-
PDF
- Split View
-
Views
-
Cite
Cite
Weliton D Silva, Lawrence M Hanks, Judith A Mongold-Diers, Anna C Grommes, José Maurício S Bento, Jocelyn G Millar, 2-Nonanone is a Critical Pheromone Component for Cerambycid Beetle Species Native to North and South America, Environmental Entomology, Volume 50, Issue 3, June 2021, Pages 599–604, https://doi.org/10.1093/ee/nvab022
Close - Share Icon Share
Abstract
An increasing body of evidence indicates that cerambycid beetles native to different continents may share pheromone components, suggesting that these compounds arose as pheromone components early in the evolution of the family. Here, we describe the identification and field testing of the pheromone blends of two species in the subfamily Cerambycinae that share 2-nonanone as an important component of their male-produced aggregation-sex pheromones, the South American Stizocera consobrina Gounelle (tribe Elaphidiini) and the North American Heterachthes quadrimaculatus Haldeman (tribe Neoibidionini). Along with 2-nonanone, males of S. consobrina also produce 1-(1H-pyrrol-2-yl)-1,2-propanedione, whereas males of H. quadrimaculatus produce 10-methyldodecanol. Field bioassays conducted in Brazil (targeting S. consobrina) and Illinois (targeting H. quadrimaculatus) demonstrated that adults of both species were attracted only by the blends of both their pheromone components, and not to the individual components. The use of the pyrrole as a critical component for the former species is further evidence that this compound is a common pheromone structure among cerambycines in different biogeographical regions of the world.
Research over the last 15 yr has revealed that reproduction in cerambycid beetles of the large subfamily Cerambycinae is mediated by volatile aggregation-sex pheromones produced by males, which attract both sexes (reviewed in Millar and Hanks 2017). Some pheromone structures appear to be broadly shared among closely related species (e.g., congeners), and even among species in different tribes (Hanks and Millar 2016). There also is increasing evidence that pheromone structures are shared by species native to different continents. For example, 3-hydroxyalkan-2-ones and the related 2,3-alkanediols are pheromone components of cerambycine species native to North and South America, Eurasia, and Africa (Hanks and Millar 2016), suggesting these structures evolved early in the evolution of the subfamily. Conversely, pheromones of some cerambycine species appear to be less common and shared only among congeners, whereas others may be species-specific (Ray et al. 2011; Zou et al. 2015; Silva et al. 2016a,b; Millar et al. 2017).
Here, we report the identification of pheromone blends for two cerambycine species, Stizocera consobrina Gounelle (tribe Elaphidiini) native to South America (Monné 2020) and Heterachthes quadrimaculatus Haldeman (tribe Neoibidionini) from eastern North America (Monné and Nearns 2020). To our knowledge, there is no published information about the host species of S. consobrina. Hickories (Carya species) are the most common hosts of H. quadrimaculatus, and there is some evidence that larvae develop in dead hosts (Linsley 1963a, MacRae and Rice 2007). Both species have 2-nonanone as a pheromone component, but each has a second, entirely different pheromone component that is essential for strong attraction: 1-(1H-pyrrol-2-yl)-1,2-propanedione (henceforth semanopyrrole) for S. consobrina, and 10-methyldodecanol for H. quadrimaculatus. Attraction of adults of S. consobrina to semanopyrrole alone in an earlier study (Silva et al. 2017) was the first evidence of its being an important pheromone component for any South American cerambycid species. The data reported here show that the attraction of this species to semanopyrrole is synergized by 2-nonanone.
Materials and Methods
Sources of Chemicals
2-Nonanone was purchased from Aldrich Chemical Co. (Milwaukee, WI). Semanopyrrole was synthesized as described in Zou et al. (2016), and 10-methyldodecanol as described in Silva et al. (2016a).
Study Sites
Stizocera consobrina was targeted with a field experiment conducted in a forest remnant of Cerrado (Brazilian savanna) located at the Forest Science Experimental Station of the University of São Paulo (Anhembi SP, Brazil; −22.7060, −48.1669; https://www.esalq.usp.br/svee/Anhembi/). The experimental site comprised a ~5 ha area covered with original forest remnants and mature, reforested tree species native to Cerrado biome, including representatives from several botanical families, primarily Fabaceae. Immediately surrounding the area, there were pasture lands with scattered trees of the species Senegalia polyphylla (D.C.) Britton & Rose and Eucalyptus grandis Hill ex Maiden.
The field experiment targeting H. quadrimaculatus was conducted at Forest Glen Preserve in Vermilion Co., Illinois (40.0152, −87.5677; Vermilion County Conservation District). The preserve is ~730 ha in area and consists primarily of beech-maple and oak-hickory forest types that have been unmanaged since 1968 (http://www.vccd.org/).
General Methods of Trapping
Attraction of beetles to synthesized candidate pheromones was tested with black cross-vane panel traps (corrugated plastic; AlphaScents, Portland, OR in Illinois, and traps of similar design that were custom-built of similar materials in Brazil; Silva et al. 2020). Efficiency of both types of traps was improved by coating interior surfaces with the fluoropolymer dispersion Fluon PTFE (AGC Chemicals Americas, Inc., Exton, PA; Graham et al. 2010). Traps were suspended from frames of polyvinylchloride irrigation pipe and positioned ~0.5 m above the ground. The frames were mounted on 1-m steel reinforcing bars driven partway into the ground. Basins of traps used in field bioassays contained saturated aqueous NaCl solution with a few drops of dish detergent to preserve captured beetles. Sentinel traps were modified to capture live beetles for collection of headspace volatiles by drilling holes (~ 2 mm) in collection jars (Brazil) or by replacing trap basins with plastic jars with aluminum window screen bottoms (Illinois), allowing rainwater to drain. Pheromone lures were prepared from polyethylene pouches (press-seal bags, Bagette model 14770, 5.1 × 7.6 cm, 0.05 mm thick, Cousin Corp., Largo, FL) containing a cotton wick and charged with solutions of test compounds in 1 ml isopropyl alcohol. The control lures were the same pouches with cotton wicks loaded with 1 ml of neat isopropyl alcohol.
Beetles captured in Brazil were identified to species by taxonomists at the Zoology Museum of the University of São Paulo, and beetles captured in Illinois were identified with the key in Lingafelter (2007). Voucher specimens of beetle species captured in Brazil have been deposited in the museum of the Department of Entomology and Acarology-USP/ESALQ, Piracicaba, SP, Brazil. Representative specimens of beetle species captured in Illinois are available from the laboratory collection of LMH and have been submitted to the insect collection at the Illinois Natural History Survey.
Collection and Analysis of Beetle-Produced Compounds
Adults of S. consobrina that were used for the collection of headspace volatiles were caught with sentinel traps, outfitted with dry collection jars, deployed in Anhembi throughout October 2018. Traps were baited with semanopyrrole because this species was attracted in significant numbers to this compound during field bioassays that targeted other cerambycine species (Silva et al. 2017). Traps were serviced daily, and beetles were sent on the date of capture to the Laboratory of Chemical Ecology and Insect Behavior, University of São Paulo, Piracicaba (~85 km from Anhembi). Sex of beetles was determined based on the characteristic punctation on the propleura of males (Martins 2005). Beetles were held under laboratory conditions (25 ± 2°C, 60 ± 10% RH, 12:12 (L:D) h, and 5000 lux light intensity) for 24 h prior to collecting volatiles. Headspace volatiles were collected from males and females held individually in 500 ml cylindrical glass chambers with the internal surfaces of half the chamber lined with paper toweling to provide a surface for perching and hiding. Beetles were supplied with 10% sucrose solution in glass vials for nourishment. Headspace volatiles were collected with glass pipettes (8.5 cm long × 0.5 cm i.d.) containing the adsorbent HayeSep Q (150 mg of 80/100 mesh; Supelco, Bellefonte, PA) held in place with glass wool plugs. Collectors were connected to the outlets of chambers with screw caps fitted with PTFE ferrules. Air purified with activated charcoal was pushed with an oil-free compressor pump through the chamber at constant flow of ~150 ml/min regulated by flowmeters. Individual beetles (four males and five females) were continuously aerated for periods of 48 h as many as three times. System contaminants were monitored by aerating chambers that contained only paper towel liners and feeder vials. Volatiles were stripped from collectors by washing with three successive aliquots of 500 μl of dichloromethane into 2-ml silanized amber glass vials, which were stored at −30°C. Aeration extracts were not spiked with an internal standard because there was no plan to quantify emission of pheromone.
Extracts of volatiles from adults of S. consobrina initially were analyzed in Brazil by gas chromatography with flame ionization detection (GC-FID) to identify those which contained compounds absent in controls, specifically any sex-specific compounds. Two-microliter aliquots were injected into a GC-2010 gas chromatograph (Shimadzu Corp., Kyoto, Japan) fitted with a capillary column (Rtx-1; 30 m × 0.25 mm i.d. × 0.25 μm film; Restek, Bellefonte, PA). Injections were made splitless (purge valve off for 1 min), injector temperature 250°C, helium carrier gas (linear velocity of 30 cm/s). The GC oven program was 35°C (hold 1 min), increased to 40°C at 2°C/min (hold 1 min), increased to 250°C at 10°C/min (hold 15 min). Extracts containing sex-specific compounds were sent to the University of California, Riverside (UCR), where they were reanalyzed on an Agilent 7820A GC interfaced to a 5977E mass selective detector, with helium carrier gas. The GC was fitted with an HP-5 column (30 m × 0.25 mm i.d. × 0.25 μm film; Agilent Technologies, Santa Clara, CA), with splitless injections of 1 µl aliquots of samples. The GC oven program was, initial temperature 40°C (hold 5 min), 10°C/min to 280°C (hold 10 min). Quadrupole, ion source, and injector temperatures were 150, 230, and 250°C, respectively. Mass spectra were obtained with electron impact ionization (EI, 70 eV; mass range from 40 to 400 Da).
Adults of H. quadrimaculatus for collection of beetle-produced odors originally were captured alive as bycatch in traps baited with pheromones of other cerambycid species during field bioassays conducted during 2008–2017 (e.g., Hanks et al. 2019). Beetles were sexed by antennal length relative to body length (longer in males; Linsley 1963a). Males and females were caged separately in the laboratory (~14:10 (L:D) h, ~20°C) and provided 10% aqueous sucrose solution as nourishment. Beetle-produced volatiles were collected from six males and three females by holding beetles in glass Mason-style canning jars near closed exterior windows (natural photoperiod, ~14:10 (L:D) h, ~20°C). Charcoal-purified air was pushed through the jars at a rate of ~1 liter/min for 24–48 h under house air pressure, with individual flow regulators for each jar. Volatiles were collected on a glass tube cartridge containing HayeSep Q (150 mg) between plugs of glass wool. Aeration equipment was connected with Teflon tubing. In tandem, volatiles were collected from empty jars as controls for system contaminants. Headspace volatiles were recovered by extracting the adsorbent cartridges with 1.5 ml of dichloromethane containing eicosane (38 µg) as an internal standard.
In Illinois, extracts of headspace collections were analyzed on an Agilent 7890B GC coupled to a 5977A mass selective detector (Agilent), in splitless mode and with helium carrier gas. The GC was fitted with an HP-5 column (30 m × 0.25 mm ID, Agilent), and the oven temperature program was 30°C (hold 1 min), 10°C/min to 250°C (hold 5 min). Compounds were tentatively identified by matches with database spectra if available (NIST database), or by spectral interpretation. Chemical identifications were confirmed with authentic standards, matching mass spectra and retention times.
Field Bioassays of Candidate Pheromones
For the experiment targeting S. consobrina in Brazil, treatment lures were assigned randomly to traps (positioned 15 m apart) in four transects (30 m apart) and each transect contained one trap per treatment. The treatments, each diluted in 1 ml isopropanol, were: 1) 2-nonanone (25 mg), 2) semanopyrrole (25 mg), 3) natural blend, i.e., blend of 2-nonanone (4.5 mg) + semanopyrrole (25 mg) at same ratio produced by adult beetles (see Results), 4) 1:1 blend of 2-nonanone + semanopyrrole (25 mg each), 5) solvent control (1 ml neat isopropanol). Traps were serviced weekly, at which time treatment lures were shifted one position down transects to control for positional effects. Beetles were collected from 14 November to 28 December 2018. Lures were replaced every 2 wk.
For the experiment targeting H. quadrimaculatus in Illinois, four traps were set up ~10 m apart in each of five transects (separated by at least 20 m) at the Forest Glen Preserve site on 8 July 2019. Within each transect, four treatments, each in 1 ml isopropanol, were assigned randomly to traps, as follows: 1) 2-nonanone (25 mg), 2) racemic 10-methyldodecanol (50 mg), 3) 1:1 blend of 2-nonanone + racemic 10-methyldodecanol (i.e., 25 and 50 mg, respectively), 4) solvent control. Beetles were collected every 2–3 d, at which time treatments were shifted one position down transects. Trapping was stopped on 31 August 2019. Lures were replaced every 2 wk.
Statistical Analysis
Treatment effects were analyzed separately for each species represented by at least 10 specimens, with replicates defined by the number of transects and collection date (i.e., replication over space and time). Because the data violated assumptions of ANOVA (Sokal and Rohlf 1995), differences between treatment means were tested with the non-parametric Friedman’s Test (PROC FREQ, option CMH; SAS Institute 2011). Replicates with no beetles in any trap, for example as a result of inclement weather, were not included in analyses. Significance levels were adjusted in the bioassays for S. consobrina (α = 0.017; n = 3 independent analyses for three species), according to the Bonferroni procedure (Quinn and Keough 2002). For each analysis, pairs of treatment means were compared using the nonparametric Ryan-Einot-Gabriel-Welsch Q (REGWQ) multiple comparison test (SAS Institute 2011). The sex ratios of adults of S. consobrina and H. quadrimaculatus captured by traps baited with the optimal attractants (2-nonanone + semanopyrrole and 2-nonanone + 2-methyldodecanol, respectively; see Results) were compared to a nominal proportion of 0.5 with 95% Clopper–Pearson exact confidence intervals (Newcombe 1998).
Results
Collection and Analysis of Beetle-Produced Compounds
Analysis of headspace volatiles from adult males of S. consobrina revealed the presence of two compounds that were not present in equivalent extracts of conspecific females or controls (Fig. 1A). The first compound was readily identified as 2-nonanone from its EI mass spectrum, with a good match to a database spectrum, and the match was confirmed with an authentic standard. The mass spectrum of the second compound was very simple, consisting of essentially three ions, an apparent molecular ion at m/z 137 (21%), the base peak at m/z 94 (100%), and a third fragment at m/z 66 (48%). From our recent work with other cerambycid species (Table 1), it was readily identified as the known compound semanopyrrole, and confirmed with a standard. The average ratio of 2-nonanone:semanopyrrole in three representative aeration extracts was ~1:5.6 (estimated from peak area integration of compounds).
Species in the subfamily Cerambycinae which are known to have semanopyrrole as a pheromone component, and/or that are attracted by this compound alone or in blends
| Tribe/ Species . | Native continent . | Pheromone composition . | Attractant . | Reference . |
|---|---|---|---|---|
| Callidiini | ||||
| Callidiellum rufipenne (Motschulsky) | Asia | 3R/S-ketol, 2R/S-ketol | pyrrole + 3R-ketol | Zou et al. 2016 |
| Callidiellum villosulum (Fairmaire) | Asia | unknown | pyrrole + 3-ketol | Wickham et al. 2016 |
| Callidium antennatum hesperum Casey | North America | pyrrole, 3R-ketol | pyrrole + 3-ketol | Millar et al. 2019 |
| Callidium pseudotsugae Fisher | North America | pyrrole, 3R-ketol | not confirmed | Millar et al. 2019 |
| Semanotus amethystinus (LeConte) | North America | pyrrole | pyrrole | Millar et al. 2019 |
| Semanotus bifasciatus Motschulsky | Asia | unknown | pyrrole | Wickham et al. 2016 |
| Semanotus ligneus (F.) | North America | pyrrole, acetoin, minor compounds | not confirmed | Millar et al. 2019 |
| Semanotus litigiosus (Casey) | North America | pyrrole, acetoin, minor compounds | not confirmed | Millar et al. 2019 |
| Clytini | ||||
| Xylotrechus buqueti (Castelnau & Gory) | Asia | unknown | pyrrole + 3-ketol | Wickham et al. 2016 |
| Dryobiini | ||||
| Dryobius sexnotatus Linsley | North America | pyrrole, 3R-ketol | pyrrole + 3R-ketol | Diesel et al. 2017 |
| Elaphidiini | ||||
| Ambonus distinctus (Newman) | South America | pyrrole, 3R-ketol, methionol | pyrrole + 3-ketol + methionol | Silva et al. 2017 |
| Ambonus electus (Gahan) | South America | pyrrole, 3R-ketol | pyrrole + 3-ketol | Silva et al. 2017 |
| Amorupi fulvoterminata (Berg) | South America | unknown | pyrrole | Silva et al. 2017 |
| Stizocera consobrina Gounelle | South America | pyrrole, 2-nonanone | pyrrole + 2-nonanone | present article |
| Dichophyiini | ||||
| Chrysoprasis aurigena (Germar) | South America | unknown | pyrrole + 3-ketol | Silva et al. 2017 |
| Phoracanthini | ||||
| Allotraeus asiaticus (Schwarzer) | Asia | unknown | pyrrole | Wickham et al. 2016 |
| Tribe/ Species . | Native continent . | Pheromone composition . | Attractant . | Reference . |
|---|---|---|---|---|
| Callidiini | ||||
| Callidiellum rufipenne (Motschulsky) | Asia | 3R/S-ketol, 2R/S-ketol | pyrrole + 3R-ketol | Zou et al. 2016 |
| Callidiellum villosulum (Fairmaire) | Asia | unknown | pyrrole + 3-ketol | Wickham et al. 2016 |
| Callidium antennatum hesperum Casey | North America | pyrrole, 3R-ketol | pyrrole + 3-ketol | Millar et al. 2019 |
| Callidium pseudotsugae Fisher | North America | pyrrole, 3R-ketol | not confirmed | Millar et al. 2019 |
| Semanotus amethystinus (LeConte) | North America | pyrrole | pyrrole | Millar et al. 2019 |
| Semanotus bifasciatus Motschulsky | Asia | unknown | pyrrole | Wickham et al. 2016 |
| Semanotus ligneus (F.) | North America | pyrrole, acetoin, minor compounds | not confirmed | Millar et al. 2019 |
| Semanotus litigiosus (Casey) | North America | pyrrole, acetoin, minor compounds | not confirmed | Millar et al. 2019 |
| Clytini | ||||
| Xylotrechus buqueti (Castelnau & Gory) | Asia | unknown | pyrrole + 3-ketol | Wickham et al. 2016 |
| Dryobiini | ||||
| Dryobius sexnotatus Linsley | North America | pyrrole, 3R-ketol | pyrrole + 3R-ketol | Diesel et al. 2017 |
| Elaphidiini | ||||
| Ambonus distinctus (Newman) | South America | pyrrole, 3R-ketol, methionol | pyrrole + 3-ketol + methionol | Silva et al. 2017 |
| Ambonus electus (Gahan) | South America | pyrrole, 3R-ketol | pyrrole + 3-ketol | Silva et al. 2017 |
| Amorupi fulvoterminata (Berg) | South America | unknown | pyrrole | Silva et al. 2017 |
| Stizocera consobrina Gounelle | South America | pyrrole, 2-nonanone | pyrrole + 2-nonanone | present article |
| Dichophyiini | ||||
| Chrysoprasis aurigena (Germar) | South America | unknown | pyrrole + 3-ketol | Silva et al. 2017 |
| Phoracanthini | ||||
| Allotraeus asiaticus (Schwarzer) | Asia | unknown | pyrrole | Wickham et al. 2016 |
pyrrole = semanopyrrole, 3-ketol = racemic 3-hydroxyhexan-2-one, 3R-ketol = (R)-3-hydroxyhexan-2-one, 3S-ketol = (S)-3-hydroxyhexan-2-one, 2R-ketol = (R)-2-hydroxyhexan-2-one, 2S-ketol = (S)-2-hydroxyhexan-2-one, methionol = 3-methylthiopropan-1-ol.
Species in the subfamily Cerambycinae which are known to have semanopyrrole as a pheromone component, and/or that are attracted by this compound alone or in blends
| Tribe/ Species . | Native continent . | Pheromone composition . | Attractant . | Reference . |
|---|---|---|---|---|
| Callidiini | ||||
| Callidiellum rufipenne (Motschulsky) | Asia | 3R/S-ketol, 2R/S-ketol | pyrrole + 3R-ketol | Zou et al. 2016 |
| Callidiellum villosulum (Fairmaire) | Asia | unknown | pyrrole + 3-ketol | Wickham et al. 2016 |
| Callidium antennatum hesperum Casey | North America | pyrrole, 3R-ketol | pyrrole + 3-ketol | Millar et al. 2019 |
| Callidium pseudotsugae Fisher | North America | pyrrole, 3R-ketol | not confirmed | Millar et al. 2019 |
| Semanotus amethystinus (LeConte) | North America | pyrrole | pyrrole | Millar et al. 2019 |
| Semanotus bifasciatus Motschulsky | Asia | unknown | pyrrole | Wickham et al. 2016 |
| Semanotus ligneus (F.) | North America | pyrrole, acetoin, minor compounds | not confirmed | Millar et al. 2019 |
| Semanotus litigiosus (Casey) | North America | pyrrole, acetoin, minor compounds | not confirmed | Millar et al. 2019 |
| Clytini | ||||
| Xylotrechus buqueti (Castelnau & Gory) | Asia | unknown | pyrrole + 3-ketol | Wickham et al. 2016 |
| Dryobiini | ||||
| Dryobius sexnotatus Linsley | North America | pyrrole, 3R-ketol | pyrrole + 3R-ketol | Diesel et al. 2017 |
| Elaphidiini | ||||
| Ambonus distinctus (Newman) | South America | pyrrole, 3R-ketol, methionol | pyrrole + 3-ketol + methionol | Silva et al. 2017 |
| Ambonus electus (Gahan) | South America | pyrrole, 3R-ketol | pyrrole + 3-ketol | Silva et al. 2017 |
| Amorupi fulvoterminata (Berg) | South America | unknown | pyrrole | Silva et al. 2017 |
| Stizocera consobrina Gounelle | South America | pyrrole, 2-nonanone | pyrrole + 2-nonanone | present article |
| Dichophyiini | ||||
| Chrysoprasis aurigena (Germar) | South America | unknown | pyrrole + 3-ketol | Silva et al. 2017 |
| Phoracanthini | ||||
| Allotraeus asiaticus (Schwarzer) | Asia | unknown | pyrrole | Wickham et al. 2016 |
| Tribe/ Species . | Native continent . | Pheromone composition . | Attractant . | Reference . |
|---|---|---|---|---|
| Callidiini | ||||
| Callidiellum rufipenne (Motschulsky) | Asia | 3R/S-ketol, 2R/S-ketol | pyrrole + 3R-ketol | Zou et al. 2016 |
| Callidiellum villosulum (Fairmaire) | Asia | unknown | pyrrole + 3-ketol | Wickham et al. 2016 |
| Callidium antennatum hesperum Casey | North America | pyrrole, 3R-ketol | pyrrole + 3-ketol | Millar et al. 2019 |
| Callidium pseudotsugae Fisher | North America | pyrrole, 3R-ketol | not confirmed | Millar et al. 2019 |
| Semanotus amethystinus (LeConte) | North America | pyrrole | pyrrole | Millar et al. 2019 |
| Semanotus bifasciatus Motschulsky | Asia | unknown | pyrrole | Wickham et al. 2016 |
| Semanotus ligneus (F.) | North America | pyrrole, acetoin, minor compounds | not confirmed | Millar et al. 2019 |
| Semanotus litigiosus (Casey) | North America | pyrrole, acetoin, minor compounds | not confirmed | Millar et al. 2019 |
| Clytini | ||||
| Xylotrechus buqueti (Castelnau & Gory) | Asia | unknown | pyrrole + 3-ketol | Wickham et al. 2016 |
| Dryobiini | ||||
| Dryobius sexnotatus Linsley | North America | pyrrole, 3R-ketol | pyrrole + 3R-ketol | Diesel et al. 2017 |
| Elaphidiini | ||||
| Ambonus distinctus (Newman) | South America | pyrrole, 3R-ketol, methionol | pyrrole + 3-ketol + methionol | Silva et al. 2017 |
| Ambonus electus (Gahan) | South America | pyrrole, 3R-ketol | pyrrole + 3-ketol | Silva et al. 2017 |
| Amorupi fulvoterminata (Berg) | South America | unknown | pyrrole | Silva et al. 2017 |
| Stizocera consobrina Gounelle | South America | pyrrole, 2-nonanone | pyrrole + 2-nonanone | present article |
| Dichophyiini | ||||
| Chrysoprasis aurigena (Germar) | South America | unknown | pyrrole + 3-ketol | Silva et al. 2017 |
| Phoracanthini | ||||
| Allotraeus asiaticus (Schwarzer) | Asia | unknown | pyrrole | Wickham et al. 2016 |
pyrrole = semanopyrrole, 3-ketol = racemic 3-hydroxyhexan-2-one, 3R-ketol = (R)-3-hydroxyhexan-2-one, 3S-ketol = (S)-3-hydroxyhexan-2-one, 2R-ketol = (R)-2-hydroxyhexan-2-one, 2S-ketol = (S)-2-hydroxyhexan-2-one, methionol = 3-methylthiopropan-1-ol.
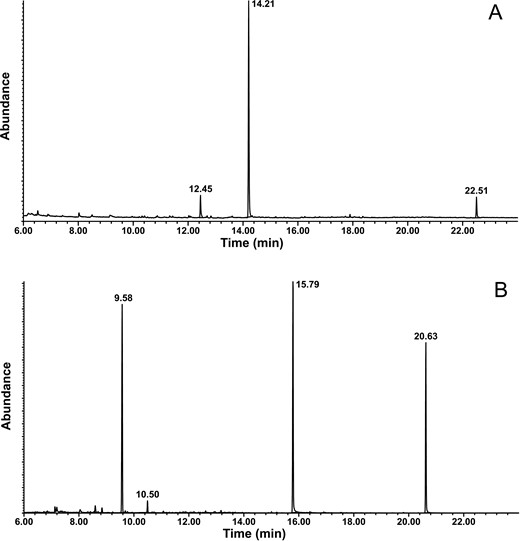
Representative total ion chromatograms of headspace extracts showing two major male-specific compounds for (A) Stizocera consobrina (retention times 12.45 and 14.21 min corresponding to 2-nonanone and semanopyrrole, respectively; peak at 22.51 min is a system contaminant), and (B) Heterachthes quadrimaculatus (retention times 9.58 and 15.79 min corresponding to 2-nonanone and 10-methyldodecanol, respectively, the internal standard eicosane at 20.63 min, and a system contaminant at 10.50 min).
Extracts of headspace volatiles from adult males of H. quadrimaculatus contained two compounds that were not present in the three headspace extracts of females, nor in control aerations (Fig. 1B). Both compounds were detected in five of the six headspace collections from males. The first peak was readily identified from its mass spectrum and retention time as 2-nonanone (see above), whereas the second peak was tentatively identified as 10-methyldodecanol from its mass spectrum, because we had recently found this compound in extracts from another South American cerambycid species (Silva et al. 2020). In particular, there was a trace ion at m/z 182 from loss of water from the parent compound, and further losses of 29 (ethyl group) or 57 mass units (sec-butyl group) to give diagnostic ions at m/z 153 and 125, respectively, characteristic of a methyl-branched compound with the methyl on the third carbon from the end of the chain. The identification was confirmed by retention time and mass spectral matches with a standard. The ratio of 2-nonanone and 10-methyldodecanol in the five extracts was ~1.15:1.
Field Bioassays of Candidate Pheromone Components
A total of 199 adults of S. consobrina were captured during the field experiment in Brazil. Only the natural blend of 2-nonanone and semanopyrrole (1:5.6) and the 1:1 blend attracted significant numbers of beetles (Fig. 2A; means significantly different Q4,75 = 54.8, P < 0.0001). The sex ratio of captured beetles in traps baited with 2-nonanone + semanopyrrole was slightly female-biased (65% females; 95% Clopper–Pearson exact confidence interval of 0.5391–0.7417, P = 0.0051). Although 16 adults of Chrysoprasis auriventris auriventris Redtenbacher (tribe Dichophyiini) and 15 adults of Eburodacrys sexguttata Lameere (tribe Eburiini) were caught during the trials, treatment means were not significantly different than control means in either case.
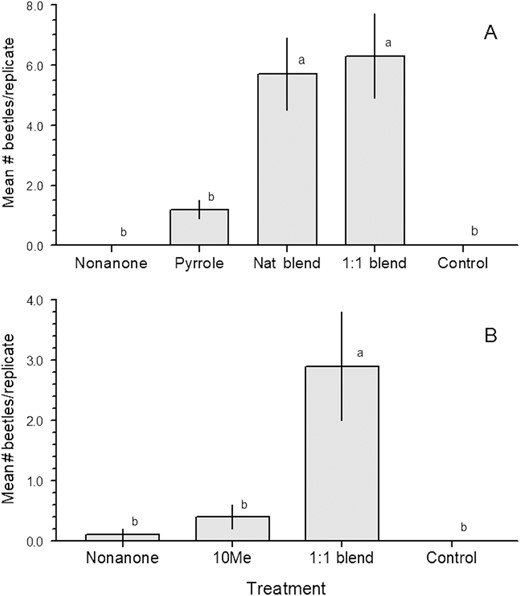
Mean (±SE) numbers of adult beetles captured by traps baited with synthesized compounds for the species (A) Stizocera consobrina in Brazil (n = 199 beetles), and (B) Heterachthes quadrimaculatus in Illinois (24 beetles). Treatments: Nonanone = 2-nonanone, Pyrrole = semanopyrrole, 10Me = racemic 10-methyldodecanol. The natural blend of 2-nonanone and semanopyrrole in A was 1:5.6. Means with different letters within species are significantly different (REGWQ test, P < 0.05).
During the field experiment in Illinois, 24 adults of H. quadrimaculatus were captured, and very few beetles of any other species. Of the total number, 20 adults of H. quadrimaculatus (~83%) were in traps baited with the blend of 2-nonanone + 10-methyldodecanol (Fig. 2B; means significantly different; Q3,28 = 13.1, P = 0.0003). The sex ratio of the trapped beetles was exactly 1:1 (50% female; Clopper–Pearson P = 1).
Discussion
The field experiments confirmed that both of the study species have two-component pheromone blends, with males of the Brazilian species S. consobrina producing semanopyrrole with 2-nonanone, and males of the North American species H. quadrimaculatus also producing 2-nonanone, but paired with 10-methyldodecanol. Adults of both species were significantly attracted only by the blends of both of their pheromone components, and not to the individual components. The field experiment targeting S. consobrina further demonstrated that adults were similarly attracted by a 1:5.6 blend of components matching that produced by males, and by a 1:1 blend of the two components, suggesting that the presence of both compounds was important, but the ratio was not.
Despite the apparent synergistic role of 2-nonanone as a pheromone component for S. consobrina, adults of this species had been attracted in significant numbers to traps baited solely with semanopyrrole in an earlier study (Silva et al. 2017). This finding is consistent with research on other species of cerambycids which has demonstrated that even weak attraction to certain individual pheromone components can result in statistically significant treatment effects when more powerful attractants are not included in bioassays (e.g., Millar et al. 2018). Furthermore, although semanopyrrole as a single component was not statistically more attractive than the control (or 2-nonanone), traps baited with semanopyrrole did catch 18 beetles, whereas traps baited with 2-nonanone or the controls caught no beetles at all.
The studies reported here contribute to the growing evidence that semanopyrrole is a pheromone component of cerambycine species in multiple tribes, and native to many biogeographical regions of the world (summarized in Table 1). To date, it has been found to be the sole or a critical pheromone component, or at least an attractant, for another 15 cerambycine species in the tribes Callidiini, Clytini, Dryobiini, Elaphidiini, Dichophyiini, and Phoracanthini native to South America, North America, and Asia. Based on these data, we anticipate that it will turn up in additional species, possibly from new continents, as additional species are examined.
The genus Heterachthes comprises 75 species, nearly all of which are native to Central and South America (Monné 2020, Monné and Nearns 2020), and its tribe, Neoibidionini, is considered Neotropical in origin (Monné et al. 2017). The South American ancestry of H. quadrimaculatus may account for its use of 10-methyldodecanol as a pheromone component, because to date, the only other species known to use this compound as a pheromone component are the South American cerambycines Compsibidion graphicum (Thomson) and Compsibidion sommeri (Thomson), also of the Neoibidionini (Silva et al. 2020). It should be noted that no other cerambycid species were attracted to traps baited with 10-methyldodecanol during the field experiment in Illinois, suggesting that this compound is not shared with any sympatric species.
The use of 2-nonanone by H. quadrimaculatus and S. consobrina also might suggest a South American ancestry for the former species. However, this compound, along with (R)-3-hydroxyhexan-2-one, comprises the pheromone of the North American cerambycine Cyrtophorus verrucosus (Olivier) (tribe Anaglyptini; Mitchell et al. 2015). The genus Cyrtophorus is monotypic, but it is thought to have a Eurasian ancestry (Linsley 1963b). Thus, time may reveal that 2-nonanone comprises another conserved pheromone component within the subfamily Cerambycinae.
These hypotheses about the biogeographical origins of pheromone chemistry obviously are hindered by our limited knowledge of the pheromone chemistry of the Cerambycinae (Hanks et al. 2016), which is represented by more than 12,200 species in 119 tribes worldwide (Tavakilian and Chevillotte 2020), in comparison to the ~200 species for which pheromones now are known or suspected. Nevertheless, the research presented and discussed here lends further support to the hypothesis that much of the pheromone chemistry of cerambycine species appears to be conserved, with species native to different continents, and separated by millions of years since they diverged from common ancestors, still sharing compounds that serve as critical components of their pheromones.
Acknowledgments
We thank Aaron Blue for assistance in the laboratory and field in Illinois, Steve Buck and the University of Illinois Committee on Natural Areas, the Champaign County Forest Preserves District, Vermilion County Conservation District, and the Illinois Department of Natural Resources for access to field sites. We also thank Dr. João Carlos Mendes (Department of Forest Science, USP-ESALQ) for access to the Experimental Station in Anhembi. A special thank you to Antonio Santos-Silva and Francisco Nascimento (Museum of Zoology of USP) for identifying the cerambycid species from Brazil, and to Dr. Emiliana Romagnoli and Hugo Rainho for assisting with the field experiments in Brazil. We gratefully acknowledge financial support from INCT-Semiochemicals in Agriculture (Fundação de Amparo à Pesquisa do Estado de São Paulo and Conselho Nacional de Desenvolvimento Científico e Tecnológico, grants: 2014/50871-0 and 465511/2014-7) to WDS and JMSB and United States Department of Agriculture - Animal and Plant Health Inspection Service (grants 15, 16, 17, and 18-8130-1422-CA) to JGM and LMH. Field collections of the study species in Brazil were conducted under SISBIO permit nº #46395 from the Brazilian Ministry of the Environment. This work was registered with the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen, Brazil) under nº# AE3897B.



