-blockers used in previous trials, ≥50% of the target β-blocker dose was not associated with superior cardiovascular outcomes up to 5 years as compared with <50% of the target dose. Contemporary randomized clinical trials are needed to clarify the optimal dose of β-blockers after MI.
Introduction
For decades, β-blocker therapy has been a cornerstone of medical treatment of all patients after acute myocardial infarction (MI). This practice is based on trials from the 1980s, where β-blocker therapy improved survival by about 20%.1–6 Back then, patients commonly suffered from larger MIs with reduced left ventricular ejection fraction (LVEF) resulting in heart failure (HF). Standard of care did not yet include modern treatments such as reperfusion therapy, antithrombotic agents, statins, and angiotensin-converting enzyme (ACE) inhibitors. Regardless of LVEF, β-blockers are still used today in secondary prevention in the majority of all MI cases,7–9 which is also supported by guidelines, notably, based on expert opinion.10,11
Treatment effects of β-blockers have been attributed to heart rate reduction, prevention of malignant arrhythmias, and possible anti-inflammatory effects on post-MI remodelling.1–6
On the other hand, clinically relevant adverse effects of hypotension, bradycardia,1–6 and more subjective patient reported symptoms like fatigue, exercise intolerance, and deterioration of psychosocial functioning due to nightmares, depression, and sexual dysfunction,12 may counterbalance the potential benefits.
β-blocker therapy for patients with reduced LVEF below 40% is well accepted and established in guidelines.13,14 However, controversy exists regarding MI patients with LVEF ≥40% as several recent meta-analyses have reported conflicting results,14–16 in particular in the post-reperfusion era where the use of β-blocker has not been associated with a lower risk of cardiovascular events.17
In general, current guidelines do not recommend a specific β-blocker or dose.10,11
Available data indicate that prescribed doses of β-blockers in clinical practice are below the targeted doses in reported trials.18,19 Patient adherence is rarely reported at all. Available observational research on a possible dose–response relationship regarding the risk–benefit ratio of β-blocker therapy is restricted by selected samples with relatively small size and limited data on possible confounding factors.20–23
To date, β-blocker therapy has not been investigated in contemporary randomized clinical trials (RCTs) in MI patients with normal or mildly depressed LVEF and guideline authorities and experts request conclusive new data.10,24
To address some of these knowledge gaps, we have performed a nationwide cohort study based on linked registry data from six national registries to investigate whether there is an association between dose of β-blocker and cardiovascular outcomes after acute MI.
Methods
Registries and data
This observational cohort study utilized data from several mandatory Swedish national registries linked to the nationwide comprehensive Swedish Web System for Enhancement and Development of Evidence-based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry.25 The 10th International Classification of Diseases system (ICD-10) is used for all diagnoses reported herein.
SWEDEHEART encompasses over 100 variables on hospital admissions for MI at all Coronary Care Units (CCUs) and other specialized facilities in Sweden. Data include time and site variables, diagnosis of MI, past and present medications, hospital treatment variables, comorbidities, demographic data, and more. The diagnosis of MI (ICD-10, I21–I23) is decided by the study-independent local cardiologist according to current guidelines. SWEDEHEART has excellent nationwide coverage (100% of CCUs, >90% of patients with MI <80 years). Data are monitored continuously with >95% agreement between the registry and health records.25 The national Patient Registry (PR) registers all admissions in Sweden including dates, diagnoses, and treatments.26 The national Prescribed Drug Registry (PDR) registers all dispensed drugs from pharmacies in the country, including variables such as type of medication, dose, prescription date, and dispensation date. The Swedish National Population Registry includes annual measurements on several socioeconomic status variables, including income, education, and country of birth. The Cause-of-death Registry has complete nationwide coverage of date and cause of death.27
Individual patient-level data from these registries were linked via the unique personal identification number by the National Board of Health and Welfare in Sweden safeguarding the identifier key and only delivering pseudonymized data to the researchers. Data processing and analysis were performed thereafter. The present study adheres to the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Uppsala, Sweden (2013/478).
Study population
The flow of patients through the study is available in Figure 1. In summary, of all 192 059 MI admissions registered in SWEDEHEART from 1 January 2006 through 31 December 2015, the main study population included 97 575 unique patients with first-time MI >18 years of age, whom survived until hospital discharge, were discharged with β-blocker, and had a subsequent first post-discharge β-blocker dispensation registered in the PDR.
Exposures and outcomes
The main exposure was β-blocker dose taken from the first dispensation post-discharge. The follow-up started at discharge and ended with event, end of follow-up or censoring. Hence, β-blocker dose is inferred to be sufficiently stable at discharge in the main analysis. The target doses of commonly used β-blockers were defined as follows and in agreement with prior studies1–6: metoprolol 200 mg/day; bisoprolol 10 mg/day; atenolol 100 mg/day. The most common discharge drug and dose was metoprolol 50 mg (N = 64 449, reference dose), followed by metoprolol 100 mg (N = 28 902). In agreement with prior studies,21,22 the daily β-blocker dose was described as a proportion of the respective target dose and dichotomized in two β-blocker dose groups: <50% of the target dose (reference dose) and ≥50% of the target dose.
In secondary sensitivity analyses, we (i) select the second β-blocker dispensation to ascertain dose stability (exposure misclassification), (ii) in a landmark analysis, postpone the start of follow-up with 30 days of increments and correspondingly condition on those surviving, to assess immortal time bias, (iii) run the analysis with the alternative exposure of three-level β-blocker dose [low dose (25 mg atenolol, 1.25–2.5 mg bisoprolol, or 25–50 mg metoprolol)], moderate dose (26–50 mg atenolol, 2.6–5.0 mg bisoprolol, or 51–100 mg metoprolol), high dose (>50 mg atenolol, >5 mg bisoprolol, or >100 mg metoprolol), (iv) stratify main exposure analysis in relevant subgroups by sex (male/female), age (<65 years/≥65 years/<75 years), LVEF (≥50%/40–49%/<40%), infarct type [ST-segment elevation MI (STEMI), non-STEMI (NSTEMI)], and (v) run a complete case analysis.
All doses assumed a patient consumption of one pill per day with the same reference dose across all analyses.
The primary outcome was post-discharge 1-year time to re-infarction or all-cause death. Secondary outcomes used (vi) a 5-year follow-up, and (vii) also evaluated the alternative extended composite of time to re-infarction/all-cause death/stroke (I60–I64)/HF hospitalization (I50)/new-onset AF (I48). All-cause mortality was defined by a patient having a registered death date in the Cause-of-death Registry. Re-infarction/stroke/new-onset HF/AF diagnoses and dates were taken from the PR and the SWEDEHEART registry.
Covariates
To control for confounding and increase the precision of modelling estimates, covariates were modelled based on a directed acyclic graph (Supplementary material online, Figure SA1). Included in-hospital variables were hospital site, hospital size (small/medium/large), admission year, hospital stay, sex, age, smoking status (never/former/current smoker), occupational status (working/sick-leave/unemployed/retired/student/other), diabetes, body mass index (BMI), hypertension, previous stroke, LVEF, heart rate, systolic blood pressure (SBP), infarct type (STEMI/NSTEMI), reperfusion, and revascularization. Discharge medicine variables were angiotensin-II receptor blockers (ARB), ACE inhibitors, oral anticoagulation, other antiplatelets, aspirin, calcium channel blockers (CCB), digitalis, diuretics, statins, other lipid-lowering agents, and nitrates. Prior diagnoses included asthma, bronchitis, emphysema, other chronic respiratory disease, peripheral artery disease, depression, and anxiety. Socioeconomic history variables were country of birth (foreign/Sweden), prior year household income adjusted for family composition (quintiles), and highest attained education (primary/secondary/higher).
Statistical analysis
Numerical variables are presented as their arithmetic mean (SD) and categorical variables as their count (%) unless otherwise specified. Missing data are reported and multiple imputation via chained equations and predictive mean matching was performed, followed by pooled effect estimation across the resulting five imputed datasets according to Rubin’s rules. Imputation worked satisfactorily across analyses and variables with most missing data were infarct type (23.2%), LVEF (20.2%), and BMI (15.4).
Mixed effects Cox regression was applied to compute hazard ratios (HR) with robust 95% confidence intervals (CI) which are interpreted as relative risks (RR). The proportional hazards and other assumptions were reasonably met after modelling diagnostics, mainly inspection of Schoenfeld and normalized Martingale (deviance) residuals. Models included linear main effects with age also modelled as a quadratic term, and a categorical term (above or below the population median), and hospital site as a random effect. For stratified analyses, the variable stratified on was not adjusted for with the exception of numerical age which still had to be adjusted for within categorical age strata.
Data pre-processing and analysis were performed in R version 3.4.3.
Results
The total study population consisted of 97 575 unique patients with first-time MI prospectively recruited into the SWEDEHEART registry. At baseline, 33 126 (33.9%) patients were discharged with ≥50% of the target β-blocker dose and 64 449 (66.1%) with <50% of the target β-blocker dose at first dispensation. Over an average follow-up time of 953 days within the 5-year follow-up span, 38 649 (39.6%) patients suffered either re-infarction or all-cause death. Extending the composite outcome by adding stroke, HF hospitalization, or new-onset AF shortened the average follow-up time to 795 days during which 50 008 (51.3%) patients experienced an event. Correspondingly, 16 268 (16.7%) patients died by all-causes, 28 215 (28.9%) had a re-infarction, 5600 (5.7%) suffered a stroke, 2185 (2.2%) had new-onset AF, and 22 626 (23.2%) were hospitalized for HF.
Descriptive summary statistics for patients discharged alive are available in Table 1 and Supplementary material online, Table SA1. Compared to patients with <50% of the target β-blocker dose, patients with ≥50% of the target dose were more likely to be taking Atenolol and Bisoprolol, have a longer hospital stay, be retired, have diabetes, hypertension, display a higher heart rate at admission, suffer an NSTEMI, have received reperfusion or revascularization therapy, and be discharged with more concomitant medication (ARB, oral anticoagulation, CCB, digitalis, and diuretics). Patients with ≥50% of the target dose were also less likely to have a prior mood disorder diagnosis, and fewer had attained higher education.
Descriptive data by β-blocker dose for the total patient population (N = 97 575)
| . | ≥50% of the target β-blocker dose (n = 33 126) . | <50% of the target β-blocker dose (n = 64 449) . | P-valuea . | Missing datab . |
|---|---|---|---|---|
| β-blocker | ||||
| Metoprolol | 22 453 (67.8) | 55 282 (85.8) | <0.001 | 0 |
| Bisoprolol | 8203 (24.8) | 8015 (12.4) | <0.001 | 0 |
| Atenolol | 2470 (7.5) | 1152 (1.8) | <0.001 | 0 |
| In-hospital | ||||
| Hospital stayc | 4.0 [3.0, 7.0] | 4.0 [3.0, 6.0] | <0.001 | 0 |
| Male | 21 350 (64.5) | 41 845 (64.9) | 0.142 | 0 |
| Age | 68.7 (12.0) | 68.8 (12.4) | 0.081 | 0 |
| Current smoker | 7537 (24.2) | 15 368 (25.2) | 0.001 | 5531 |
| Occupation status | <0.001 | 5037 | ||
| Working | 8586 (27.6) | 17 978 (29.3) | ||
| Retired | 21 182 (68.1) | 40 963 (66.7) | ||
| Diabetes | 7038 (21.3) | 9536 (14.8) | <0.001 | 129 |
| BMI | 27.6 (4.7) | 26.7 (4.4) | <0.001 | 15014 |
| Hypertension | 18 630 (56.6) | 26 608 (41.5) | <0.001 | 574 |
| Previous stroke | 2455 (7.7) | 3872 (6.1) | <0.001 | 1988 |
| LVEF | <0.001 | 19722 | ||
| ≥50% | 15 339 (57.9) | 31 795 (61.9) | ||
| 40–49% | 6214 (23.4) | 11 057 (21.5) | ||
| 30–39% | 3712 (14.0) | 6230 (12.1) | ||
| <30% | 1244 (4.7) | 2262 (4.4) | ||
| Heart rate | 83.6 (23.0) | 79.4 (20.9) | <0.001 | 2876 |
| SBP | 152.2 (29.2) | 148.5 (28.1) | <0.001 | 3451 |
| Infarct type | <0.001 | 22618 | ||
| NSTEMI | 14 934 (62.9) | 31 009 (60.5) | ||
| STEMI | 8794 (37.1) | 20 220 (39.5) | ||
| Reperfusion | 11 175 (33.7) | 22 956 (35.6) | <0.001 | 7 |
| Revascularization | 10 419 (31.5) | 21 470 (33.3) | <0.001 | 7 |
| Other discharge medicines | ||||
| ACE inhibitors | 21 213 (64.1) | 40 193 (62.4) | <0.001 | 75 |
| ARB | 5816 (17.8) | 8319 (12.9) | <0.001 | 654 |
| Other antiplatelets | 27 499 (83.0) | 55 171 (85.6) | <0.001 | 27 |
| Aspirin | 30 726 (92.8) | 61 090 (94.8) | <0.001 | 20 |
| CCB | 6253 (18.9) | 6988 (10.8) | <0.001 | 29 |
| Digitalis | 1158 (3.5) | 990 (1.5) | <0.001 | 17 |
| Diuretics | 10 216 (30.9) | 14 014 (21.7) | <0.001 | 24 |
| Statins | 29 573 (89.3) | 57 650 (89.5) | 0.446 | 36 |
| Nitrates | 4046 (12.2) | 5652 (8.8) | <0.001 | 125 |
| Comorbidities | ||||
| Asthma | 1613 (4.9) | 2674 (4.1) | <0.001 | 10 |
| PAD | 859 (2.6) | 1262 (2.0) | <0.001 | 10 |
| Depression | 1407 (4.2) | 2910 (4.5) | 0.057 | 10 |
| Anxiety | 1360 (4.1) | 2744 (4.3) | 0.272 | 10 |
| Socioeconomic history | ||||
| Foreign born | 5084 (15.4) | 9474 (14.7) | 0.007 | 10 |
| Education | <0.001 | 1864 | ||
| Higher | 5421 (16.6) | 11 618 (18.4) | ||
| Primary | 13 755 (42.2) | 25 537 (40.4) | ||
| Secondary | 13 390 (41.1) | 25 990 (41.2) |
| . | ≥50% of the target β-blocker dose (n = 33 126) . | <50% of the target β-blocker dose (n = 64 449) . | P-valuea . | Missing datab . |
|---|---|---|---|---|
| β-blocker | ||||
| Metoprolol | 22 453 (67.8) | 55 282 (85.8) | <0.001 | 0 |
| Bisoprolol | 8203 (24.8) | 8015 (12.4) | <0.001 | 0 |
| Atenolol | 2470 (7.5) | 1152 (1.8) | <0.001 | 0 |
| In-hospital | ||||
| Hospital stayc | 4.0 [3.0, 7.0] | 4.0 [3.0, 6.0] | <0.001 | 0 |
| Male | 21 350 (64.5) | 41 845 (64.9) | 0.142 | 0 |
| Age | 68.7 (12.0) | 68.8 (12.4) | 0.081 | 0 |
| Current smoker | 7537 (24.2) | 15 368 (25.2) | 0.001 | 5531 |
| Occupation status | <0.001 | 5037 | ||
| Working | 8586 (27.6) | 17 978 (29.3) | ||
| Retired | 21 182 (68.1) | 40 963 (66.7) | ||
| Diabetes | 7038 (21.3) | 9536 (14.8) | <0.001 | 129 |
| BMI | 27.6 (4.7) | 26.7 (4.4) | <0.001 | 15014 |
| Hypertension | 18 630 (56.6) | 26 608 (41.5) | <0.001 | 574 |
| Previous stroke | 2455 (7.7) | 3872 (6.1) | <0.001 | 1988 |
| LVEF | <0.001 | 19722 | ||
| ≥50% | 15 339 (57.9) | 31 795 (61.9) | ||
| 40–49% | 6214 (23.4) | 11 057 (21.5) | ||
| 30–39% | 3712 (14.0) | 6230 (12.1) | ||
| <30% | 1244 (4.7) | 2262 (4.4) | ||
| Heart rate | 83.6 (23.0) | 79.4 (20.9) | <0.001 | 2876 |
| SBP | 152.2 (29.2) | 148.5 (28.1) | <0.001 | 3451 |
| Infarct type | <0.001 | 22618 | ||
| NSTEMI | 14 934 (62.9) | 31 009 (60.5) | ||
| STEMI | 8794 (37.1) | 20 220 (39.5) | ||
| Reperfusion | 11 175 (33.7) | 22 956 (35.6) | <0.001 | 7 |
| Revascularization | 10 419 (31.5) | 21 470 (33.3) | <0.001 | 7 |
| Other discharge medicines | ||||
| ACE inhibitors | 21 213 (64.1) | 40 193 (62.4) | <0.001 | 75 |
| ARB | 5816 (17.8) | 8319 (12.9) | <0.001 | 654 |
| Other antiplatelets | 27 499 (83.0) | 55 171 (85.6) | <0.001 | 27 |
| Aspirin | 30 726 (92.8) | 61 090 (94.8) | <0.001 | 20 |
| CCB | 6253 (18.9) | 6988 (10.8) | <0.001 | 29 |
| Digitalis | 1158 (3.5) | 990 (1.5) | <0.001 | 17 |
| Diuretics | 10 216 (30.9) | 14 014 (21.7) | <0.001 | 24 |
| Statins | 29 573 (89.3) | 57 650 (89.5) | 0.446 | 36 |
| Nitrates | 4046 (12.2) | 5652 (8.8) | <0.001 | 125 |
| Comorbidities | ||||
| Asthma | 1613 (4.9) | 2674 (4.1) | <0.001 | 10 |
| PAD | 859 (2.6) | 1262 (2.0) | <0.001 | 10 |
| Depression | 1407 (4.2) | 2910 (4.5) | 0.057 | 10 |
| Anxiety | 1360 (4.1) | 2744 (4.3) | 0.272 | 10 |
| Socioeconomic history | ||||
| Foreign born | 5084 (15.4) | 9474 (14.7) | 0.007 | 10 |
| Education | <0.001 | 1864 | ||
| Higher | 5421 (16.6) | 11 618 (18.4) | ||
| Primary | 13 755 (42.2) | 25 537 (40.4) | ||
| Secondary | 13 390 (41.1) | 25 990 (41.2) |
Data are mean (SD), median [IQR], or count (%).
ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blockers; BMI, body mass index; CCB, calcium channel blockers, LVEF, left ventricular ejection fraction; NSTEMI, non-ST-elevation myocardial infarction; PAD, peripheral artery disease; SBP, systolic blood pressure; STEMI, ST-elevation myocardial infarction.
Bivariate comparisons are t-test, Kruskal–Wallis test, and chi-square test for Gaussian numeric, non-Gaussian numeric, and categorical variables, respectively.
Imputed before main analysis.
Time in days from hospital admission to discharge.
Descriptive data by β-blocker dose for the total patient population (N = 97 575)
| . | ≥50% of the target β-blocker dose (n = 33 126) . | <50% of the target β-blocker dose (n = 64 449) . | P-valuea . | Missing datab . |
|---|---|---|---|---|
| β-blocker | ||||
| Metoprolol | 22 453 (67.8) | 55 282 (85.8) | <0.001 | 0 |
| Bisoprolol | 8203 (24.8) | 8015 (12.4) | <0.001 | 0 |
| Atenolol | 2470 (7.5) | 1152 (1.8) | <0.001 | 0 |
| In-hospital | ||||
| Hospital stayc | 4.0 [3.0, 7.0] | 4.0 [3.0, 6.0] | <0.001 | 0 |
| Male | 21 350 (64.5) | 41 845 (64.9) | 0.142 | 0 |
| Age | 68.7 (12.0) | 68.8 (12.4) | 0.081 | 0 |
| Current smoker | 7537 (24.2) | 15 368 (25.2) | 0.001 | 5531 |
| Occupation status | <0.001 | 5037 | ||
| Working | 8586 (27.6) | 17 978 (29.3) | ||
| Retired | 21 182 (68.1) | 40 963 (66.7) | ||
| Diabetes | 7038 (21.3) | 9536 (14.8) | <0.001 | 129 |
| BMI | 27.6 (4.7) | 26.7 (4.4) | <0.001 | 15014 |
| Hypertension | 18 630 (56.6) | 26 608 (41.5) | <0.001 | 574 |
| Previous stroke | 2455 (7.7) | 3872 (6.1) | <0.001 | 1988 |
| LVEF | <0.001 | 19722 | ||
| ≥50% | 15 339 (57.9) | 31 795 (61.9) | ||
| 40–49% | 6214 (23.4) | 11 057 (21.5) | ||
| 30–39% | 3712 (14.0) | 6230 (12.1) | ||
| <30% | 1244 (4.7) | 2262 (4.4) | ||
| Heart rate | 83.6 (23.0) | 79.4 (20.9) | <0.001 | 2876 |
| SBP | 152.2 (29.2) | 148.5 (28.1) | <0.001 | 3451 |
| Infarct type | <0.001 | 22618 | ||
| NSTEMI | 14 934 (62.9) | 31 009 (60.5) | ||
| STEMI | 8794 (37.1) | 20 220 (39.5) | ||
| Reperfusion | 11 175 (33.7) | 22 956 (35.6) | <0.001 | 7 |
| Revascularization | 10 419 (31.5) | 21 470 (33.3) | <0.001 | 7 |
| Other discharge medicines | ||||
| ACE inhibitors | 21 213 (64.1) | 40 193 (62.4) | <0.001 | 75 |
| ARB | 5816 (17.8) | 8319 (12.9) | <0.001 | 654 |
| Other antiplatelets | 27 499 (83.0) | 55 171 (85.6) | <0.001 | 27 |
| Aspirin | 30 726 (92.8) | 61 090 (94.8) | <0.001 | 20 |
| CCB | 6253 (18.9) | 6988 (10.8) | <0.001 | 29 |
| Digitalis | 1158 (3.5) | 990 (1.5) | <0.001 | 17 |
| Diuretics | 10 216 (30.9) | 14 014 (21.7) | <0.001 | 24 |
| Statins | 29 573 (89.3) | 57 650 (89.5) | 0.446 | 36 |
| Nitrates | 4046 (12.2) | 5652 (8.8) | <0.001 | 125 |
| Comorbidities | ||||
| Asthma | 1613 (4.9) | 2674 (4.1) | <0.001 | 10 |
| PAD | 859 (2.6) | 1262 (2.0) | <0.001 | 10 |
| Depression | 1407 (4.2) | 2910 (4.5) | 0.057 | 10 |
| Anxiety | 1360 (4.1) | 2744 (4.3) | 0.272 | 10 |
| Socioeconomic history | ||||
| Foreign born | 5084 (15.4) | 9474 (14.7) | 0.007 | 10 |
| Education | <0.001 | 1864 | ||
| Higher | 5421 (16.6) | 11 618 (18.4) | ||
| Primary | 13 755 (42.2) | 25 537 (40.4) | ||
| Secondary | 13 390 (41.1) | 25 990 (41.2) |
| . | ≥50% of the target β-blocker dose (n = 33 126) . | <50% of the target β-blocker dose (n = 64 449) . | P-valuea . | Missing datab . |
|---|---|---|---|---|
| β-blocker | ||||
| Metoprolol | 22 453 (67.8) | 55 282 (85.8) | <0.001 | 0 |
| Bisoprolol | 8203 (24.8) | 8015 (12.4) | <0.001 | 0 |
| Atenolol | 2470 (7.5) | 1152 (1.8) | <0.001 | 0 |
| In-hospital | ||||
| Hospital stayc | 4.0 [3.0, 7.0] | 4.0 [3.0, 6.0] | <0.001 | 0 |
| Male | 21 350 (64.5) | 41 845 (64.9) | 0.142 | 0 |
| Age | 68.7 (12.0) | 68.8 (12.4) | 0.081 | 0 |
| Current smoker | 7537 (24.2) | 15 368 (25.2) | 0.001 | 5531 |
| Occupation status | <0.001 | 5037 | ||
| Working | 8586 (27.6) | 17 978 (29.3) | ||
| Retired | 21 182 (68.1) | 40 963 (66.7) | ||
| Diabetes | 7038 (21.3) | 9536 (14.8) | <0.001 | 129 |
| BMI | 27.6 (4.7) | 26.7 (4.4) | <0.001 | 15014 |
| Hypertension | 18 630 (56.6) | 26 608 (41.5) | <0.001 | 574 |
| Previous stroke | 2455 (7.7) | 3872 (6.1) | <0.001 | 1988 |
| LVEF | <0.001 | 19722 | ||
| ≥50% | 15 339 (57.9) | 31 795 (61.9) | ||
| 40–49% | 6214 (23.4) | 11 057 (21.5) | ||
| 30–39% | 3712 (14.0) | 6230 (12.1) | ||
| <30% | 1244 (4.7) | 2262 (4.4) | ||
| Heart rate | 83.6 (23.0) | 79.4 (20.9) | <0.001 | 2876 |
| SBP | 152.2 (29.2) | 148.5 (28.1) | <0.001 | 3451 |
| Infarct type | <0.001 | 22618 | ||
| NSTEMI | 14 934 (62.9) | 31 009 (60.5) | ||
| STEMI | 8794 (37.1) | 20 220 (39.5) | ||
| Reperfusion | 11 175 (33.7) | 22 956 (35.6) | <0.001 | 7 |
| Revascularization | 10 419 (31.5) | 21 470 (33.3) | <0.001 | 7 |
| Other discharge medicines | ||||
| ACE inhibitors | 21 213 (64.1) | 40 193 (62.4) | <0.001 | 75 |
| ARB | 5816 (17.8) | 8319 (12.9) | <0.001 | 654 |
| Other antiplatelets | 27 499 (83.0) | 55 171 (85.6) | <0.001 | 27 |
| Aspirin | 30 726 (92.8) | 61 090 (94.8) | <0.001 | 20 |
| CCB | 6253 (18.9) | 6988 (10.8) | <0.001 | 29 |
| Digitalis | 1158 (3.5) | 990 (1.5) | <0.001 | 17 |
| Diuretics | 10 216 (30.9) | 14 014 (21.7) | <0.001 | 24 |
| Statins | 29 573 (89.3) | 57 650 (89.5) | 0.446 | 36 |
| Nitrates | 4046 (12.2) | 5652 (8.8) | <0.001 | 125 |
| Comorbidities | ||||
| Asthma | 1613 (4.9) | 2674 (4.1) | <0.001 | 10 |
| PAD | 859 (2.6) | 1262 (2.0) | <0.001 | 10 |
| Depression | 1407 (4.2) | 2910 (4.5) | 0.057 | 10 |
| Anxiety | 1360 (4.1) | 2744 (4.3) | 0.272 | 10 |
| Socioeconomic history | ||||
| Foreign born | 5084 (15.4) | 9474 (14.7) | 0.007 | 10 |
| Education | <0.001 | 1864 | ||
| Higher | 5421 (16.6) | 11 618 (18.4) | ||
| Primary | 13 755 (42.2) | 25 537 (40.4) | ||
| Secondary | 13 390 (41.1) | 25 990 (41.2) |
Data are mean (SD), median [IQR], or count (%).
ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blockers; BMI, body mass index; CCB, calcium channel blockers, LVEF, left ventricular ejection fraction; NSTEMI, non-ST-elevation myocardial infarction; PAD, peripheral artery disease; SBP, systolic blood pressure; STEMI, ST-elevation myocardial infarction.
Bivariate comparisons are t-test, Kruskal–Wallis test, and chi-square test for Gaussian numeric, non-Gaussian numeric, and categorical variables, respectively.
Imputed before main analysis.
Time in days from hospital admission to discharge.
Main analysis
Adjusted 1-year follow-up estimates showed that patients treated with ≥50% of the target β-blocker dose had a similar risk of all-cause death (HR 1.05; 95% CI, 0.99–1.12), re-infarction (HR 1.03; 95% CI, 0.99–1.09), and the composite endpoint (HR 1.03; 95% CI 0.99–1.08), and a somewhat higher risk when stroke, HF hospitalization, and new-onset AF were added to the composite (HR 1.08; 95% CI 1.04–1.12, P < 0.001), compared with patients with <50% of the target dose. Results remained unchanged up to 5 years of follow-up except for the hazard of all-cause death that was independently slightly higher in the patients treated with ≥50% of the target β-blocker dose (HR 1.05; 95% CI 1.02–1.09) (Take home figure, Table 2).
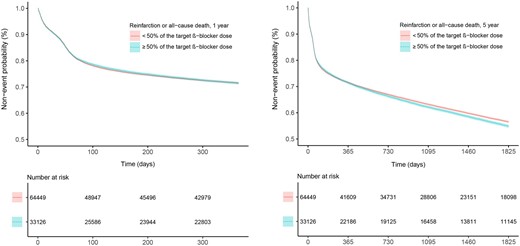
Kaplan–Meier estimate for the primary composite endpoint of re-infarction or all-cause death, by dose level, ≥50% or <50% of the target β-blocker dose, for the 1- and 5-year follow-up time in 97 575 unique patients with MI.
Main analysis of ≥50% of the target β-blocker dose vs. <50% of the target dose on outcomes and follow-up time
| Outcome . | 1 year . | 5 years . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Re-infarction or all-cause death | 1.03 (0.99–1.08) | 0.18 | 1.03 (0.99–1.07) | 0.09 |
| Re-infarction, all-cause death, stroke, heart failure hospitalization, or new-onset atrial fibrillation | 1.08 (1.04–1.12) | <0.001 | 1.07 (1.04–1.11) | <0.001 |
| All-cause death | 1.05 (0.99–1.12) | 0.09 | 1.05 (1.02–1.09) | 0.01 |
| Re-infarction | 1.03 (0.99–1.09) | 0.18 | 1.04 (1.00–1.09) | 0.06 |
| Stroke | 1.08 (0.98–1.18) | 0.09 | 1.05 (0.99–1.11) | 0.08 |
| New-onset atrial fibrillation | 1.30 (1.17–1.44) | <0.001 | 1.29 (1.18–1.41) | <0.001 |
| Heart failure hospitalization | 1.17 (1.13–1.22) | <0.001 | 1.16 (1.12–1.20) | <0.001 |
| Outcome . | 1 year . | 5 years . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Re-infarction or all-cause death | 1.03 (0.99–1.08) | 0.18 | 1.03 (0.99–1.07) | 0.09 |
| Re-infarction, all-cause death, stroke, heart failure hospitalization, or new-onset atrial fibrillation | 1.08 (1.04–1.12) | <0.001 | 1.07 (1.04–1.11) | <0.001 |
| All-cause death | 1.05 (0.99–1.12) | 0.09 | 1.05 (1.02–1.09) | 0.01 |
| Re-infarction | 1.03 (0.99–1.09) | 0.18 | 1.04 (1.00–1.09) | 0.06 |
| Stroke | 1.08 (0.98–1.18) | 0.09 | 1.05 (0.99–1.11) | 0.08 |
| New-onset atrial fibrillation | 1.30 (1.17–1.44) | <0.001 | 1.29 (1.18–1.41) | <0.001 |
| Heart failure hospitalization | 1.17 (1.13–1.22) | <0.001 | 1.16 (1.12–1.20) | <0.001 |
Adjusted associations (HR, robust 95% CI) for the total study population (n = 97 575).
CI, confidence interval; HR, hazard ratio.
Main analysis of ≥50% of the target β-blocker dose vs. <50% of the target dose on outcomes and follow-up time
| Outcome . | 1 year . | 5 years . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Re-infarction or all-cause death | 1.03 (0.99–1.08) | 0.18 | 1.03 (0.99–1.07) | 0.09 |
| Re-infarction, all-cause death, stroke, heart failure hospitalization, or new-onset atrial fibrillation | 1.08 (1.04–1.12) | <0.001 | 1.07 (1.04–1.11) | <0.001 |
| All-cause death | 1.05 (0.99–1.12) | 0.09 | 1.05 (1.02–1.09) | 0.01 |
| Re-infarction | 1.03 (0.99–1.09) | 0.18 | 1.04 (1.00–1.09) | 0.06 |
| Stroke | 1.08 (0.98–1.18) | 0.09 | 1.05 (0.99–1.11) | 0.08 |
| New-onset atrial fibrillation | 1.30 (1.17–1.44) | <0.001 | 1.29 (1.18–1.41) | <0.001 |
| Heart failure hospitalization | 1.17 (1.13–1.22) | <0.001 | 1.16 (1.12–1.20) | <0.001 |
| Outcome . | 1 year . | 5 years . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Re-infarction or all-cause death | 1.03 (0.99–1.08) | 0.18 | 1.03 (0.99–1.07) | 0.09 |
| Re-infarction, all-cause death, stroke, heart failure hospitalization, or new-onset atrial fibrillation | 1.08 (1.04–1.12) | <0.001 | 1.07 (1.04–1.11) | <0.001 |
| All-cause death | 1.05 (0.99–1.12) | 0.09 | 1.05 (1.02–1.09) | 0.01 |
| Re-infarction | 1.03 (0.99–1.09) | 0.18 | 1.04 (1.00–1.09) | 0.06 |
| Stroke | 1.08 (0.98–1.18) | 0.09 | 1.05 (0.99–1.11) | 0.08 |
| New-onset atrial fibrillation | 1.30 (1.17–1.44) | <0.001 | 1.29 (1.18–1.41) | <0.001 |
| Heart failure hospitalization | 1.17 (1.13–1.22) | <0.001 | 1.16 (1.12–1.20) | <0.001 |
Adjusted associations (HR, robust 95% CI) for the total study population (n = 97 575).
CI, confidence interval; HR, hazard ratio.
Sensitivity analyses
Multiple sensitivity analyses with adjusted hazard estimates for 1 and 5 years of follow-up were performed. Modelling the alternative exposure of the second pharmaceutical dispensation revealed no signal of exposure misclassification (Supplementary material online, Table SA2). The landmark analysis showed no signal of immortal time bias (Supplementary material online, Appendix). When analysing predefined population strata for the composite endpoints (Figure 2, Supplementary material online, Figure SA2) including the alternative three-class β-blocker dose exposure (Supplementary material online, Tables SA1 and Supplementary material online, Tables SA1 and SA3), a similar pattern of estimated risks was observed. Finally, results remained stable in the complete case analysis (N = 50 141) (Supplementary material online, Table SA4). Thus, associations observed in all sensitivity analyses were in agreement with the primary findings.
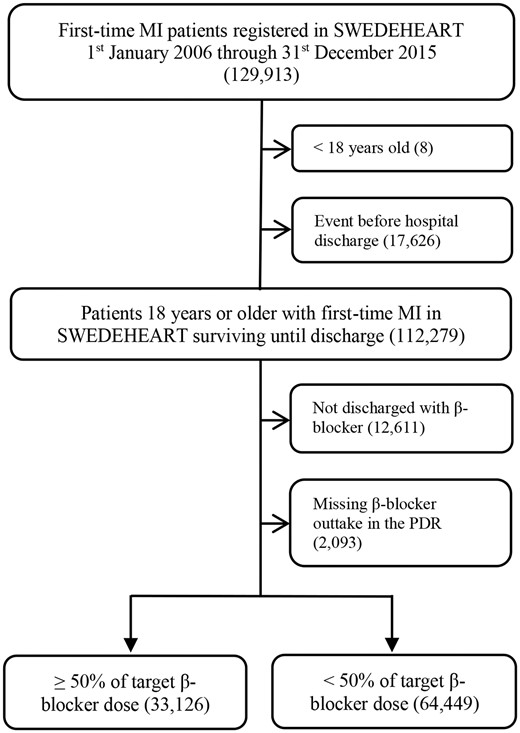
Flowchart. LVEF, left ventricular ejection fraction; MI, myocardial infarction; PDR, Prescribed Drug Registry; SWEDEHEART, Swedish Web System for Enhancement and Development of Evidence-based Care in Heart Disease Evaluated According to Recommended Therapies.
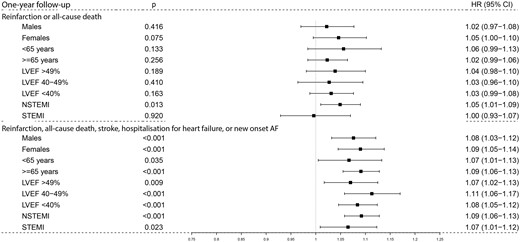
Hazard ratios of the primary and secondary composite endpoints in subgroups of patients during 1-year follow-up. Boxes and lines are the adjusted point estimates with 95% robust confidence intervals for the main composite outcome of re-infarction or all-cause death, and the secondary extended composite outcome of re-infarction, all-cause death, stroke, hospitalization for heart failure, or new-onset atrial fibrillation in patients with ≥50% of the target β-blocker dose vs. <50% of the target β-blocker dose during 1-year follow-up. HR, hazard ratio.
Discussion
This nationwide observational study is based on unselected consecutive patients with MI, who were prospectively recruited into the SWEDEHEART registry. We evaluated the association between β-blocker doses and cardiovascular outcomes up to 5 years after the index MI. Our key results include the following: First, two out of three patients were treated with <50% of the target β-blocker dose, i.e. substantially lower doses compared with previous randomized trials.1–6 Second, up to 1 year, there was no difference in outcome between the two groups, i.e. ≥50% of the target β-blocker dose and <50% of the target dose, when assessing the primary composite endpoint of all-cause death or re-infarction. When adding stroke, HF hospitalization or new-onset AF to the composite endpoint, there was a signal of greater risk if treated with ≥50% of the target β-blocker dose that remained unchanged up to 5 years of follow-up. Third, the results remained consistent in multiple sensitivity analyses and across relevant patient subgroups, importantly, even including patients who developed HF during the index hospitalization.
The observed lower dosing of β-blockers in our data compared with target doses in previous randomized trials is in line with published smaller studies.18,19 The underlying reasons are unclear. Current MI patients may not tolerate higher doses of β-blockers due to unwanted side effects of bradycardia or hypotension. Limited patient acceptance of subjective symptoms involving fatigue, mood changes, or sexual dysfunction may also play a larger role today. Moreover, drug non-adherence has been shown to increase with polypharmacy, in particular with β-blockers, which may contribute to the physicians’ reluctance to increase doses.28,29 Organizational challenges are also important to consider. With the hospital stay approaching 2–3 days for many patients, the time is simply not long enough to allow for dose titration as compared with previous eras.
Thus, it is possible that the lack of efficacy may partly be explained by sub-therapeutic doses of β-blocker, but in our data, we do not find any such signal. On the contrary, a slight but significant increase in the risk of cardiovascular events in higher β-blocker doses was observed, in accordance with two previously published observational studies from the USA (N = 7000)20 and Korea (N = 12 000).22 As current MI patients suffer a smaller myocardial injury with less commonly manifested HF during index hospitalization, it is possible that the proclaimed positive treatment effects of β-blockers are already achieved by the lower doses and dose titration does not improve outcome, or may even add risk. In fact, even the pivotal trials of the past did not systematically assess the optimal β-blocker doses by titration.1–6 Instead, fixed titration schemes up to high doses were used which makes it possible that the optimal dose of β-blockers for secondary prevention is lower than the selected doses in those trials. Notably, patients treated with ≥50% of the target β-blocker dose had more comorbid conditions at baseline, a longer hospital stay and were discharged with more concomitant medication indicating a more severe course of disease. The observed difference between the compared groups was driven by the risk over time of re-hospitalization for AF, HF, or all-cause death. Thus, it cannot be ruled out—despite our extensive efforts to control for confounding—that the higher event rates in patients on higher β-blocker doses indicate a frailer group of patients rather than a true dose-dependent detrimental effect.
Other patient factors may impact on dose selection of β-blocker therapy, but previous studies were too small and lacked important possible covariates. Thanks to the wealth and completeness of our data from multiple national registries, we were able to include relevant contributing information such as comorbidities, concomitant pharmacotherapies, and socioeconomic factors in combination with comprehensive in-hospital characteristics. However, the overall results remained consistent.
Of particular interest are the subgroups stratified by LVEF. β-blocker therapy has been shown to have a survival benefit in patients with HF and are recommended as first-line therapeutic agents with similar target doses as described above.13 In our data, the degree of HF did not alter the association between β-blocker dose and outcome. Interestingly, a meta-analysis of β-blocker therapy with HF patients stratified for reduced, mid-range, and preserved ejection fraction demonstrated no survival benefit when LVEF was >40% in sinus rhythm when compared with no β-blocker therapy.14
We did not analyse outcomes in MI patients discharged without β-blockers. By tradition originating in the Scandinavian β-blocker trials from the past1,2 and strong local recommendations, >90% of all patients with MI were discharged with β-blockers during the observation period, as compared with ∼80% in average in Europe.7,9 It is reasonable to believe that it was not at random but driven by clear reasons to omit β-blockers in those 12 611 individuals discharged without β-blockers. Thus, a direct comparison to β-blocker-treated patients would have implied an inherent risk for confounding. However, four large RCTs are currently ongoing: the Swedish Randomized Evaluation of Decreased Usage of betablockers after MI in the SWEDEHEART registry (REDUCE-SWEDEHEART, NCT03278509), the Danish betablocker (DANBLOCK, NCT03778554) trial, the Norwegian BEtablocker Treatment after MI (BETAMI, NCT03646357),30 and a Spanish-Italian trial tREatment With Beta-blockers After myOcardial Infarction withOut Reduced Ejection fracTion (REBOOT, NCT 03596385). Although the rationale of these trials is to compare the effectiveness of β-blockers vs. no β-blockers, more prospective data from randomized trials will become available to further evaluate a potential dose-dependent relationship of β-blockers therapy on cardiovascular outcomes.
The strengths of our study are that it was performed in a large contemporary cohort of patients with MI prospectively recruited into the national SWEDEHEART registry thus minimizing the risk of selection bias. Linkage to multiple other registries provided us with a unique wealth of relevant cofactors which may influence the dose–response relationship of β-blockers. However, some limitations merit consideration. First, the possibility of residual confounding and selection bias based on unaccounted factors or treatment decision by the caring physician cannot be excluded. Furthermore, our analysis was based on ICD-10 codes for morbidity data and no adjudication of events was performed. Therefore, coding errors can cause misclassification bias although we overall estimate the risk to be low.25,26 Second, the primary survival analysis was indexed to the β-blocker dose dispensed after discharge and we may have missed subsequent dose adjustments. Although dose adjustments occur over time, the majority of patients remained on the discharge dose during the 1st year in line with data from other cohorts.8,31 Third, we based our analysis on dispensed doses of β-blockers as a proxy for treatment adherence. For obvious reasons, it was not possible to check actual treatment compliance by other means such as surveys, pill-counts or even drug concentrations as proposed in an ongoing RCT.30
Conclusion
In contrast to β-blockers doses used in previous randomized trials, ≥50% of the target β-blocker dose was not associated with improved cardiovascular outcomes up to 5 years as compared with <50% of the target dose in patients with MI. The association was consistent across relevant subgroups including patients with HF. Future randomized trials need to redefine the optimal dose of β-blocker therapy in long-term secondary prevention post-MI.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Conflict of interest: none declared.
Acknowledgements
C.H., T.J., and R.H. report grants from the Swedish Heart-Lung Foundation. T.J. and R.H. were supported by the Stockholm County Council.
References
Norwegian Multicenter Study Group.
Author notes
Katarina Mars and John Wallert authors contributed equally to this study.




Comments