-
Views
-
Cite
Cite
Yuki Ikeda, Shunsuke Ishii, Shohei Nakahara, Saeko Iikura, Teppei Fujita, Yuichiro Iida, Takeru Nabeta, Nobuhiro Sato, Junya Ako, J-PVAD Investigators, Device-related adverse events and flow capacity of percutaneous ventricular assist devices, European Heart Journal. Acute Cardiovascular Care, Volume 14, Issue 2, February 2025, Pages 93–103, https://doi.org/10.1093/ehjacc/zuae132
Close - Share Icon Share
Abstract
Complication management is crucial in patients receiving mechanical circulatory devices. However, there are limited data on the association between the risks of complications and device type in patients with percutaneous ventricular assist devices (PVAD).
The Japanese registry for PVAD (J-PVAD) is a nationwide ongoing registry that enrols consecutive patients with cardiogenic shock treated with PVAD. We analysed 5717 patients in the J-PVAD from 1 February 2020 to 31 December 2022, to compare the incident risks of device-related problems and all-cause mortality within 30 days after PVAD introduction based on flow capacities of first-line PVAD (low: Impella 2.5/CP, n = 5375; high: Impella 5.0/5.5, n = 342). The overall incidence of major device-related problems, including haemolysis, major bleeding, kidney injury, sepsis, and pump stop, was 13%, 21%, 7%, 3%, and 1%, respectively. The all-cause mortality rate was 34%. The incident risks of haemolysis [hazard ratio (HR) 0.38, 95% confidence interval (CI) 0.24–0.58], kidney injury (HR 0.32, 95% CI 0.18–0.57), and pump stop (HR 0.38, 95% CI 0.16–0.91) were lower in patients with high-flow PVAD compared with those with low-flow PVAD. The risks of major bleeding or sepsis did not differ significantly between groups. The risk of all-cause mortality was lower in patients with high-flow PVAD compared with those with low-flow PVAD (HR 0.79, 95% CI 0.65–0.96).
Compared with those with low-flow PVAD, patients with high-flow PVAD had lower incident risks of device-related problems, including haemolysis, kidney injury, and pump stop, as well as lower risk of all-cause mortality.
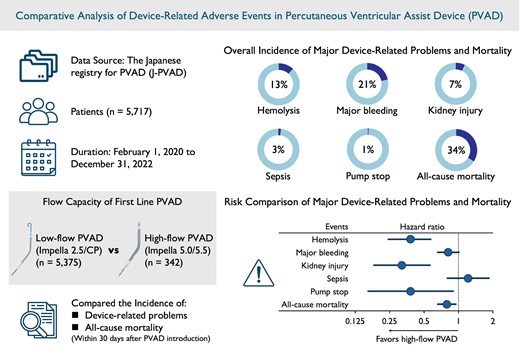
This study revealed that high-flow percutaneous ventricular assist device had lower incident risk of device-related adverse events including haemolysis, kidney injury, and pump stop, as well as lower risk of all-cause mortality, compared with low-flow percutaneous ventricular assist device.






Comments