-
PDF
- Split View
-
Views
-
Cite
Cite
Marcus E Pembrey, Does cross-generational epigenetic inheritance contribute to cultural continuity?, Environmental Epigenetics, Volume 4, Issue 2, April 2018, dvy004, https://doi.org/10.1093/eep/dvy004
Close - Share Icon Share
Abstract
Human studies of cross-generational epigenetic inheritance have to consider confounding by social patterning down the generations, often referred to as ‘cultural inheritance’. This raises the question to what extent is ‘cultural inheritance’ itself epigenetically mediated rather than just learnt. Human studies of non-genetic inheritance have demonstrated that, beyond foetal life, experiences occurring in mid-childhood before puberty are the most likely to be associated with cross-generational responses in the next generation(s). It is proposed that cultural continuity is played out along the axis, or ‘payoff’, between responsiveness and stability. During the formative years of childhood a stable family and/or home permits small children to explore and thereby learn. To counter disruptions to this family home ideal, cultural institutions such as local schools, religious centres and market places emerged to provide ongoing stability, holding the received wisdom of the past in an accessible state. This cultural support allows the growing child to freely indulge their responsiveness. Some of these prepubertal experiences induce epigenetic responses that also transfer molecular signals to the gametes through which they contribute to the conception of future offspring. In parallel co-evolution with growing cultural support for increasing responsiveness, ‘runaway’ responsiveness is countered by the positive selection of genetic variants that dampen responsiveness. Testing these ideas within longitudinal multigenerational cohorts will need information on ancestors/parents’ own communities and experiences (Exposome scans) linked to ongoing Phenome scans on grandchildren; coupled with epigenome analysis, metastable epialleles and DNA methylation age. Interactions with genetic variants affecting responsiveness should help inform the broad hypothesis.
Introduction
The title Isabelle Mansuy chose for the 2017 Latsis Symposium was ‘Transgenerational Epigenetic Inheritance: Impact for Biology and Society’. It was the inclusion of ‘… and Society’ that made me think again about what underpins the enduring nature of human cultures. This was a topic I discussed at length with my friend, the late David Hart, who would pose such questions as: among early Greek cultures, how did the state of Sparta maintain its distinctive militaristic nature for 400 years? We considered epigenetics among other processes and wrote an article in 2009, but never published it—principally because environmental epigenomics was still in its infancy.
The degree to which culture is part and parcel of our biological makeup has been debated since the times of ancient Greece, yet remains an open question. The word culture can have many meanings—a debate I will put aside. For the purposes of this paper, culture can be defined as ‘The ideas, customs, and social behaviour of a particular people or society [1]; or artefacts, social and symbolic behaviour, taste, intellectual development at a particular time or place. It is characterized by the transmission of ideas from one generation to the next. Not just the transmission of information but ‘ways of thinking’ that lead to social and intellectual coherence—‘solidarity between generations’. But why and how do cultures endure—often for many generations? The answer seems to lie in the formative years of childhood. Most people would say that the core features of a particular culture are acquired from birth by imitation and associative learning via the immediate family, and then also through behavioural adaptations to the ways of the local community, and through the wider society and the cultural institutions that underpin it.
There is a feeling that cultural transmission is more than imitation and learning, yet evolution based on inherited DNA sequence differences operates over too long a timescale to be the main explanation. Many authors express this sentiment without explicitly considering possible mechanisms. For example, in his authoritative 2017 book ‘How Language Began’ [2], Daniel Everett refers (p171) to the brain’s preparedness for language whilst rejecting any (genetic) prespecified mental capacity (p205). Everett does accept that the recent primate evolution of the FOXP2 gene sequence [3] ‘allows for better control of the vocal apparatus and mental processing of the kind used in modern human’s language’ (p. 194) but also adds later ‘Maybe humans passed a lot of grammar down by example from millennium to millennium as the species continued to evolve (p. 202). Towards the end he writes ‘Because language is a cultural artefact, we must understand what culture is in order to understand language … … The larger issue is how cultures hang together at all (p. 273). It is this larger issue of hanging together that the current paper is attempting to address. I use the term ‘continuity’ to imply an ongoing process of information transfer between generations that tends to counter marked behavioural deviations in the face of rapidly changing circumstances. Continuity has been defined as ‘a connection or line of development with no sharp breaks’ [4] or ‘the fact that something continues to happen or exist, with no great changes or interruptions’ [5]. My use of the term does not imply that no changes whatsoever can occur, or that an ongoing tendency across generations is either beneficial or adverse.
In terms of adversity, there is a recent example that again reveals a feeling that cultural transmission is more biologically embedded than just learning. It comes from the paper titled ‘In the shadow of coal: How large-scale industries contributed to present-day regional differences in personality and well-being’ [6]. ‘There are compelling theoretical and empirical reasons for supposing that the massive concentration of large-scale coal-based industries could have left a lasting psychological imprint in the local cultures [my emphasis] of the old industrial regions’. And later, ‘We argue that psychological adversity “runs deep” [my emphasis] in these regions, expressed not only in the actual well-being of these people but also in their personality traits that are linked to well-being’. The phrases ‘lasting psychological imprint’ and ‘runs deep’ capture the point I am making about an ill-defined sense that cultural transmission is more than just learning. The above quotes come from recent academic treatises on aspects of culture. The media has, for a decade or more, used the phrase ‘in the DNA’ (as a metaphor of stable, heritable difference) to qualify some cultural activity that ‘runs deep’. ‘Carols are part of our cultural DNA’ said the presenter of the BBC’s Today radio programme at Christmas time 2008; also ‘Siding with protesters is in the Lib Dem DNA’ [7]; and ‘Opera is in the Italian DNA. It’s not ornamental. It’s vital for the success of the state of Italy’ [8]. Thus, the phrase ‘in the DNA’ has become a signifier for, not just being biologically embedded, but also inherited. Could this deeply entrenched idea of ‘genetic memory’ (as some lay people call it) be cross-generational epigenetic inheritance? This is a difficult question to answer, but it is time to make a start.
What If Cross-Generational Epigenetic Inheritance Did Contribute to Human Development?
For many, learning from the prevailing social and various physical environments, plus our inherited genetic background, seems sufficient for cultures to endure for generations. The concept of the ‘meme’ introduced by Richard Dawkins in 1976 [9] as a unit of imitation does not challenge this view that culture is learnt. It just suggests that replication of behaviours by imitation has some parallels with genetic replication and can evolve by natural selection. So cultural transmission is the passing of information by non-genetic means whether or not you subscribe to the somewhat questionable meme-based view that there are discreet ‘units’ of culture. And for most people ‘by non-genetic means’ in this context equates with learning. Daniel Everett rightly points out, at least where language is concerned, that cultural continuity is not ‘set in stone’ (but see later discussion about cultural institutions) or hardwired into the DNA sequence. It is dynamic, like life in general. ‘Culture is dynamic, shifting, reinterpreted moment by moment. The roles, knowledge and values of culture are only found in the bodies (the brain is part of the body) and behaviors of its members’ [2, 10] (see p. 67). Despite this plasticity we are back to the current view that there is learning and there is genetic inheritance through the DNA code. In my experience, such neat dichotomies, are usually wrong. Just because there appears to be a sufficient explanation does not mean that is the whole explanation. Many different threads from the distant evolutionary past have contributed to cultural homo sapiens.
One such thread is epigenetic regulation of gene expression during development and in response to the environment. This additional layer of (epi)genomic information can last a lifetime, and there is increasing evidence that epigenetic responses to (early-life) experience may also influence the development of the next generation(s) [11]. Mammalian research and human studies into the role of epigenetic processes in development, plus the enduring impact of early life exposures throughout adulthood, have been underway for over two decades, much under the umbrella of the Developmental Origins of Health and Disease (DOHaD) paradigm [12, 13]. The impact of early-life adversity on later neurobehavioural outcomes has been one focus [14], and more recently these studies have incorporated the interactions with the (gut) microbiome [15]. There is still a lot to learn about the role of epigenetics in DOHaD [16], but it is already clear that epigenetic processes contribute to many experiences being biologically embedded or ‘getting under the skin’. Human observations are in line with animal studies. For the purposes of this paper (and in this journal), I will take a role for epigenetics in the biological embedding of developmental experience within a single individual as a given, and just consider the impact on the subsequent generation(s).
If some form of cross-generational epigenetic inheritance is common in humans, it would contribute a biological transmission to descendants that is very different from genetic inheritance. Genetic inheritance comes just from the parents and their respective genetic lineages; and chromosomal behaviour during meiosis leads to genetic diversity in the offspring. On the other hand, in theory at least, non-genetic intergenerational signals (whatever their molecular basis), that were induced by epigenetic responses to (early-life) experiences in the previous generation, will tend to produce comparable developmental adjustments within the offspring. By definition this information that is passed to the offspring is ‘in at the beginning’—inherited at conception and potentially able to modify development thereafter. This starting point has important implications for epigenome analysis later in the life of the offspring e.g. analysis of metastable epialleles [17] that are considered later. But even more importantly, the initial epigenetic response is not just garnered from the biological parents, but from interactions with any (and perhaps many) significant others in the community, e.g. a school teacher. To this must be added the shared experience of natural disasters (e.g. Canadian 1998 ice storm [18, 19]) or blessings that befall their society as a whole (Fig. 1). Herein lies the epigenetic potential for social cohesion across the generations; that is, an enduring culture.
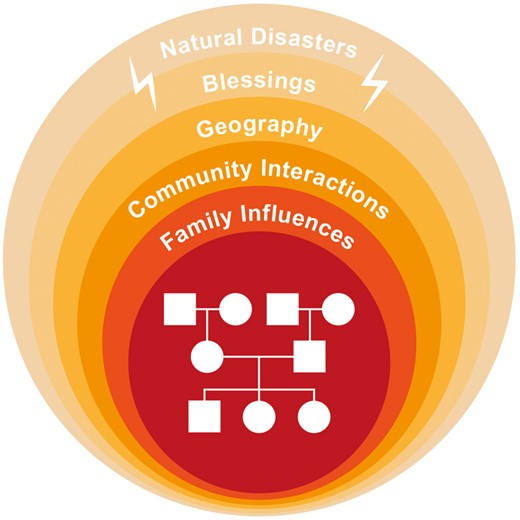
An indication of the multiple, ever-widening sources of experience that may induce epigenetic responses, and therefore potentially influence the next generation(s) through cross-generational epigenetic inheritance, contributing to cultural continuity. By contrast genetic inheritance just comes from the parents via their ancestors; here represented by the central pedigree.
Brief Update on Relevant Cross-Generational (Epigenetic) Responses in Humans and Mammals
There is growing evidence that some effects of exposures can be biologically transmitted to the next or subsequent generations in some way. These effects are called intergenerational if the exposure (or importantly the organism’s response to it, e.g. response to DNA damage) could have reached the germ cells leading to the next generation(s), or transgenerational if this is not the case. The latter implies some molecular ‘memory’ of the ancestral experience, or its response to it, is being passed down via the gametes. And here, a prime candidate is inter/trans-generational epigenetic inheritance [20]. Clearly the transmission of ideas, attitudes and ways of thinking that underpin a particular culture could be through several different routes, a number not involving the germline. Some of these non-genetic transmission pathways may still involve epigenetic responses in a fundamental way, so for the purposes of discussing culture, I will use ‘cross-generational responses’ as a catch-all term. One such cross-generational pathway is exemplified by the classic rat studies of the influence of maternal care style on offspring stress response and their own nurturing behaviour [21–23]. Thus, nurturing behaviour is perpetuated in what could be called a cross-generational epigenetic cascade.
Non-genetic intergenerational inheritance has recently focused on paternal pre-conceptional effects on offspring development—an emerging field labelled POHaD [24]. The few human studies are supported by relevant animal experiments. In summary, in addition to environmentally induced changes to the sperm epigenome, it is clear that sperm also carry induced non-coding RNAs (including tRNA-derived small RNAs) [25–29]. Non-coding RNAs can be transferred to maturing post-testis sperm from the somatic epididymal epithelial cells [30]. This by-passes meiosis and the testis-blood barrier—in other words breaches August Weismann’s historic ‘barrier’. Teleologically speaking, this appears to help address the apparent imbalance between the parents in non-genetic influences on their developing foetus. It now seems information can sail through from both of them.
In further consideration of inter/trans-generational (epigenetic) inheritance and cultural continuity, there are some general points to make first. It should be emphasized that cross-generational responses need not result in ‘inheritance of acquired characteristics’ although they often do. The outcome in the next generation can be in the opposite direction, as if it were an adaptation. We have observed this in our intergenerational studies of maternal smoking in pregnancy, when the maternal grandmother smoked in pregnancy, but the study mother did not. Against expectations (direct prenatal tobacco exposure leads to a smaller birth weight) the grandsons were bigger at birth than if neither grandmother nor mother had smoked [31]. These grandsons had greater cardiovascular fitness and lean mass when followed to adolescence [32]. There was no such outcome in granddaughters, illustrating a feature often seen in animal experiments [33] and human observations [11, 34]; namely, there are often sex-specific (but not sex-limited) transmission routes and offspring/descendant outcomes.
Human inter/trans-generational studies up to 2014 have been reviewed in detail [11]. This includes all the transgenerational associations of paternal grandparent’s food supply in childhood on the grandchild’s longevity plus cardiovascular and diabetic deaths in the Överkalix population in northern Sweden. Since that time a large Taiwan study of betel-quid chewing and smoking (after childhood) reported that longer duration of paternal betel-quid chewing and of smoking, pre-fatherhood, independently predicted early occurrence of incident metabolic syndrome in their offspring [35]. An intergenerational Swedish study [36] of the association between the death of a parent at different periods in childhood and the birth characteristics of their own offspring is discussed below.
Exposure-Sensitive Period for Inducing Human Cross-Generational Responses; Relevance to Cultural Continuity
Various stages of foetal development have long been regarded as exposure-sensitive periods in terms of developmental ‘programming’ within the DOHaD paradigm [37]. However for inducing human inter/trans-generational responses, the mid-childhood period leading up to the growth spurt going into puberty is also a critical exposure-sensitive period. First demonstrated in the Överkalix studies [34, 38, 39], it has observed in other studies [40]. This period was 8 or 9–12 years a century or more ago but taken as 6–10 years for contemporary studies. More recently, a transgenerational study of exposure in mid-childhood to the German 1916-18 famine looked at economic and related outcomes in later generations [41]. They assessed height, mental health and educational achievement in the descendants of boys exposed at 9–12 and girls at 8–10 years. Among the third generation, males tend to have better mental health scores if their paternal grandfather was exposed at 9–12 years. The Swedish study of the death of a parent found parental (F1) death occurring at age 8–12 years of F2 boys (and to a lesser extent at 13–17 years) was associated with premature birth and reduced birth weight in their own offspring (F3) [36].
This replication of the association of human cross-generational responses with initial exposures in mid childhood (in different populations at different periods in history) suggests the underlying processes evolved because biological transfer of such information to the next generation was a selective advantage. If so, it makes sense that social and environmental information (or a protective response to it) is captured before puberty, so potentially all future offspring can benefit. By this reasoning the upper age limit of the sensitive period being roughly the onset of puberty, makes sense, but what about the lower age limit of 6 years, or 8 years historically? It may simply be due to many aspects of immaturity, including the development of the testes and ovaries, or the fact that the period from birth to 6 years is primarily geared to survival per se. In this context it is interesting that phenotypic concordance between monozygotic twins is relatively low at this age, reaching its maximum in adolescence or later, for example with IQ [42]. Again this could be interpreted as surviving first, before then revealing your genetic potential ready for the mating game. What can be said, in terms of culture, is that mid childhood represents the latter part of ‘the formative years’. The Jesuit maxim ‘Give me the child until he is seven, and I will give you the man’ makes the point; and this is echoed by UNICEF who regard the formative years as birth until 8 years [43]. One can argue that for the first 5 years the cultural input is primarily from the family, but then, at least for settled communities the cultural institutions, such as market places, schools, religious centres come more into play, enhancing social cohesion. For example, school experience in mid-childhood has been shown to predict health in adulthood [44]. Importantly these increased societal influences coincide in timing with the exposure sensitive period for inducing cross-generational responses. The interplay between families and the local cultural institutions also needs to be considered. These elements include security—city walls, food supply/markets; sense of belonging—religions, gangs, sports; information—trading, tablets of stone, writing, schools, etc. These institutions hold the received wisdom of the past in an accessible state—available to all, or perhaps selected members or families, or at a price. The stability of a community over time depends on both the constituent families and the cultural institutions. Importantly, the cultural institutions may provide fall-back support and encouragement for children from dysfunctional families or other crises that occur in that critical mid-childhood ‘transfer window’ for cross-generational (epigenetic) inheritance.
The Pay off between Responsiveness and Stability
There is a fundamental pay off between stability and responsiveness. Reputedly, the Jaguar (∼1975–2004) was the last fighter jet to be really flown by the pilot. Modern fighter jets are now built so unstable in the air (needing constant computer adjustments of the flaps to stay airborne) in order to be responsive enough to take avoiding action against missiles [45]. I suspect this issue is also an important matter for cultural continuity. One definition of responsiveness is ‘The quality of reacting quickly and strongly [46]. Alternatively, the word responsive can be defined as ‘showing interest or emotion in reaction to someone or something’ [47]. This captures the sensitivity element—whether one reacts easily or, at the other extreme, remain unresponsive. If cultural institutions encourage stability within a community and across the generations, then this permits the individuals within that community to adopt more responsive behaviours. This may be crucial for children in their formative years in order to adopt new ideas and technologies on which the future of their communities may depend—and perhaps transmit epigenetic adjustments to their gametes. However, such a permissive culture may also carry the risk of what one could call ‘runaway’ responsiveness’; ignoring the past for a horizontal drift into an unstable melting pot of behaviours ravaged by wave after wave of fashion, rather like the ‘crazes’ that sweep today’s primary schools. Too large a swing of the pendulum and the latest fighter jet falls out of the sky! From a population genetics point of view it is likely the population would hedge its bets through the positive selection of DNA variants that reduce (extreme) responsiveness. A comparable evolutionary scenario has been modelled in a paper titled Evolutionary emergence of responsive and unresponsive personalities [48]. Their evolutionary model is based on two key ingredients; the benefits of responsiveness are frequency dependent, and positive-feedback mechanisms reduce the cost of responsiveness, permitting some selective balance. There are indeed DNA variants of dopamine and serotonin pathway genes that result (at the extreme combinations) in what I call ‘responders’ or ‘non-responders’ to early-life stress in terms of later adolescent self-regulation [49] or telomere length [50]. Past studies of depression and post-traumatic stress disorder (PTSD) after child abuse have identified a role for the glucocorticoid receptor as a pivotal nuclear receptor of the stress hormone system. The gene encoding the FK506-binding-protein-5(FKBP5) is an important functional regulator of the glucocorticoid receptor complex and a SNP (rs1360780) moderates the risk for PTSD after early trauma [51]. Furthermore, this SNP results in childhood trauma-dependent DNA demethylation in functional glucocorticoid response elements of FKBP5 [52]. Interestingly, although barely commented upon, the non-abused controls with what they called the ‘protective allele’ had more lifetime PTSD that the non-abused with the so called ‘risk’ allele. Perhaps ‘protective allele’ carriers are actually ‘non-responders’, less responsive to typical, healthy social support, as well as abuse when it happens? This general field of differential susceptibility of the developing brain to contextual adversity and stress was recently reviewed [53].
The Broad Hypothesis
I propose that cultural continuity and some adjustments over time are played out along the axis, or ‘payoff’, between responsiveness and stability. During the formative years of childhood a stable family and/or home permits small children to explore and thereby learn. To counter disruptions to this family home ideal, cultural institutions such as local schools, religious centres and market places emerged to provide ongoing stability, holding the received wisdom of the past in an accessible state. This cultural support allows the growing child to freely indulge their responsiveness. Some of these prepubertal experiences induce epigenetic responses that also transfer molecular signals to the gametes through which they contribute to the conception of future offspring. In parallel co-evolution with growing cultural support for increasing responsiveness, ‘runaway’ responsiveness is countered by the positive selection of genetic variants that dampen responsiveness. It is proposed that such genetic variants would reduce the biological embedding of the early life experience and thus the measurable intergenerational effects on offspring development.
Testing the Hypothesis
The first thing to say is that testing these ideas in humans is a huge challenge, and will need a number of comprehensive, multigenerational cohorts followed from before birth throughout development, into adulthood and on to the next generation. In the first instance, an assessment of just how plausible this cultural continuity hypothesis is, will be built up piecemeal. This type of patchwork approach has already paid off in testing the whole idea of human non-genetic inheritance in the first place. It is worth remembering that 10 years ago, studies of specific maternal peri-conceptional and intrauterine exposures on foetal development would regard lack of a paternal exposure effect as a useful ‘control’ for unknown social confounders [54, 55]. This is because finding a paternal association would cast doubt on the maternal findings. Now we could be turning this view on its head and pose the question—can established paternal, pre-conceptional POHaD effects contribute to ‘social patterning’ down the generations through intergenerational epigenetic inheritance? Less speculatively, the demonstration of mid childhood as an exposure-sensitive period for cross-generational associations does help to reduce concern about a number of unknown confounders, including genetic pleiotropy. For example [40], are DNA variants associated with the father’s mid childhood smoking habit, if transmitted, also associated with the risk of obesity in his sons at 17 years? If so, then why not with father’s onset of smoking at 14 years? That an association was not found with father’s onset at 14 years makes the genetic pleiotropy explanation less likely. The other aspect of animal and human cross-generational (epigenetic) inheritance is the often observed sex-specific outcomes in the next generation(s). Over time, the observed epidemiological correlates with such cross-generational associations have been building a more specific picture of the phenomenon. One is moving slowly from a hypothesis-free exploration to some more specific prior hypotheses. However, we should not be complacent. A study [56] of Social Genetic Effects in mice detected associations between a trait of one mouse and the genetic makeup of its cage mates. It turned out that such social genetic effects explained up to 29% of variation in anxiety, wound healing, body weight and immune function, and in several cases contributed more to phenotypic variation than the mouse’s own genes. These correlations appear to hold for both in-bred and out-bred strains. Recently Social Genetic Effects of friends and school classmates have been reported to contribute to educational achievement [57]. Such correlations presumably depend on phenotypic expression—ranging from direct effects like body odour to complex behavioural variations. Social Genetic Effects, of course, can play both ways in terms of social cohesion and cultural continuity, but much is likely to be mediated by epigenetics. Yes, it is going to be complicated!
One way of reducing complications in the interpretation of human observational studies has been to include the father and his ancestors, in addition to the maternal side, in multigenerational prospective cohorts; as has been the case in the Avon Longitudinal Study of Parents and Children (ALSPAC) UK [58], for example. Paternal cross-generational associations are less confounded than maternal associations with respect to several potential intergenerational transmission routes including mitochondrial inheritance, cross-placental transfer of biochemical signals, and via the microbiome and breast milk. But perhaps most importantly for epigenomic studies, sperm are potentially available. As alluded to earlier, sperm-mediated transmission is by definition ‘in at the beginning’—contributing to the conceptus. This coupled with the improved identification of metastable epialleles (epigenetic variants set ‘stochastically’ in the early embryo and maintained during subsequent cellular differentiation [59]) will facilitate human epigenetic analysis in the next generation(s), because metastable epialleles are not tissue specific. Recent data [17] from the study of monozygotic twins indicate that they share more metastable epialleles than expected from being genetically identical. This ‘supersimilarity’ implies the generation of such epialleles in the earliest stages of the embryo—or perhaps the arrival of such epialleles (or something that induces them) in the egg or sperm. More broadly, the chromatin state of the early embryo could potentially correlate with general genomic biomarkers such as epigenetic (DNA methylation) age [60] and chromosomal telomere length [61, 62], in the new born and later in life. They are increasingly recognized as a measure of biological ageing. For human studies, variation in these two measures might provide useful outcome measures (that are clearly biologically embedded) in cross-generational analysis. Additionally, the role of genetic variants that influence responsiveness to stress, for example, could be tested on the above (epi)genomic markers in the next generation(s). However, the impact of genetic unresponsiveness on society will be complex and context-dependent. One could speculate that in the trenches of the First World War being a ‘non-responder’ helped maintain courage and therefore social cohesion and survival. This contributed to restoration of a core cultural way of life post-war; but then ‘responders’ contributed in turn to the adoption of important innovations. Whatever the approaches adopted for human studies going forward, there is always the need for replication of cross-generational associations with early-life experience in different populations. This can prove a particular challenge when considering cultural continuity because this needs ongoing quantitative measures of well-being and neurobehavioral traits right across the population and not just a (nested) case-control design focused on disease and adverse outcomes. Ideally multigenerational cohorts should start with an ‘open’, specific hypothesis-free approach through Phenome Scans [63] and Exposome scans on the previous generation(s) [64].
Before concluding this final section, it is worth standing back to look at the unknowns. Famously, Donald Rumsfeld stated [65] ‘There are known knowns. These are things we know that we know. There are known unknowns. That is to say, there are things that we know we don't know. But there are also unknown unknowns. There are things we don't know we don't know’. Mindful of this, in any pie chart of factors contributing to developmental variation in humans, I usually add a ‘yet to be discovered’ slice, the size of which can be debated. What are the known unknowns in the current field of non-genetic cross-generational inheritance with respect to culture? There are three that I have followed with interest. Human intelligence, Pavlovian conditioning and genomic imprinting. There is consistent evidence in a number of industrialized countries that the IQ of the population has risen over many decades, known as the Flynn effect [66–68]. What combination of factors are responsible for this rise remains an open question. Interestingly from the cultural continuity perspective, Mark Shlyankevich hypothesized in 2011 that the relatively high intelligence of the Ashkenazi Jews is not so much a result of genetic selection, but a consequence of studying religious texts for at least 100 generations [69]. He speculates that intense early study had a cumulative inter-generational epigenetic effect resulting in a higher average intelligence. This idea is in line with the Flynn effect and implies some epigenetic neurological ‘priming’ of the next generation. In the context of ‘priming’, it has been reported [70] that fear conditioning with a novel odour in mice produces similar behaviour (and neuroanatomy) in the unconditioned offspring and is associated with sperm DNA methylation changes in the relevant specific olfactory receptor gene. If replicated, this would have real implications for cultural continuity; experiences in one generation ‘priming’ as it were, the next. It happens that there is a notorious history of this idea of cross-generational effects of Pavlovian conditioning. Ivan Pavlov himself felt that repeated conditioning in each successive generation might eventually lead to unconditioned descendent mice (not dogs in this particular study) who react to a bell with food seeking behaviour as a ‘hard-wired’ instinct, just as new born chicks instinctively peck at seeds on the ground. Initial studies from his laboratory, performed by Nikolai Studentsov, showed a progressive reduction across the generations in the number of conditioning episodes needed to induce food seeking. This was reported in 1923 (under the spelling I. P. Pawlow) in Science [71]. Challenged by others Pavlov’s laboratory eventually repeated the study, this time with controls, and the previously reported transgenerational ‘priming’ was not replicated, in the sense there was no difference from controls. An account of all this is discussed in Loren Graham’s 2016 book Lysenko’s Ghost: Epigenetics and Russia [72]. In terms of testing the cultural continuity hypothesis outlined in this paper, it is crucial that this conditioning work is pursued. Proving a negative is difficult, so we need several laboratories to invest in trying to answer this ‘for once and all’, combining modern neuro-behavioural research techniques, imaging, gene expression and of course epigenetics. Hopefully, in years to come this particular idea will move into the known known category; it is shown to be extremely unlikely or very likely to be true.
Genomic imprinting also qualifies as a known unknown principally on the grounds that it is an established epigenetic gene-silencing process that involves two generations. Genomic imprinting (gene silencing dependent on the parent of origin) establishes the principle that epigenetic marks such as DNA methylation placed (in the developing gametes) in one generation can influence gene expression in the next [73]. This is possible because imprinted genes are protected from the genome wide de-methylation that occurs after conception in the pre-implantation period. It was my research on human imprinting disorders in the early 1990’s that made me speculate that imprinting plays a key role in human transgenerational responses [74]. It has been a long wait! However, genomic imprinting remains a known unknown because the phenomenon has not been ruled out as a player; and there are hints that genomic imprinting networks may be involved in a new field that doesn’t really have a name yet, i.e. just emerging from the unknown unknown category. A study published a couple of years ago [75] demonstrated that mice with TRIM28 insufficiency resulted in polyphenism, wherein lean and obese phenotypes can arise from the identical genotypes through dysregulation of an imprinted gene network. They showed that human BMI distributions and transcriptomes also suggest TRIM28-associated subpopulations. They conclude that perturbation of imprinted genes Peg3 and Nnat trigger stochastic bi-stable obesity. It is interesting from the POHaD perspective that the long range physical interaction of several imprinted genes, is particularly marked in spermatogonia; ‘suggesting that the ‘imprintome’ is primarily operating in stem cells of the male germline’ [76]. Indeed, paternal obesity is associated with altered DNA methylation patterns at MEST, PEG3 and NNAT imprinted genes in his offspring [77]. Polyphenism implies that genetically identical embryos carry two or more potential developmental trajectories. The switch to one trajectory is currently regarded as ‘stochastic’—that means a known unknown in my book. Watch this space!
Conclusion
It is time to seriously examine the potential role of cross-generational epigenetic inheritance in our cultural continuity. It is recognized that this is a multifaceted question, and as such will involve diverse academic disciplines. It calls for what one could call the ‘patchwork’ approach, in the hope of one day realizing the whole; just like those tackling other big questions in science. The cultural patchwork is stitched together by history and experience. This is not my phrase—I heard diplomat Alexander Downer [78] on BBC radio discuss how one got to understand a country to which one had been posted. ‘They feel as they feel because of their own history and experience. People are defined by these experiences’. The question ‘where did I come from?’ and its reciprocal ‘deep sense of belonging’ might relate to being tied to one’s ancestors, not so much through genetic inheritance, but through their experiences. It may not all be down to storytelling, but underpinned by non-genetic gametic inheritance.
Acknowledgements
First and foremost I would like to thank the late David Hart (1944–2011) for wonderful, stimulating conversations about culture and his encouragement to write our ideas down. I am also grateful for ongoing discussions with Jean Golding and Marilyn Monk over many decades and for discussions within the Network in Epigenetic Epidemiology (2009–14) led by Lars Olov Bygren. Thanks also to the reviewers for their constructive suggestions, David Smithson for the artwork and Nick Dennis for telling me about the Jaguar jet fighter many years ago! This work was funded by The Templeton Foundation (grant no. 60828).
Conflict of interest statement. None declared.
References
https://www.brainyquote.com/quotes/donald_rumsfeld_148142 (



