-
PDF
- Split View
-
Views
-
Cite
Cite
A. Hakeem, J. F. Grant, G. J. Wiggins, P. L. Lambdin, F. A. Hale, D. S. Buckley, J. R. Rhea, J. P. Parkman, G. Taylor, Factors Affecting Establishment and Recovery of Sasajiscymnus tsugae (Coleoptera: Coccinellidae), an Introduced Predator of Hemlock Woolly Adelgid (Hemiptera: Adelgidae) on Eastern Hemlock (Pinales: Pinaceae), Environmental Entomology, Volume 42, Issue 6, 1 December 2013, Pages 1272–1280, https://doi.org/10.1603/EN13017
Close - Share Icon Share
Abstract
To reduce populations of hemlock woolly adelgid, Adelges tsugae Annand (Hemiptera: Adelgidae), >500,000 Sasajiscymnus tsugae (Sasaji and McClure) (Coleoptera: Coccinellidae) have been released in the Great Smoky Mountains National Park since 2002. To determine factors affecting establishment and recovery of these predatory beetles, 65 single release sites were sampled using beat sheets from 2008 to 2012. Several abiotic and biotic factors were evaluated for their association with establishment and recovery of S. tsugae. Information on predatory beetle releases (location, year of release, number released, and season of release), topographic features (elevation, slope, Beers transformed aspect, and topographic relative moisture index), and temperature data (minimum and maximum temperatures 1 d after release and average minimum and maximum temperatures 7 d after release) were obtained from Great Smoky Mountains National Park personnel. These factors were evaluated using stepwise logistic regression and Pearson correlation. S. tsugae was recovered from 13 sites 2 to 10 yr after release, and the greatest number was recovered from 2002 release sites. Regression indicated establishment and recovery was negatively associated with year of release and positively associated with the average maximum temperature 7 d after release and elevation (generally, recovery increased as temperatures increased). Several significant correlations were found between presence and number of S. tsugae and year of release, season of release, and temperature variables. These results indicate that releases of S. tsugae should be made in warmer (≍10-25°C) temperatures and monitored for at least 5 yr after releases to enhance establishment and recovery efforts.
Eastern hemlock, Tsuga canadensis Carrière, is an important component of many eastern forest types (Eyre 1982) and has been designated a foundation species. Eastern hemlock provides fundamental structure to the forest ecosystem, facilitates communities of organisms through habitat creation, is irreplaceable by another species, and sustains ecosystem services (i.e., habitat for fish, reduced erosion, water filtration, and timber production) (Dayton 1972, Ellison et al. 2005, Sackett et al. 2011). Healthy hemlock stands also regulate forest floor moisture, decomposition, and nitrogen cycling, and restrict vines and shrubs by limiting light and other resources (Kizlinski et al. 2002). A large number of vertebrates, birds, fish, and insects are associated with eastern hemlock and the forests of which they are a component (Reay et al. 1990, DeGraaf et al. 1992, Buck et al. 2005, Dilling et al. 2007).
Eastern hemlock is distributed throughout the northeastern and Appalachian regions of North America extending from Nova Scotia to Georgia and westward to Minnesota (Ward et al. 2004, U.S. Department of Agriculture–Natural Resources Conservation Service [USDA–NRCS] 2012). The Great Smoky Mountains National Park (GRSM) is located in the southern Appalachians and comprises 210,876 ha (National Park Service [NPS] 2012). Within GRSM, the total hemlock resources cover ≈55,442 ha with >5,665 ha of hemlock-dominated forests (Yost et al. 1994, Soehn et al. 2005, Webster 2010).
Hemlock woolly adelgid, Adelges tsugae Annand (Hemiptera: Adelgidae), was first reported in the GRSM in 2002, but may have been present and undetected for several years (Soehn et al. 2005). In the eastern United States, hemlock woolly adelgid has three generations, two of which (the sistens and progrediens) are parthenogenic and reproduce on hemlock (McClure 1989, Deal 2007). The third generation (the sexuparae) has winged adults, and immatures develop in conjunction with progrediens on hemlock from heterogeneous egg masses oviposited by the sistens. However, adult sexuparae, which require an alternate host unavailable in the United States, are not known to reproduce in this region (McClure 1989, Deal 2007). In Tennessee, sistens generally begin to oviposit in early February, with egg hatch initiating in early March (Deal 2007). On hatching, progrediens and sexuparae quickly develop to adults, and oviposition of sisten eggs by progrediens begins in 6 to 8 wk. Winged adult sexuparae are usually observed in May. Sisten eggs hatch into crawlers that settle and begin aestivation in mid to late June, and development resumes in early to mid-October. Owing to climatic differences, sistens may oviposit and progrediens and sexuparae may hatch 1 mo earlier and progrediens may begin oviposition 2 mo earlier in Tennessee than in New England (McClure 1989, Deal 2007).
Hemlock woolly adelgid feeds at the base of needles on stored sugars in xylem ray parenchyma (Young et al. 1995). Owing to prolonged feeding by hemlock woolly adelgid, hemlock mortality is more rapid in southern than northern forests (Nuckolls et al. 2009, Spaulding and Rieske 2010). In the southern Appalachians, hemlock tree mortality occurs 3–6 yr after infestation of hemlock woolly adelgid, while in the northeastern United States, hemlock mortality occurs 4–10 yr after infestation (NPS 2005, Spaulding and Rieske 2010, Ford et al. 2012). Extensive mortality of eastern hemlock may be because of tree susceptibility, high numbers of hemlock woolly adelgid, and low populations of native predators of hemlock woolly adelgid (Cheah and McClure 1996).
A suite of predatory insect species, some of which feed on hemlock woolly adelgid, has been collected from various hemlock species worldwide. For example, 54 lady beetle species were collected in China from three hemlock species—Tsuga dumosa (D. Don) Eichler, Tsuga forrestii Downie, and Tsuga chinensis (Franchet) Pritzel (Yu et al. 2000). Furthermore, 55 predatory species representing 14 families were recovered from western hemlock, Tsuga heterophylla (Rafinesque-Schmaltz) Sargent, in the western United States. The abundances of larval Laricobius spp., adult Laricobius nigrinus Fender (Coleoptera: Derodontidae), and adult Leucopis argenticollis Zetterstedt (Diptera: Chamaemyiidae) on western hemlock were found to be positively correlated to hemlock woolly adelgid, and considered to be hemlock woolly adelgid specialist predators (Kohler et al. 2008). In the eastern United States, >60 generalist predatory species belonging to 21 families in five orders have been documented from eastern hemlock (Wallace and Hain 2000, Buck et al. 2005). However, none of the species found on eastern hemlock is a specialist predator of hemlock woolly adelgid.
To increase populations of natural enemies of hemlock woolly adelgid in the eastern United States, a biological control program was initiated in 1993. As part of the biological control program against hemlock woolly adelgid, >3.5 million Sasajiscymnus tsugae (Sasaji and McClure) (Coleoptera: Coccinellidae) and 102,000 L. nigrinus have been released in the eastern United States. Of these, ≈500,000 S. tsugae and ≈12,000 L. nigrinus were released in the GRSM from 2002 to 2007 and 2004 to 2007, respectively (Grant et al. 2010c, Webster 2010, Jetton et al. 2011). Adults of both predatory species and the larvae of S. tsugae feed on all stages of hemlock woolly adelgid, but larvae of L. nigrinus only feed on eggs of hemlock woolly adelgid (Cheah and McClure 1996, Cheah 2004, Salom 2004). Because it is able to feed on all stages of hemlock woolly adelgid, it is important to note that suitable food resources are available year-round for S. tsugae.
Periodic recoveries of these two predatory species in areas of release are encouraging. In a study examining establishment of L. nigrinus throughout the eastern United States, L. nigrinus was recovered from 13 of 22 release sites, and its establishment was positively associated with minimum winter temperature and number of beetles released (Mausel et al. 2010). In addition, L. nigrinus was recovered from three sites (27.3%; 3 of 11 sampled sites) from GRSM (Grant et al. 2010b), and the number of adult L. nigrinus collected at one site in North Carolina increased from ≈50 in November 2009 to >550 in April 2010 (McDonald et al. 2010).
Likewise, S. tsugae has been recovered in several areas of release in the United States. In the northeastern United States, adults and larvae of S. tsugae were recovered from several sites 5 yr after release and had dispersed 0.93 km from the original release site (Blumenthal 2002, McClure and Cheah 2002, Cheah et al. 2005). In North Carolina, adult S. tsugae were recovered from several release sites 1 to 2 yr after releases (McDonald et al. 2008). In a study conducted in Tennessee near GRSM, S. tsugae released in whole-tree canopy enclosures were recovered for several consecutive years after the enclosures were removed (Grant et al. 2010a, Hakeem et al. 2010, Wiggins et al. 2010). Furthermore, coexistence of S. tsugae and L. nigrinus has been documented in some release sites (Hakeem et al. 2010, 2011), which indicates that these predatory beetles are compatible and may enhance biological control efforts against hemlock woolly adelgid.
Despite the widespread releases of S. tsugae and its sporadic recovery in several areas of the eastern United States, little is known about the abiotic and biotic factors that may influence establishment and recovery of populations of S. tsugae. Knowledge of these factors would enhance current biological control programs against hemlock woolly adelgid by increasing the probability of establishment of S. tsugae. In 2008, a study was initiated in GRSM to determine the incidence of S. tsugae at release sites and to identify abiotic and biotic factors that affect establishment and recovery of these introduced predatory beetles.
Materials and Methods
Assessment of Establishment of S. tsugae.
To evaluate the extent of establishment of S. tsugae released against hemlock woolly adelgid between 2002 and 2007 in GRSM, 65 beetle release sites were sampled from 2008 to 2012. It is important to note that only one beetle release was made at each site. Location and release information, such as release date and number released, for each site was obtained from GRSM personnel, and sites were located using a Garmin global positioning system (GPS) map 60 CSx GPS unit. In the sites examined during this study, releases of S. tsugae were made from 2 June to 10 July 2002, 9 April to 5 June 2003, 24 February to 24 May 2004, 24 January to 30 June 2005, 26 January to 22 June 2006, and 24 January to 9 May 2007. Sampling was conducted from 1 May to 17 July 2008, 24 February to 16 June 2009, 12 April to 8 July 2010, 4 May to 14 June 2011, and 12 June to 26 June 2012, and some sites were sampled multiple (as many as three) times. These sampling dates coincided with expected activity of S. tsugae in the field, as adults and larvae of S. tsugae have been observed in release sites in Tennessee from February through November (Deal 2007, Grant et al. 2010b, Wiggins et al. 2010). At each site, beat-sheet sampling was conducted for four person-hours. Sampling was conducted within 100 m of the release site, and beat-sheet sampling was performed by striking accessible branches from ground level to a height of ≈2.5 m five to eight times with a wooden rod while holding a canvas beat sheet (71 by 71 cm) beneath the branch to catch adults and larvae of dislodged predators. Depending on the size of the tree, one to three beat-sheet samples were collected per tree. Voucher specimens were placed in 2-ml vials and taken to the laboratory for identification and confirmation. Vouchers of suspected larvae were maintained in a jar (2.64 liters) with hemlock twigs (10–15 cm) infested with hemlock woolly adelgid until emergence as an adult. Specimens were identified and confirmed by the authors (A.H. and G.J.W.) and staff at the Lindsay Young Beneficial Insects Laboratory (J.P.P. and other personnel, University of Tennessee). Voucher specimens were stored at the Integrated Pest Management and Biological Control Laboratory at the University of Tennessee.
Assessment of Abiotic and Biotic Factors Affecting Establishment and Recovery of S. tsugae.
Several factors associated with releases of S. tsugae were assessed to evaluate their influence on establishment and recovery of predatory beetle populations. The number of adult S. tsuage released, the year of release, and the season of release, which was designated by release date (Winter: 24 January–17 March [season=1]; Spring: 22 March–16 June [season=2]; Summer: 25 June–10 July [season=3]; no releases from 11 July to 23 January were assessed), were evaluated for their influence on establishment. Topographic variables that characterize physical site conditions (such as aspect, elevation, and slope) were obtained from GRSM personnel for each site. Elevation data (10-m resolution) were included, and slope was calculated from the elevation data for all release sites in ArcMap 10 (Environmental Systems Resource Institute [ESRI] 2010; elevations for all recovery sites are provided in Table 1). Aspect was transformed using Beers method (Beers et al. 1966), which provides a weighted value to indicate site exposure to sunlight, and was included in analyses. Topographic relative moisture index (TRMI; Parker 1982), which is a measure of potential soil moisture calculated from elevation and slope, also was analyzed for influence on establishment of S. tsugae. Climate data are continually collected from several weather stations throughout GRSM, and temperature data for the release period of each study year were obtained from 10 weather stations from GRSM personnel (Fig. 1). Temperature data, including minimum and maximum temperatures for the day after releases and the average minimum and maximum temperatures for the 7-d period after releases, were acquired from the weather station closest to each release site.
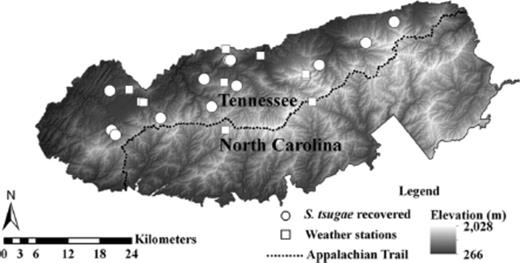
Locations of weather stations (n=10) and S. tsugae recovery sites (n=13) at the GRSM, 2008–2012.
Sites (n=13) from where S. tsugae were recovered after releases between 2002 and 2007 at GRSM

Adults were released at all sites except for Jakes Creek, where eggs were placed in the field.
Sites (n=13) from where S. tsugae were recovered after releases between 2002 and 2007 at GRSM

Adults were released at all sites except for Jakes Creek, where eggs were placed in the field.
Data Analysis.
Data were analyzed using logistic regression and Pearson correlation in SPSS 18.0 (SPSS 2009). All repeated samples were entered as a separate sampling date. Backward stepwise logistic regression was used to select significant variables (P=0.05) associated with establishment of S. tsugae. The dependent variable was presence of S. tsugae (i.e., recovery at a release site) and the independent variables were year of release, number of S. tsugae released, season of release, elevation, slope, Beers transformed aspect, TRMI, minimum and maximum temperatures 1 d after release, and average minimum and maximum temperatures for the 7-d period after release. To further evaluate the association of these factors with establishment of S. tsugae, Pearson correlations were performed between the independent variables listed above and presence of S. tsugae, number of adult S. tsugae, number of larval S. tsugae, and total number of S. tsugae at recovery sites.
Results and Discussion
Assessment of Establishment of S. tsugae.
From 2008 to 2012, 614 S. tsugae (n=316 adults and n=298 larvae) were recovered in beat-sheet sampling from 13 of 65 (20%) sites in GRSM (Fig. 1; Table 1). Sites where releases were made during 2002 had the greatest percent recovery of S. tsugae (8 of 10 sites, 80%), followed by 2003 (2 of 5 sites, 40%), 2006 (1 of 15 sites, 7%), and 2007 (2 of 19 sites, 11%; Table 1). No recoveries were made from 2004 (n=7) or 2005 (n=9) release sites.
These findings underscore the importance of continued and repeated monitoring of biological control agents after releases. Recoveries of S. tsugae were made 2 to 10 yr after releases, illustrating the importance of monitoring several years after releases. Furthermore, S. tsugae was not recovered from 6 of the 13 recovery sites the first time they were sampled, but was recovered from all six of these sites after a second sampling was conducted, illustrating the importance of repeated sampling at the same site. Of the sites where S. tsugae was not recovered, four release sites were sampled twice, and all others were sampled once. Lack of recovery of S. tsugae from these sites does not necessarily mean that S. tsugae is not established, and continued sampling at these sites may yield S. tsugae in the future.
This study also documented several recoveries of S. tsugae in areas where multiple or clusters of release sites occurred. Three release sites near Laurel Falls were in proximity to each other (≈500 m), and the total number of S. tsugae released in 2002 at these sites was 4,827 (Table 1). In 2008, S. tsugae was recovered from two of these sites, and S. tsugae was recovered from all three sites in 2009 and 2012. In addition, in 2012 sampling, the numbers of S. tsugae collected were 37% higher (n=336) than in 2008 (n=123; Fig. 2), which suggests that populations of S. tsugae are increasing over time (Table 1; Fig. 2). Larvae and adults of S. tsugae also were recovered from two of three release sites along the Gregory Ridge Trail (within ≈1 km of each other) and one of four release sites near Buckhorn Gap (within 1.5 km of each other). Documentation of S. tsugae in these areas of multiple releases may indicate that multiple or clusters of releases in proximity can aid these beetles in finding mates and prey, promote reproduction, and increase the likelihood of establishment and recovery of S. tsugae.
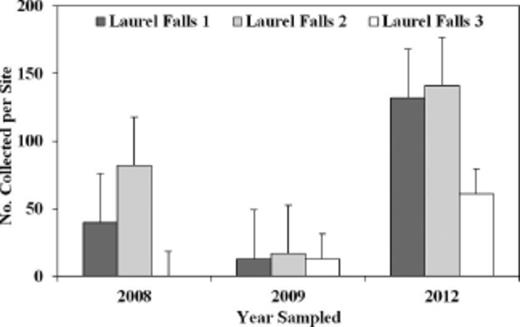
Recovery of S. tsugae on eastern hemlock from repeated sampling in release sites near Laurel Falls at GRSM, 2008–2012.
Assessment of Abiotic and Biotic Factors Affecting Establishment and Recovery of S. tsugae.
Several factors were identified as important to establishment and recovery of S. tsugae in GRSM. Backward stepwise logistic regression selected the year of release (P=0.001), average maximum temperature 7 d after release (P=0.035), and elevation (P=0.031) as significant variables associated with establishment and recovery of S. tsugae on eastern hemlock in GRSM (Table 2). This model explains 58% (R2=0.580) of S. tsugae recovery on eastern hemlock in GRSM. No other variables were significant.
Variables that influenced establishment and recovery of S. tsugae at the GRSM selected by backward stepwise logistic regression (R2=0.580)
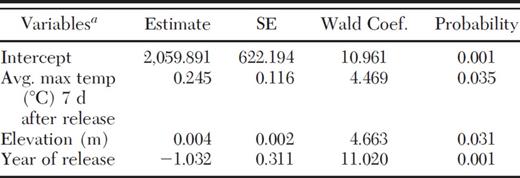
Specific information on elevation and temperature data associated with recovery sites is provided inTables 1 and 4, respectively.
Variables that influenced establishment and recovery of S. tsugae at the GRSM selected by backward stepwise logistic regression (R2=0.580)

Specific information on elevation and temperature data associated with recovery sites is provided inTables 1 and 4, respectively.
The significant inverse relationship between year of release and presence of S. tsugae suggests that the likelihood of detecting establishment of S. tsugae increases as the duration of time since release increases. A similar association between year of release and presence of S. tsugae was previously documented (Hakeem et al. 2010). When coccinellid beetles, such as S. tsugae, are released, they may disperse and have difficulty finding mates. This dispersal may slow the rate of population growth, thus, requiring a longer period of time for populations to grow and attain detectable levels. For example, no S. tsugae was recovered from the 2002 release sites during sampling conducted in 2003 (Lambdin et al. 2006), yet S. tsugae was recovered from eight of these sites 6 to 10 yr after releases. In addition, S. tsugae was not recovered from Lynn Camp (2002 release site) in 2008 sampling, but was recovered in 2012 sampling, which suggests that S. tsugae may have been present in 2008, but population levels were undetectable.
The significance of the average maximum temperature 7 d after release indicates that warmer temperatures (≈10 to 25°C) in the field are more conducive to establishment. This finding also may illustrate the necessity of acclimatizing beetles to field conditions before release. Insectaries rear and maintain colonies of S. tsugae at warm (≈24 to 30°C) temperatures. Thus, temperatures similar to rearing conditions at the time of field release may enhance initial survival of S. tsugae. Early season releases may require acclimatization to minimize inactivity, mortality, or both, because of sudden exposure to low temperatures. Releases made in April through June may not require acclimatization, as temperatures in the field during this time of year may be similar to rearing conditions, allowing predators to adjust to any minor temperature differences between insectaries and the field.
The significance of elevation in the regression model suggests that certain elevations may be more suitable for establishment of S. tsugae. It is important to note, however, that S. tsugae were recovered from elevations ranging from 669 to 1,086 m (Table 1). Seven of the 13 recovery sites were above 915 m (3,000 feet), and the three sites from which the most S. tsugae were recovered (Laurel Falls 1, 2, and 3) are all above this elevation. However, no significant associations were identified between establishment or recovery of S. tsugae and elevation using Pearson correlation (Table 3). Interactions between temperature and elevation of certain release sites may have enhanced establishment, as warmer temperatures (i.e., above 0°C) contribute to establishment of S. tsugae. For example, releases in all but one recovery site were made in the spring or summer of the year (Table 1). The temperatures during this time of year at the corresponding elevations may have been more conducive to survival of S. tsugae than other temperature–elevation interactions and led to population establishment. Perhaps the climate associated with the higher elevations at Laurel Falls contributed to establishment, recovery, or both, of S. tsugae at these sites. Owing to the disparity of the importance of elevation between regression and correlation analyses, and the uncertainty of the interactions between temperature and elevation, further research is needed to determine more precisely the role of elevation in establishment of introduced predators of hemlock woolly adelgid.
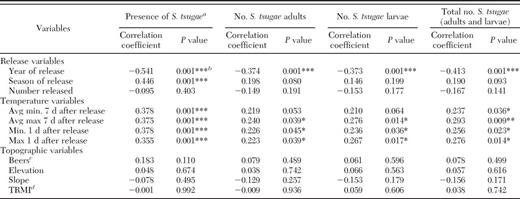
Correlation between abiotic and biotic factors and S. tsugae establishment and recovery at the GRSM, 2008–2012
Specific information on elevation and temperature data associated with recovery sites is provided inTables 1 and 4, respectively.
Probabilities: * P < 0.05, ** P < 0.01, *** P < 0.001; if no symbol is present, no significant correlation was found.
Beers transformed aspect is a measure of exposure to direct sunlight based on original aspect (Beers et al. 1966).
Topographic relative moisture index is an estimate of soil moisture based on topography (Parker 1982).
Correlation between abiotic and biotic factors and S. tsugae establishment and recovery at the GRSM, 2008–2012

Specific information on elevation and temperature data associated with recovery sites is provided inTables 1 and 4, respectively.
Probabilities: * P < 0.05, ** P < 0.01, *** P < 0.001; if no symbol is present, no significant correlation was found.
Beers transformed aspect is a measure of exposure to direct sunlight based on original aspect (Beers et al. 1966).
Topographic relative moisture index is an estimate of soil moisture based on topography (Parker 1982).
Pearson correlation analysis supports the results obtained from regression analysis and identified several other abiotic and biotic factors significantly associated with presence and numbers of S. tsugae at release sites (Table 3). A negative association was documented between presence of S. tsugae and year of release (P=0.001), and a positive association was documented between presence of S. tsugae and season of release (P=0.001). Temperature (average minimum [P=0.001] and maximum [P=0.001] temperatures 7 d after release and minimum [P=0.001] and maximum [P=0.001] temperatures 1 d after release) also was positively associated with presence of S. tsugae. The number of adult S. tsugae recovered was negatively associated with year of release (P=0.001) and positively associated with the average maximum (P=0.039) temperature 7 d after release and the minimum (P=0.045) and maximum (P=0.039) temperatures 1 d after release. The number of larval S. tsugae recovered was negatively associated with year of release (P=0.001) and positively associated with the average maximum (P=0.014) temperature 7 d after release and the minimum (P=0.036) and maximum (P=0.017) temperatures 1 d after release. The total number of S. tsugae (adults and larvae) recovered was negatively associated with year of release (P=0.001) and positively associated with the average minimum (P=0.036) and maximum (P=0.009) temperatures 7 d after release, as well as the minimum (P=0.023) and maximum (P=0.014) temperature 1 d after release. No other significant associations were documented (Table 3).
These findings further suggest that temperature after releases significantly influences establishment and time since release significantly increases the ability to detect S. tsugae. The significant negative relationship between year of release and the presence and number of S. tsugae supports the finding that a longer time period is required for populations of S. tsugae to increase to detectable levels than previously anticipated. The positive associations among various measures of S. tsugae and temperature at release sites further indicate that temperatures consistently above freezing within 1 wk of release increase the likelihood of establishment. The association between the presence of S. tsugae and the season of release also supports this finding. The importance of temperature after beetle releases seems intuitive, and this finding may be the most important factor for successful establishment of S. tsugae. The general activity (feeding, mating, predator avoidance, etc.) of most predatory beetles may be limited by cold temperatures. Studies in New England documented that adults of S. tsugae were able to survive temperatures consistently between −4 to 1°C (Cheah and McClure 2000) and were able to withstand minimum temperatures as low as −21.6°C (Cheah 2004); however, all S. tsugae observed were inactive during these cold periods. In addition, these beetles were not released in cold temperatures, rather they were released in warmer seasons and were monitored throughout the winter months. If cold temperatures prompt S. tsugae to remain inactive for too long after being accustomed to artificial rearing conditions, these beetles may starve or die because of sudden exposure, despite their purported ability to withstand cold temperatures. In the current study, in all but one site S. tsugae established from single releases made when the average minimum and maximum temperatures 7 d after releases ranged from 7.5 and 28.6°C, respectively (Table 4). The one exception was a release with average temperatures 7 d after release ranging from −1.3 to 6.1°C, but the coldest of these temperatures occurred 5 to 7 d after releases (minimum and maximum temperatures the day after release were 3.9 and 7.1°C, respectively), possibly giving the beetles time to feed and seek shelter before subzero temperatures occurred (Table 4). These findings may have implications for releases of other biological control agents of hemlock woolly adelgid. Keena and Montgomery (2010) found that larvae of Scymnus camptodromus (Yu and Liu) (Coleoptera: Coccinellidae), a potential predator of hemlock woolly adelgid imported from China and currently being studied in quarantine, may develop at temperatures as low as 10°C but may survive best at 15 to 20°C. Based on current findings, field studies incorporating releases at different temperature ranges should be conducted to document the optimum conditions for the survival of this species in the field after release.
Sites where S. tsugae were recovered, and the corresponding temperatures at the initial time of release at GRSM, 2002–2007
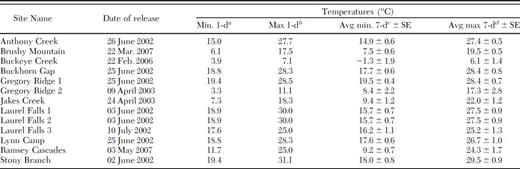
Minimum temperature 1 d after release.
Maximum temperature 1 d after release.
Minimum average temperature for the 7-d period after release.
Maximum average temperature for the 7-d period after release.
Sites where S. tsugae were recovered, and the corresponding temperatures at the initial time of release at GRSM, 2002–2007

Minimum temperature 1 d after release.
Maximum temperature 1 d after release.
Minimum average temperature for the 7-d period after release.
Maximum average temperature for the 7-d period after release.
Reduction of impacts of hemlock woolly adelgid to eastern hemlock is important, as no other tree species is available to replace this long-lived evergreen and shade-tolerant tree. Loss of eastern hemlock because of hemlock woolly adelgid may have negative impacts on riparian and terrestrial communities associated with eastern hemlock. Area-wide application of pesticides on eastern hemlock against hemlock woolly adelgid is not feasible because of high cost, inaccessibility, and practical constraints. The most feasible and economical long-term option to control hemlock woolly adelgid is through release of biological control agents, such as S. tsugae and other predators, that have limited nontarget effects. In general, abotic and biotic factors play an important role in the success of biological control programs. This research demonstrated that several temperature variables affect establishment and recovery of these introduced predatory beetles. Temperature at the time of release is critical for long-term establishment of S. tsugae, and releases may be most successful if made when temperatures average 10 to 25°C 7 d after release. Accordingly, establishment may be enhanced by releasing in spring and early summer when temperatures are consistently within this range. These findings may assist land managers, foresters, and other professionals engaged in biological control of hemlock woolly adelgid to determine the most effective time of year to release predatory beetles to increase their survival in the field. The efficacy and survival of S. tsugae in the eastern United States will be enhanced by these findings, and will lead to better management of hemlock woolly adelgid in GRSM and elsewhere. Long-term monitoring and repeated sampling at release sites will provide greater estimations of establishment of S. tsugae, and continued monitoring in GRSM will provide a better understanding of the factors influencing establishment of S. tsugae.
We thank Kristine Johnson, Thomas Colson, Jesse Webster, and Jim Renfro from the Great Smoky Mountains National Park for assistance with locating predator release sites, coordinating sampling, and acquiring data for analysis for this project. We thank Ann Reed for statistical guidance and assistance. We also thank Renee Follum, Josh Grant, Monica MacCarroll, Jared Oakes, David Paulsen, and Coleman Timberlake (University of Tennessee) for assisting with data collection. This project was funded in part by Cooperative Agreements with the USDA Forest Service, Southern Region, U.S. Department of Agriculture–Animal and Plant Health Inspection Service (USDA–APHIS; agreement 10-8247-0723-CA), and state and Federal appropriations.
References



