-
PDF
- Split View
-
Views
-
Cite
Cite
Laura J Nixon, Heather Leach, Caitlin Barnes, Julie Urban, Danielle M Kirkpatrick, Dalton C Ludwick, Brent Short, Douglas G Pfeiffer, Tracy C Leskey, Development of Behaviorally Based Monitoring and Biosurveillance Tools for the Invasive Spotted Lanternfly (Hemiptera: Fulgoridae), Environmental Entomology, Volume 49, Issue 5, October 2020, Pages 1117–1126, https://doi.org/10.1093/ee/nvaa084
Close - Share Icon Share
Abstract
The spotted lanternfly, Lycorma delicatula White, is an invasive planthopper (Hemiptera: Fulgoridae) that was first detected in the United States in Berks County, PA, in 2014, and has since spread in the mid-Atlantic region. This phloem-feeding pest has a broad host range, including economically important crops such as grape where their feeding causes dieback of infested plants. Monitoring the presence and abundance of L. delicatula is of utmost importance to develop pest management approaches. Current monitoring practices include sticky bands deployed on tree trunks, sometimes paired with commercially available methyl salicylate lures. A drawback associated with sticky bands is the high numbers of nontarget captures. Here, we developed traps for L. delicatula based on a circle trap originally designed for weevils. These traps are comprised of a screen funnel that wraps around the trunk of a tree and guides individuals walking up the trunk into a collection device. In 2018 and 2019, we compared circle trap designs with sticky bands in Pennsylvania and Virginia. In both years, circle trap designs yielded captures that were equivalent to or exceeded captures of L. delicatula on sticky bands. Nontarget captures were significantly lower for circle traps compared with sticky bands. Presence of a methyl salicylate lure in association with traps deployed on host trees or vertical tree-mimicking posts did not increase L. delicatula captures compared with unbaited traps. Circle traps, modified using vinyl screen and a larger collection device, present an alternative to the current approach with reduced nontarget capture for monitoring L. delicatula.
The spotted lanternfly, Lycorma delicatula White, is an invasive pest originating from China, India, and Vietnam. Its invaded range currently includes South Korea, Japan, and the mid-Atlantic region of the United States. In the United States, L. delicatula was initially detected in Berks County, PA in 2014 (Barringer et al. 2015, Dara et al. 2015). Predictive models have shown that much of the Eastern United States is climactically suitable for this species and continued spread is likely (Jung et al. 2017, Wakie et al. 2020). Lycorma delicatula is univoltine, overwintering as eggs. First hatch in the native range is reported to be mid-April, with nymphs present throughout the summer months, and adults emerging in June and July to deposit eggs from mid-August through fall (Lee et al. 2019). Populations in the mid-Atlantic have, thus far, followed a similar developmental pattern (Liu 2019).
Lycorma delicatula feed on a broad range of hosts, with common hosts including tree of heaven (Ailanthus altissima (Mill.) Swingle), black walnut (Juglans nigra L.), maple species (Acer spp.), black locust (Robinia pseudoacacia L.), and both wild and cultivated grapes (Vitis spp.) (Barringer et al. 2015, Dara et al. 2015). Like other phloem feeders, L. delicatula produce copious amounts of liquid excrement, called honeydew, which predominantly consists of sugar and amino acids (Douglas 2006). This substance coats surfaces below feeding sites and attracts other insects such as Vespidae and serves as a substrate for sooty mold growth, which interferes with photosynthesis and can result in plant decline and death (Han et al. 2008, Dara et al. 2015). Particular concerns for L. delicatula impact on specialty crops including grapes and tree fruit (Lee et al. 2019) have led to studies identifying effective insecticides for their management and evaluation of potential biological controls, such as parasitoids (Yang et al. 2014, Liu and Mottern 2017, Leach et al. 2019).
In an effort to prevent further spread and eradicate L. delicatula populations, several states have established quarantines and eradication programs. These programs utilize cultural practices to remove host trees as well as foliar sprays and systemic trunk drenches (Parra et al. 2018). However, as L. delicatula continues to spread, it is essential to develop tools for monitoring population presence, relative abundance, and seasonal activity. Indeed, monitoring for insect populations is considered a foundation of developing integrated pest management tactics (Flint 2012). As L. delicatula are known to exhibit negative gravitaxis (Kim et al. 2011), placing sticky bands to serve as traps around the trunks of host trees has become a common practice for monitoring in infested areas. Although these sticky bands are easy to obtain and install, they have frequent nontarget vertebrate and invertebrate captures and require new sticky material regularly (Penn State Extension 2019). Moreover, recent studies have identified attractive host plant volatiles (Cooperband et al. 2019) and alternative trap designs (Francese et al. 2020) including circle traps used to monitor weevil species such as plum curculio, Conotrachelus nenuphar (Herbst) (Coleoptera: Curculionidae) (Leskey and Wright 2004a,b). However, these lures combined with alternative trap styles have not been widely evaluated and compared with standard sticky bands in the United States. Therefore, we conducted a 2-yr study in Pennsylvania and Virginia to: 1) develop and evaluate modified circle trap designs with standard sticky bands; and 2) evaluate the attractiveness of recently identified and commercialized methyl salicylate lures to foraging L. delicatula populations deployed with traps or vertical tree-mimicking posts.
Materials and Methods
2018 Trapping Studies
Standard brown sticky bands (width: 21.5 cm, Web-Cote Industries Inc., Hamburg, NJ) 1) were compared with two additional trap designs, 2) a small circle trap (AgBio Inc., Westminster, CO) made of vinyl-coated polyester screen (‘Pet Resistant’, Saint-Gobain, Malvern, PA) with a circular plastic collection top (diameter: 5.5 cm, height: 5 cm), as previously described for plum curculio (Leskey and Wright 2004a), and 3) a modified protruding circle trap with a larger, square collection jar top (12.5 × 9.5 × 9.5 cm) (AgBio Inc.) and folded vinyl screen funnel with horizontal wooden supports beneath the jar entrance to enable larger L. delicatula to enter (see Fig. 1 for trap designs). Sites included a community park in Winchester, VA (39°12′22″N, 78°9′18″W) and a memorial park in Reading, PA (40°20′50.3″N, 75°54′08.1″W). At each site, three replicates of each trap type, either baited with a methyl salicylate lure (Alpha Scents Inc., Syracuse, NY) or left unbaited, were installed on host trees: Virginia traps were installed on A. altissima and Pennsylvania traps were installed on to a mix of host trees including A. altissima, Acer spp., and Quercus spp. All circle traps contained DDVP kill strips (Vaportape II, Hercon, PA) in the collection device. Each week, traps were checked and the number of nymphal and adult L. delicatula captured was recorded, and all sticky bands, kill strips, and lures were replaced. Trapping data were collected from 22 August to 17 October in Virginia and 10 August to 9 October in Pennsylvania. The weekly captures of L. delicatula at each site were analyzed with a repeated-measures generalized linear model. The model included presence of lure, trap design, and time as explanatory factors, with interaction terms (lure × trap, time × trap); the replicate was included as a nested variable with trap design to account for random variation from location. These, and all subsequent analyses, were performed using JMP software 13.0 (SAS Institute, Cary, NC).

Trap designs: (A) standard sticky band, (B) small circle trap, and (C) protruding circle trap, deployed on host trees for capture of Lycorma delicatula in 2018. All affixed with methyl salicylate lure.
2019 Trapping Studies
Unbaited traps
A season-long study evaluating four unbaited trap designs was conducted in Winchester, VA (see 2018 site details) from 2 May to 14 November and in Reading, PA (40°24′43.9″N 75°59′00.9″W) from 30 May to 13 November. At each site, five replicates of each trap type were deployed onto known host trees of L. delicatula. All traps in Pennsylvania were deployed onto A. altissima (diameter at breast height, DBH, range 17–32 cm, 22.5 ± 0.7 cm), whereas traps in Virginia were deployed onto a variety of hardwood hosts including black walnut, silver maple (Acer saccharinum L.), red maple (Acer rubrum L.), black locust, and box elder (Acer negundo L.) (DBH range 13–37 cm, 21.6 ± 1.8 cm). Traps evaluated included: 1) standard sticky band as evaluated in 2018; 2) small circle trap as evaluated in 2018; 3) a modified circle trap consisting of a folded vinyl screen funnel as described for the small circle trap, but with a large collection jar top (AgBio Inc.) affixed (see Supp Material (online only) for construction details); and 4) experimental trap design TR 290 (provided by Trécé Inc., Adair, OK), consisting of a wire mesh screen funnel with a screw top jar affixed above (Fig. 2). All traps with a collection device were provisioned with a DDVP kill strip; every week these traps were emptied, nymphal and adult captures recorded, and the kill strip replaced. Capture on the sticky bands was recorded and the entire band replaced weekly. Additionally, monthly assessment of nontarget captures was recorded at the Order level from all traps at the Virginia site. Weekly capture of nontargets, categorized as predator/parasitoid, Apidae/Syrphidae, or other, was recorded at the Pennsylvania site.

Trap designs: (A) standard sticky band, (B) small circle trap, (C) modified circle trap, and (D) TR 290 experimental trap, deployed on Ailanthus altissima for capture of Lycorma delicatula in 2019.
Nymphal and adult data were analyzed separately for each site. Analysis of nymphal and adults captures in Virginia included data collected from 9 May to 1 August and 25 July to 24 October 2019, respectively. Repeated-measures generalized linear models were used to analyze each data set. Both models included tree species, trap design, and time as explanatory factors, with interaction terms (tree × trap, time × trap); the replicate was included as a nested variable with trap design to account for random variation from location. Analyses of nymphal and adult captures in Pennsylvania included data collected from 5 June to 6 September and 1 August to 7 November 2019, respectively. Repeated-measures generalized linear models for each data set included a full factorial analysis of time and trap designs, with replicate numbers nested to account for random effects.
Sticky band occlusion
To assess if the amount of occlusion of sticky bands reduced overall L. delicatula captures, sticky bands were covered with randomly distributed pieces of paper designed to occlude 25, 50, or 75% of the surface area; traps without occlusion served as controls. Three replicates were installed in Virginia on A. altissima trees where wild populations of L. delicatula were present. Each week, all sticky band treatments were replaced, and the number of adults and nymphs captured on bands was recorded from 4 June to 30 October 2019. Analyses of nymphal and adult captures included data collected from 4 June to 31 July and 24 July to 30 October 2019, respectively. Nymphal data were log-transformed to fit parametric normalcy; normalized data were analyzed using one-way ANOVA with Dunnett’s test. Adult data followed a non-normal distribution, and data were analyzed using Kruskal–Wallis with Steel test for comparisons to control.
Lure evaluation
To evaluate the efficacy of the commercially available lure containing methyl salicylate, we used a vertical tree-mimicking bioassay system. This system enabled us to separate the potential olfactory response of L. delicatula to the lure itself from the response to potential host plant volatiles if the sticky band were deployed on a host tree as in other experiments. Wooden fence posts (1.82 m tall with a 15-cm diameter) were installed every 10 m along a wood line with a L. delicatula population. Three posts were baited with methyl salicylate lures and three were left unbaited (control), and these lure positions were re-randomized weekly. Lures were attached to the post via a bungee cord directly above a sticky band affixed 1 m from the ground. Unbaited control posts had a bungee cord with an empty black clip present to keep visual cues consistent (see Fig. 3 for setup). Nymphal and adult captures were analyzed from 16 May to 31 July and 24 July to 23 October 2019, respectively. The lure evaluation data were divided into nymph and adult captures, and, as neither data set was normally distributed, comparisons were made using Wilcoxon’s rank sum test.
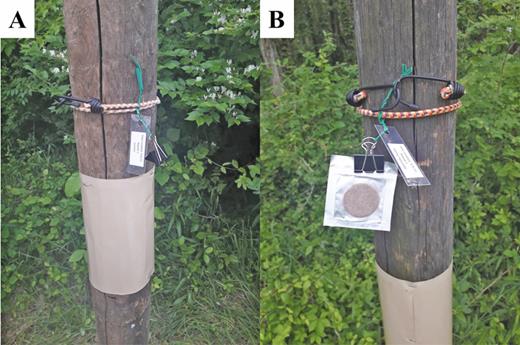
Tree-mimicking vertical posts deployed with sticky bands and (A) blank control and (B) methyl salicylate lure affixed above with a bungee cord.
Results
2018 Trapping Studies
Because traps were deployed in August 2018 and only one nymph was captured in Virginia, only adult data are presented from that site. At the Virginia site, there were no significant differences in captures between baited and unbaited traps (F = 4.7; df = 1, 126; P = 0.651) or among trap designs (F = 4.7; df = 2, 6; P = 0.060) (Fig. 4B). There was a significant interaction between trap design and time (F = 3.2; df = 16, 126; P < 0.001). In Reading, PA, nymphal capture data from 10 August to 14 September were analyzed and there were no significant differences in captures between baited and unbaited traps (F = 1.4; df = 1, 111; P = 0.245) or among trap designs (F = 4.1; df = 2, 6; P = 0.074) (Fig. 4A). However, there were significant interactions between trap design and lure (F = 6.1; df = 2, 111; P = 0.003) and trap design and time (F = 2.4; df = 14, 111; P = 0.005). There were no significant differences in adult capture among any of the trap designs in Pennsylvania (F = 1.3; df = 2, 6; P = 0.349); however, traps baited with the methyl salicylate lure captured significantly fewer adults than unbaited traps (F = 18.3; df = 1, 156; P < 0.001) (Fig. 4C). There were no significant interactions observed.
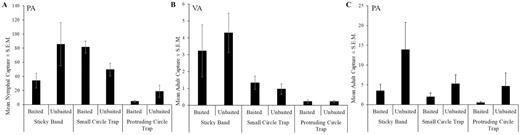
Mean number of Lycorma delicatula ± SEM (A) nymphs in Reading, PA, and adults in (B) Winchester, VA, and (C) Reading, PA captured per trap per week.
2019 Trapping Studies
Unbaited traps
At the Virginia site, the first nymphal capture was recorded on 9 May, with captures continuing until 5 September (see Fig. 5A for phenology). Trap design and tree species did not have a significant effect on nymphal captures (F = 1.5; df = 3, 11; P = 0.278, and F = 1.5; df = 5, 11; P = 0.098, respectively), whereas time did (F = 8.7; df = 12, 184; P < 0.001). The first adult was captured on 18 July with the last capture recorded on 14 November, after which no more live adult L. delicatula were observed on site (Fig. 5C). Trap design did not have a significant effect on adult capture (F = 1.2; df = 3, 11; P = 0.349) (Fig. 6C); however, tree species and time did (F = 4.5; df = 5, 11; P = 0.0179, and F = 3.1; df = 12, 184; P < 0.001, respectively).
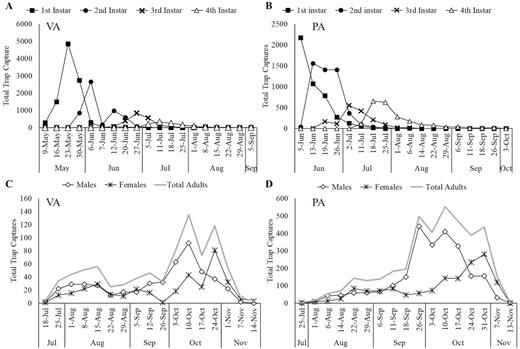
Phenology of total trap captures of nymphs in (A) Winchester, VA and (B) Reading, PA and adults in (C) Winchester, VA and (D) Reading, PA in 2019.
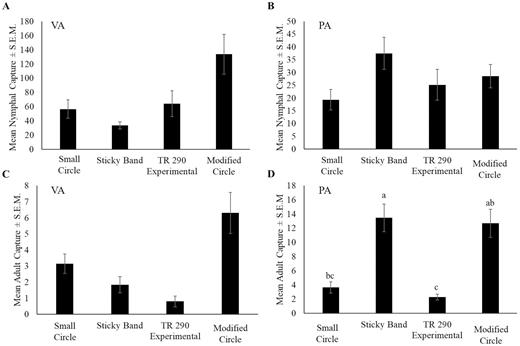
Mean number of Lycorma delicatula ± SEM nymphs in (A) Winchester, VA and (B) Reading, PA and adults in (C) Winchester, VA and (D) Reading, PA captured per trap per week in 2019. Letters denote significance levels at P < 0.05.
At the Pennsylvania site, nymphs were captured from 5 June to 3 October 2019 (Fig. 5B). Trap design did not have a significant effect on nymphal capture (F = 0.8; df = 3, 16; P = 0.516) (Fig. 6B); however, time did (F = 21.5; df = 13, 207; P < 0.001). Figure 5A and B show that overall captures decreased with each nymphal life stage at both sites. The first adult capture occurred on 25 July, with the final capture recorded on 13 November (Fig. 5D). There were significant differences in adult capture among trap designs (F = 6.6; df = 3, 16; P = 0.004) (Fig. 6D) and over time (F = 9.8; df = 14, 224; P < 0.001); there was also a significant interaction between these factors (F = 2.9; df = 42, 224; P < 0.001). Figure 5C and D show that adult capture increased in the later season. The modified trap and sticky band captured 4× more adult L. delicatula than the small circle or TR 290 experimental traps.
Additionally, trap design had a significant effect on the number of nontarget organisms captured in Virginia (F = 24.9; df = 3, 53; P < 0.0001). Sticky bands (59.7 ± 6.5 nontargets per trap) caught significantly more organisms than the modified circle trap (28.7 ± 5.8 nontargets per trap), TR 290 experimental trap (16.1 ± 3.2 nontargets per trap), and the small circle trap (5.9 ± 1.2 nontargets per trap). The sticky band was the only trap that had nontarget vertebrate captures, including skinks and birds (Fig. 7). Trap design had a significant effect on the total number of nontarget organisms captured in Pennsylvania (F = 100.7; df = 3, 414; P < 0.001). Sticky bands (34.2 ± 2.1 nontargets per trap) caught significantly more organisms than the TR 290 experimental trap (7.5 ± 8.0 nontargets per trap), modified circle trap (6.7 ± 0.7 nontargets per trap), and the small circle trap (4.3 ± 0.4 nontargets per trap) (Fig. 8).
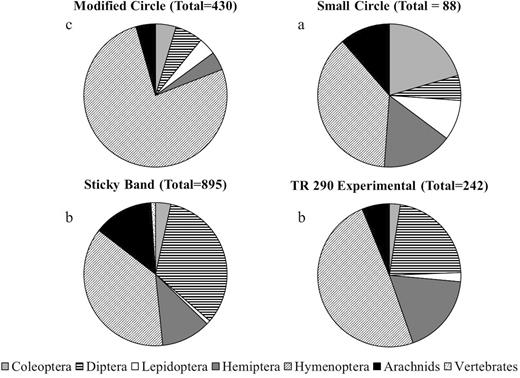
Nontarget captures for all unbaited trap designs evaluated in 2019 in Winchester, VA. Letters denote significant differences among trap treatments for total nontargets captured at P < 0.05.
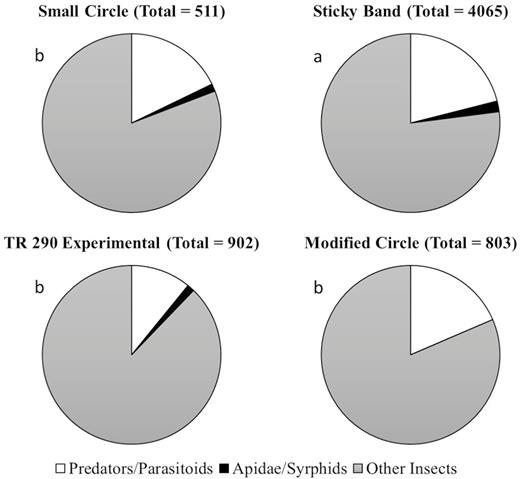
Nontarget captures for all unbaited trap designs evaluated in 2019 in Reading, PA. Letters denote significant differences among trap treatments for total nontargets captured.
Sticky band occlusion
There were no significant differences in L. delicatula nymphal capture on occluded sticky bands when compared to the control sticky bands (F = 0.5; df = 3, 80; P = 0.706) (Fig. 9A). For adults, there was a significant effect of occlusion (χ 2 = 28.6; df = 3; P < 0.001). The control band had significantly greater adult captures than bands with 25% (P = 0.001), 50% (P = 0.001), and 75% (P < 0.001) occluded areas (Fig. 9B).
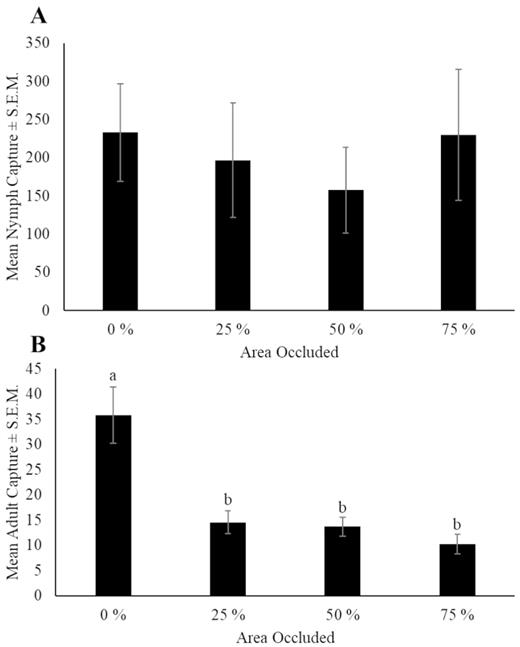
Mean number of Lycorma delicatula ± SEM (A) nymphs and (B) adults captured per trap per week on sticky bands with occluded areas in 2019 in Winchester, VA. Letters denote significance levels at P < 0.05.
Lure evaluation
There were no significant differences in captures of nymphs (χ 2 = 0.3; df = 1; P = 0.563) or adults (χ 2 = 0.1; df = 1; P = 0.723) on sticky bands baited with methyl salicylate lures compared with unbaited sticky bands (Fig. 10A and B). Season-long capture on all sticky bands deployed for the lure evaluation trial was low with 35 and 36 nymphs and 5 and 4 adults captured on baited and unbaited bands, respectively.
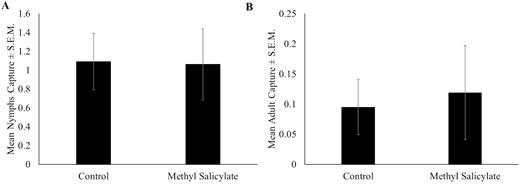
Mean number of Lycorma delicatula ± SEM (A) nymphs and (B) adults captured per week on sticky bands baited with methyl salicylate or on unbaited sticky bands in 2019 in Winchester, VA.
Discussion
It is clear that circle trap designs offer an effective alternative to standard sticky bands with reduced nontarget captures. Circle traps were originally designed to capture pecan weevil adults (Mulder et al. 1997) but have also been used to monitor plum curculio adult activity (Leskey and Wright 2004a, Leskey 2006). Francese et al. (2020) evaluated a circle trap design similar to these, but with a larger jar top and made of wire screen. Similarly, our 2018 and 2019 studies demonstrated that circle traps previously used for plum curculio research could capture adult L. delicatula in numbers comparable to standard sticky bands. These results were consistent between two sites experiencing different levels of population density, and deployment on a variety of known host trees. Ultimately, there are a number of specific differences among all circle trap designs evaluated for L. delicatula that likely contribute to overall trap efficacy.
First, the size of the collection device is critical. In 2018 and 2019, we deployed standard circle traps provisioned with a small collection funnel designed for boll weevil (Dickerson 1986). While these smaller collection devices did capture L. delicatula, the larger collection device on modified circle traps deployed in 2019 was more effective, particularly for adults (Fig. 6). Second, effectiveness of circle traps require that the large, folded screen funnel lay flush to the tree trunk and encircle the majority of the trunk. In 2018, we evaluated protruding circle traps that had a larger collection device but did not encircle the entire trunk. This design resulted in generally lower adult captures likely because the trap did not provide a continuous guiding surface around the trunk necessary to lead walking L. delicatula into the trap collection device. Similar observations have been made for both plum curculio adults (Leskey 2006) and brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentotomidae), nymphs (Acebes‐Doria et al. 2016, 2017). Moreover, like plum curculio adults (Leskey and Prokopy 2002) and H. halys nymphs (Acebes‐Doria et al. 2016), L. delicatula demonstrate negative gravitaxis (Kim et al. 2011), climbing up vertical surfaces (L. J. Nixon and T. C. Leskey, unpublished data). Finally, the screen material itself seemed to have an impact on overall captures. In 2019, we compared a modified circle trap comprised of vinyl-coated polyester screen with the TR 290 circle trap comprised of wire screen. Both were a similar design with large collection devices, but TR 290 (wire screen) captured significantly fewer L. delicatula adults compared with the modified circle trap (vinyl screen) in Pennsylvania. Based on visual observations in the field, it appears that wire screen is less acceptable to walking L. delicatula adults and nymphs as individuals attempted to walk on the screen but then subsequently dropped out of the trap. Thus, based on our data, a circle trap should: 1) utilize a larger collection jar; 2) provide a guiding edge encircling the tree trunk; and 3) be comprised of vinyl (not wire) screen to most efficiently capture L. delicatula.
While sticky bands have been used extensively to monitor L. delicatula presence (Dara et al. 2015), they have considerable drawbacks. First, glue-based traps can become saturated and prevent capture of the target species (Schmid et al. 2006). In our studies, occlusion of the sticky band itself, while not affecting nymphal captures, did have a significant effect on adult captures. Just 25% of the sticky band becoming occluded reduced adult captures significantly. Preovipositional adults can spend up to 2 mo feeding before they are ready to reproduce and may settle onto a feeding spot, only moving if their food source becomes depleted (Baker et al. 2019). This indicates that during this period, and later in the season, when adult movement is believed to be more extensive (Baker et al. 2019), sticky bands may not succeed in capturing adults if sticky bands are not in pristine condition, leading to false negatives in monitoring or detection programs. Second, some sticky band brands may not adequately be able to retain older nymphs and adults (Cooperband et al. 2019). It would be useful to quantify sticky band retention capacity to establish the overall efficacy of these traps if they continue to be used for monitoring. Third, sticky bands captured significantly more nontargets compared with circle trap designs, this result was significant at both Pennsylvania and Virginia sites with differing recording protocol. The largest proportion of nontargets captured in circle type traps deployed in Virginia were Hymenoptera, of these the majority were Formicidae (Fig. 7). This is to be expected with an indiscriminate capture mechanism, with the same sort of issues exhibited by other sticky traps aimed at capturing pestiferous Heteroptera (Blackmer et al. 2008, Rodriguez-Saona et al. 2012). In our studies, while most nontargets were invertebrates, they also included vertebrates such as reptiles and birds. Capture of these nontargets is generally unacceptable to the public; thus, providing a trap design that significantly reduces these nontarget captures is critical.
In 2018 and 2019, we evaluated the attractiveness of commercially available methyl salicylate lures in association with traps deployed on host trees and vertical tree-mimicking posts, respectively. Methyl salicylate is an herbivore-induced volatile (Tholl et al. 2006) released by a number of L. delicatula host plants, including soybean, grapevine, and black locust (Van Den Boom et al. 2004). Methyl salicylate has been identified as an attractant to a range of beneficial insects (James 2003a,b, 2006; Zhu and Park 2005), and a repellent for Aedes aegypti (Dekker et al. 2011). For L. delicatula, this compound was shown to be attractive in laboratory and field studies (Cooperband et al. 2019). However, in both of our studies, the presence of the methyl salicylate lure did not increase captures of traps deployed on host trees or on sticky bands deployed on vertical tree-mimicking posts. Posts were used to remove any host plant effect (e.g., plant volatiles) and dark, vertical stimuli are thought to be attractive to L. delicatula (Domingue and Baker 2019). One possibility for the reason for differences in these two studies is due to host plant competition. For example, plum curculio are attracted to traps baited with benzaldehyde lures in significantly greater numbers when deployed in an open field compared with those deployed within an orchard. Declines in captures were thought to be related to both competition from olfactory and visual stimuli provided by host apples trees (Leskey and Wright 2004b). Olfactory stimuli for leafhopper species are frequently combined with visual cues to produce a synergistic effect for monitoring systems (Todd et al. 1990, Bullas-Appleton et al. 2004, Patt and Sétamou 2014). Visual interception traps consisting of tall brown materials coated in Tangletrap (The Scotts Miracle-Gro Company, Marysville, OH) have been found to successfully capture adult L. delicatula (Francese et al. 2020). Thus, competition from surrounding habitat and host plants may reduce L. delicatula response to methyl salicylate lures.
It is important to note that because the lures do not increase L. delicatula capture, the traps currently developed are passive traps and collect L. delicatula that happen to crawl upwards on the host with a trap. Future study should focus on how well these passive traps estimate the surrounding population size, particularly at low densities, to enact appropriate management strategies. Additionally, more information is needed on trap performance across different life stages of L. delicatula with a known abundance. In both Pennsylvania and Virginia, more first and second instars were captured compared to later instars and adults in 2019 (Fig. 5). This could indicate either a higher population of early instars or a trap that is more efficient at this life stage.
Developing more sensitive monitoring systems for L. delicatula remains a key research priority essential to improved management of this pest. These systems may benefit from combining the current circle trap designs or upright vertical traps with other stimuli that are compatible with dispersal or other behaviors of this insect. Other systems have shown the efficacy of pairing traps with olfactory stimuli, for example, identification of the H. halys pheromone (Khrimian et al. 2014, Leskey et al. 2015) enabled season-long monitoring of this species where the previous lure (Khrimian et al. 2008) was only attractive in the late season (Leskey et al. 2012). However, synergism of stimuli employing other modalities (e.g., visual or substrate-borne) may warrant further exploration. It is clear as L. delicatula invades new regions, a cooperative and collaborative approach to research and outreach activities will be necessary to speed the answers generated by researchers, as has been done for other invasive species (Ludwick et al. 2020).
Acknowledgments
Thank you to Cameron Scorza and Elizabeth Deecher for excellent technical work in the field, and Lee Carper, Chris Hott, and Tony Rugh for trap construction. This work was funded by Plant Protection Act Section 7721 Funding, USDA-ARS Project (8080-21000-030-00-D), USDA-NIFA SCRI CAP Award (2019-51181-30014), and USDA APHIS Cooperative Agreement (AP19PPQFO000C369).
References Cited



