-
PDF
- Split View
-
Views
-
Cite
Cite
Peter J. Silk, Jon Sweeney, Junping Wu, Stephanie Sopow, Peter D. Mayo, David Magee, Contact Sex Pheromones Identified for Two Species of Longhorned Beetles (Coleoptera: Cerambycidae) Tetropium fuscum and T. cinnamopterum in the Subfamily Spondylidinae, Environmental Entomology, Volume 40, Issue 3, 1 June 2011, Pages 714–726, https://doi.org/10.1603/EN10213
Close - Share Icon Share
Abstract
Male Tetropium fuscum (F.) and T. cinnamopterum Kirby mated with live and dead (freeze-killed) conspecific females upon antennal contact, but did not respond to dead females after cuticular waxes were removed by hexane rinsing. Significantly fewer males of each species attempted to copulate with live or dead heterospecific females than with conspecifics, indicating that mate recognition was mediated by species-specific contact sex pheromones in the female's cuticular hydrocarbons. GC/MS analysis of T. fuscum elytra identified n-alkanes and mono-methyl branched alkanes of which 11-methylheptacosane and 3- and 5-methyltricosanes were dominant in females. Full male responses, including copulatory behavior, were restored with application of enantiomerically pure synthetic (S)-11-methyl-heptacosane at 40 μg /female (one female equivalent) but not with racemic or (R)-11-methyl-heptacosane. The cuticular hydrocarbons on T. cinnamopterum elytra included 11-methyl-heptacosane as well as n-alkanes, methyl-branched alkanes, mono-alkenes, and (Z, Z)-6, 9-alkadienes. (Z)-9-pentacosene, (Z)-9-heptacosene, and 11-methyl-heptacosane were female dominant, but only (Z)-9-pentacosene elicited precopulatory behaviors in conspecific males at levels similar to those behaviors elicited by unrinsed females, but elicited copulation in fewer than half of males. At female equivalent dosages (10 μg), neither (Z)-9-heptacosene nor (S)-11-methyl- heptacosane elicited responses in males that were significantly different from those responses to a rinsed female but when applied together, the proportion of males responding was significantly increased. 11-methyl-heptacosene is thus a contact pheromone component common to both species, which may explain the heterospecific mating attempts by some males.
Interest in the chemical ecology of the Cerambycidae has increased recently because of a number of interceptions of exotic cerambycids in North America (Haack and Cavey 1997, McCullough et al. 2006) and Europe. There is increased awareness of their economic importance in both forest and urban environments and in the role that they play in forest biodiversity (Hanks 1999). However, research is lacking on the role of semiochemicals as components mediating their behavior, although some recent findings concerning both short-range (contact) and long-range (volatile) sex pheromones as well as host volatiles (kairomones) have sparked a renewed interest (Allison et al. 2004, Millar et al. 2009).
The wax layer on the cuticle of insects is a complex mixture of nonpolar long-chain hydrocarbons and polar compounds (acids, aldehydes, alcohols, and ketones) that provides a hydrophobic barrier preventing desiccation (Gibbs 1998); some of these hydrocarbon components also function as contact pheromones (Howard and Blomquist 2005). Indeed, there is now some evidence showing that mate recognition in longhorn beetles is often mediated by contact chemoreception wherein males orient to live or freeze-killed females only after antennal contact, with subsequent copulatory behavior and coupling to the female genitalia (Ginzel and Hanks 2003 ; Ginzel et al. 2003a, b and 2006 ; Lacey et al. 2008). Enantiomer-specific olfactory responses are not uncommon in insects with long-range sex and aggregation pheromones (Borden et al. 1976, Tumlinson et al. 1977, Silverstein 1979, Mori 1998), but with the exception of Fukaya et al. (1997), Fukusaki et al. (1998), and Mori (2007), very few studies have been published on the effects of enantiomer-specific contact chemoreception.
Tetropium cinnamopterum Kirby (Coleoptera: Cerambycidae: Spondylidinae) (Bousquet et al. 2009) is a Nearctic, univoltine longhorn beetle with a broad longitudinal distribution across Canada (Raske 1973). Larvae feed on the phloem of spruce (Picea spp.) and occasionally pine trees (Pinus spp.) in subcortical galleries (Gardiner 1975, Drooz 1985 ,). Infestations by T. cinnamopterum are more prevalent in dying or recently killed trees (fire damaged or windblown), particularly in shaded stands (Richmond and Lejeune 1945, Connola et al. 1956, Post and Werner 1988).
Tetropium fuscum (F.) (Coleoptera: Cerambycidae: Spondylidinae) is a Palearctic species inadvertently introduced to Canada and discovered near the port of Halifax, Nova Scotia, Canada, in 1999 (Smith and Hurley 2000). In Europe, T. fuscum breeds primarily on moribund Norway spruce, Picea abies (L.) Karst. (Juutinen 1955). In Nova Scotia, it infests red spruce, P. rubens Sarg.; white spruce, P. glauca (Moench) Voss; black spruce, P. mariana (Mill.) B.S.P.; and Norway spruce, Picea abies (L.) H. Karst. (Smith and Humble 2000). In contrast to its behavior in Europe and to that of T. cinnamopterum, T. fuscum in Nova Scotia is infesting stressed and also apparently healthy spruce trees with full green crowns although stem analysis indicates susceptibility is associated with slow growth rates (O'Leary et al. 2003).
The co-occurrence of T. fuscum and T. cinnamopterum in Nova Scotia spruce forests has provided an opportunity to study the chemical ecology of both insects in terms of host interactions (kairomones), and long- and short-range chemoreception (volatile and contact sex pheromones, respectively). However, little is known about the reproductive behavior of longhorned beetles (Coleoptera: Cerambycidae) in the subfamily Spondylidinae and to date, no contact pheromones have been described. We provide evidence for contact sex pheromones in the cuticular hydrocarbons of female Tetropium fuscum (F.) and T. cinnamopterum Kirby, identify the major compounds responsible for eliciting mating behavior in males, and demonstrate chiral specificity in responses of male T. fuscum. Both intra- and inter-species interactions are also presented and discussed.
Materials and Methods
Source of Beetles.
Adult T. fuscum and T. cinnamopterum were reared from bolts (35-cm long × 20- to 40-cm diameter) cut from infested white or red spruce in Halifax, NS, and incubated at 20–22°C and 45% – 60% RH 15:9 h (L:D) cycle in a containment facility at the Atlantic Forestry Centre (AFC) in Fredericton, NB. Emerged beetles were collected 5 d per wk for 12 wk. Tetropium adults were identified to species (Smith and Humble 2000), separated by sex, and stored individually, unfed, in glass vials at 3–4°C until used. Voucher specimens have been deposited in the AFC insect collection.
Source of Chemicals and Instrumentation.
n-Alkanes (nC20 – nC29) were obtained from Sigma-Aldrich (Oakville, ON, Canada) or Alltech (IL) and were used without further purification. All other compounds were obtained from Sigma-Aldrich, Alfa-Aesar (MA) or were synthesized as noted below. All synthetic reactions were carried out under Argon.
NMR (1H and 13C) was carried out on a Varian Unity 400 MHz spectrometer in CDCl3 with TMS as internal standard. IR spectra were taken as liquid films on KBr disks on a Perkin Elmer 727B IR-spectrometer. Rotations were determined on neat samples or in hexane on a Perkin Elmer 241 polarimeter using the 589 sodium D-line.
Mass spectra were obtained by gas chromatography/mass spectrometry (GC/MS) on a Hewlett–Packard 5890 GC and a 5971 mass selective detector in the electron impact (unit resolution, EI, 70 eV) mode.
Synthesis of Methyl-branched Compounds.
Methyl-branched compounds identified on the cuticles of both T. fuscum and T. cinnamopterum were synthesized. The synthetic route chosen was tailored to the availability of starting materials.
7-Me-C23, 9-Me-C23, 11-Me-C23, 9-Me-C25, and 11-Me-C25 and 11-Me-C27 were prepared from Wittig coupling of a 2-alkanone with the appropriate (n-alkyl) triphenylphosphonium bromide and subsequent Pd/C-catalyzed hydrogenation with hydrogen gas of the resultant alkenes (Ginzel et al. 2003a). Thus, as an example, 7-Me-C23 was prepared as follows: 1.15 g (2 mmol) of n-hexadecyltriphenylphosponium bromide (Alfar-Aesar) was dissolved in 20 ml of anhydrous tetrahydrofuran (THF) in a 100-ml round-bottomed flask (rbf) then in an ice bath to 0°C. Then 2 ml (2 mmol) of the base, sodium hexamethyldisilazide (1M solution in THF), was added dropwise and the mixture stirred for 1 h and cooled to −78°C. A solution of 2-octanone (Aldrich) (0.26 g (2 mmol) in 2 ml THF) was then added dropwise and the reaction mixture slowly warmed to room temperature and stirred overnight. The mixture was quenched by the addition of water and extracted into hexane and washed with brine, dried over magnesium sulfate, filtered, and the solvent evaporated to afford 339.3 mg (50% yield) of alkene after purification by silica gel chromatography using hexane as eluent. GC/MS analysis indicated ca. equal quantities of cis- and trans-7-methyl-7-tricosenes (each M+ 336 amu). The alkene was taken up in anhydrous pentane (10 ml) and transferred to a Parr hydrogenation bottle. 5% Palladium on activated carbon was added and the bottle was flushed with hydrogen and the contents were placed under hydrogen pressure (50 psi) and shaken for 3 h. The solution was then filtered through a pad of celite and the pad was washed with diethyl ether (Ginzel et al. 2003a). The solvent was removed in vacuo to yield 237 mg (80%) of 7-methyl-C23 (>95% pure by GC/MS; M+ 338) after purification by silica gel chromatography by using hexane as eluent. Analogous reaction conditions, appropriate starting materials, and reagents yielded 9-Me-C23 (73%) after purification by silica gel chromatography by using hexane as eluent, 11-Me-C23 (42%) after purification by silica gel chromatography using hexane as eluent then purification with 5Å molecular sieve (this material was then taken up in 50 ml of iso-octane and refluxed for 3 h with 15 g activated (heated to 400°C for 6 h) 5Å molecular sieve which effectively removed most of the n-alkane side product [ O'Connor et al. 1962 ]), 9-Me-C25 (45%) after purification by silica gel chromatography by using hexane as eluent, 11-Me-C25 (31%) after purification by silica gel chromatography by using hexane as eluent, and then purification with 5Å molecular sieve and 11-Me-C27 (49%) after purification by silica gel chromatography using hexane as eluent. GC/MS analysis indicated that purity of all these methyl-branched hydrocarbons was >95%.
Synthetic 7-Me-C25 was prepared from Grignard coupling of 2-octanone with n-octadecylmagnesium chloride followed by acid-catalyzed (p-toluenesulfonic acid) dehydration of the resulting tertiary alcohol and Pd/C-catalyzed hydrogenation with hydrogen gas of the alkenes produced. Thus, a 100-ml rbf was charged with 20 ml of a 0.5 M solution of octadecylmagnesium chloride (Aldrich) in THF and cooled to 0°C. 0.64 g of 2-octanone in 2-ml dry THF was then added dropwise and the reaction mixture refluxed for 20 min and recooled to 0°C. The reaction mixture was then quenched by addition of aqueous saturated ammonium chloride solution and a 10% HCl solution was added. The solution was extracted with diethyl ether, washed with 10% NaOH, then brine, dried over magnesium sulfate and the solvent evaporated to yield 3.92 g of crude 7-methyl pentacosan-7-ol. This material was then taken up in a 100-ml rbf in 10-ml toluene and 200 mg of p-toluenesulfonic acid added. The flask was then fitted with a Dean-Stark trap (Aldrich) and the mixture was refluxed for 3 h. After cooling, 20 ml of hexane was added and the mixture washed with aqueous sodium bicarbonate (5%), then brine and dried over magnesium sulfate and concentrated on a rotary evaporater. The residue was then passed through a 2-cm plug of silica gel eluting with hexane to yield 1.84 g of alkene after purification by silica gel chromatography using hexane as eluent. GC/MS analysis indicated a mixture of 7-methyltricosenes and octacosane in about equal proportions. This mixture was subjected to Pd (5%)/C-catalyzed hydrogenation to give crude 7-methyl-C25 (≈ 40% by GC/MS; M+ 338) with octacosane as a major side-product (≈ 40%). This material was then subjected to Kugelrohr distillation (T=160°C), which removed the alkane and other minor impurities to yield 0.61 g (55%) of 7-methyl-C25. GC/MS analysis showed the final product to >95% pure. 5-Me-C23 was similarly prepared from 2-hexanone (≈94% pure; yield 84%).
5-Me-C25 was prepared from Grignard coupling of eicosylmagnesium bromide (prepared from 1-bromoeicosane) with 2-hexanone, dehydration and Pd/C-catalyzed hydrogenation with hydrogen gas as before. Thus, into a 100 ml rbf was placed 0.48 g (20 mmol) of magnesium powder, then 5 ml of 1-bromoeicosane solution (in anhydrous THF), a few crystals of iodine and 50 μl of 1.2-dibromoethane. After the Grignard reaction started, the mixture was stirred at 35°C ≈ 45°C while the remaining solution of 1-bromoeicosane (total: 3.61 g, 10 mmol in 20 ml anhydrous THF) was added dropwise. When the addition was complete, the mixture was stirred at 45°C until the bromide had been consumed. The resulting warm solution was cannulated into a clean dry flask under Argon, and 2-hexanone (0.8 g, 8 mmol) was added dropwise. The resulting mixture was stirred at room temperature overnight, quenched by adding saturated NH4Cl solution, and extracted with hexane, washed with 5% NaHCO3, brine, dried over MgSO4, and evaporated to produce 3.49 g of crude 5-methyl- pentacosan-5-ol. This material was then taken up in a 100-ml rbf in 10-ml toluene and 200 mg of p-toluenesulfonic acid added. After dehydration (procedure was the same as 7-methyl pentacosan-7-ol, described above) and Pd/C-catalyzed hydrogenation, followed by purification by activated 5Å molecular sieve and further Kugelrohr distillation (T=160°C), 704 mg (24%) of 5-Me-C25 were yielded. GC/MS analysis indicated a purity of >96%.
2-Me-C26 was prepared by using CuI-catalyzed Grignard coupling (Millar and Underhill 1986) of 5-methylhexyl magnesium iodide with 1-bromoeicosane under dry conditions. Thus, 2.49 g (9.46-mmol) triphenylphosphine and 0.64 g (9.46-mmol) imidazole were dissolved in 100 ml of a 75/25 diethylether/acetonitrile mixture in a 250-ml rbf and cooled to 0°C. Then, 2.40 g (9.46 mmol) of powdered iodine was slowly added in four portions while vigorously stirring the mixture over ≈ 20 min. The resulting yellow slurry was slowly warmed to 20°C during this process and then recooled to 0°C. 1.0 g (8.61 mmol) of 5-methylhexan-1-ol was then added dropwise over 15 min and the mixture warmed to 20°C, stirred 1 h and then cooled to 0°C when 100-ml pentane was added. The top layer was decanted off and 100 ml of a 5% aqueous sodium bicarbonate solution was added to the bottom layer and stirred. This layer was then extracted with 100 ml of pentane, the pentane extracts combined, concentrated and the residue triturated with pentane, combined and reconcentrated to yield 1.14 g (58%) of 5-methyl hexane-1-iodide after purification by silica gel chromatography using hexane as eluent. 1.14 g of this iodide was dissolved in 20 ml of dry THF under Argon in a 100-ml rbf to which was slowly added 126 mg of magnesium powder and gently stirred for 2 h at room temperature to form 5-methyl hexylmagnesium iodide. 0.91 g (2.51 mmol) of 1-bromoeicosane was placed into a separate 100-ml rbf with 20 ml of dry THF to which was added CuI (190.4 mg) and HMPA (1.8 ml). This stirred mixture was cooled to −23°C under Argon and the Grignard reagent added dropwise over 1 h and the mixture was allowed to slowly warm to room temperature and stirred overnight. To this mixture was added 100 ml of saturated aqueous ammonium chloride, extracted with diethyl ether, washed with brine and water, and dried over magnesium sulfate. Solvent was evaporated to yield 81 mg (9%) of 2-methyl hexacosane after purification by silica gel chromatography using hexane as eluent; treatment with 5Å molecular sieve in isooctane while refluxing for 3.5 h followed. GC/MS analysis showed the final product to be 96% pure but in low yield (<10%); In a similar reaction scheme, 3-Me-C23 (9%)was prepared from 1-iodo-3-methylpentane (from iodination of 3-methylpentanol) via a CuI-catalyzed Grignard alkylation with octadecylmagnesium choride in THF; 3-Me-C25 (4%) was obtained via a CuI-catalyzed Grignard coupling of 1-bromoeicosane with 3-methyl-pentylmagnesium iodide (made from 1-iodo-3-methylpentane).
Finally, 2-Me-C24 was prepared via a lithium tetrachlorocuprate (Li2CuCl4) catalyzed coupling of 5-methylhexan-1-ol tosylate with octadecylmagnesium chloride (Millar and Underhill 1986). Thus, to a 250-ml round-bottom flask was put 5-methyl-1-hexanol (1.0 g, 8.62 mmol), diethylether (50 ml), and tosyl chloride (1.8 g, 9.44 mmol) was added. The solution was cooled to 0°C and freshly powdered KOH (2.41 g) was added portion-wise over 15 min. After complete addition, the resulting mixture was stirred for 30 min and then ice water (100 ml) was added. The solution was extracted with diethyl ether (3 × 40 ml) and the combined organic phase was washed with brine, dried over MgSO4, filtered, and solvent evaporated to yield 2.11 g of 5-methylhexan-1-ol tosylate crude product. The crude 5-methylhexan-1-ol tosylate (1.01 g, 3.7 mmol) was dissolved in 50 ml THF and cooled to −78°C; octadecylmagnesium chloride (11.9 ml, 5.93 mmol) was slowly added dropwise followed by lithium tetrachlorocuprate (Li2CuCl4) (0.19 ml) and the reaction mixture was allowed to warm to room temperature and stirred overnight. After the reaction was complete, the solution was then acidified with 2N H2SO4, and 50 ml of diethylether was added, the solution washed with water twice, dried over MgSO4, filtered and the solvent evaporated to yield 0.86 g (15%) of 2-Me-C24 after purification by flash chromatography (hexane) and treatment with 4Å molecular sieve in hexane. GC/MS analysis indicated a purity of 5-Me-C25 of >96%.
Synthesis of (S)- and (R)-11-methylheptacosanes.
A Li2CuCl4 catalyzed coupling (Fouquet and Schlosser 1974) of commercially available (S)-citronellyl bromide ([α]D20=+6.8 (neat); Aldrich >98% ee; Zhao et al. 1998) with (1-tetradecyl)magnesium bromide furnished (R)-2, 6-dimethyl-2-docosene shown in Fig. 1 . This was oxidatively cleaved using ozonolysis to provide the aldehyde. The commercially available Wittig salt, (1-heptyl)phosphonium bromide, was coupled with the aldehyde above employing n-butyllithium (nBuLi) as base. Finally, palladium-catalyzed hydrogenation converted both the cis- and trans-alkene isomers into enantiomerically pure (S)-11-methylheptacosane.
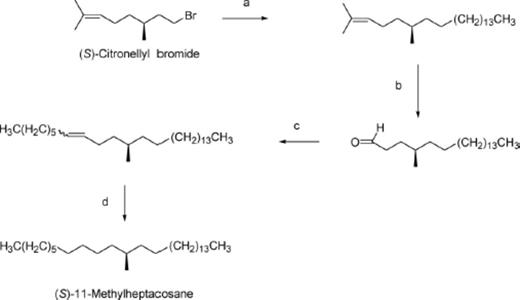
Asymmetric syntheses of (S)- and (R)-11-methylheptacosanes from (S)- and (R)-citronellyl bromides, respectively: Synthesis of (S)-11-Methylheptacosane: a) CH3(CH2)13MgBr, Li2CuCl4, −78°C – RT, 78% b) 1. O3, −78°C 2. PPh3, −78°C – RT, 57% c) CH3(CH2)6P+Ph3Br-, nBuLi, 54% d) H2, Pd/C, 17%.
(R)-11-Methylheptacosane was synthesized in an analogous fashion to (S)-11-methylheptacosane but (R)-citronellyl bromide was used as the starting material.
Spectral data for (S)-11-Me-C27: 1H NMR (CDCl3, 400 MHz): δ 1.20–1.34 (m, 49H; includes all methylene and the methine hydrogens), 0.88 (t, 6H, J=6.7 Hz), 0.84 (d, 3H, J=6.7 Hz). 13C NMR (CDCl3, 100 MHz): δ 37.1, 32.0, 30.1, 29.7 (18 signals), 29.7, 29.4, 27.1, 22.7, 19.7, 14.1 (two signals). MS: 43, 57 (base peak), 71, 85, 99, 113, 127, 140, 155, 168, 224, 252, 365, 379; [α]D20=−0.06 (3.33, hexanes). (R)-11-Me-C27: [α]D20=+0.09 (c=4.68, hexanes). The (R)-enantiomer was prepared in an identical fashion, employing (R)-citronellyl bromide ([α]D20=−6.8 (neat); Aldrich) as the starting material. It was concluded that (R)- and (S)-11-methylheptacosanes, produced from commercially available (R)- and (S)-citronellyl bromide (>98% ee) that have equal and opposite optical rotations, are also enantiomerically pure and retain the chirality of their respective starting material. The rotations of the starting materials obtained from Aldrich agree with literature values (Zhao et al. 1998).
(R)- and (S)-5-methyltricosanes were synthesized from (S)- and (R)-citronellyl bromide, respectively, in an identical fashion to the 7-methylheptacosanes, but a 2-carbon Li2CuCl4 catalyzed alkylation and a 15-carbon Wittig chain extension were employed [(S)-5-Me-C23: [α]D20=+0.77 (c=3.10, hexanes); (R)-5-methyl-C23: [α]D20=−0.70 (c=4.74, hexanes)]. (R)- and (S)-3-methyltricosanes were similarly synthesized from (S)- and (R)-citronellyl bromide, respectively; however, the ozonolysis was carried out first, followed by a 17-carbon Wittig chain extension, and then lithium aluminum hydride (LAH) reduction and hydrogenation. The spectral data for the 3- and 5-methyltricosanes were nearly identical to those of the 11-methylheptacosanes, except for the integration of the methylene protons in the 1H NMR spectra and the number of carbon signals in the 13C NMR spectra. [(S)-3-Me-C23: [α]D20=+4.0 (c=1.05, hexanes); (R)-3-Me-C23: [α]D20=−3.8 (c=1.71, hexanes)].
Olefinic Hydrocarbons.
Z9-pentacosene (Z9-C25) was synthesized in high yield by Wittig reaction of n-hexadecyltriphenylphosphonium bromide and nonanal in THF at −78°C using sodium bis(trimethylsilyl)amide (1.0 M solution in THF) as base (Ginzel et al. 2003a). Similarly, Z9-C27 was made starting with (1-nonadecyl)triphenylphosphonium bromide coupled to nonanal. The alkadienes (Z, Z)-6, 9- C25 and (Z, Z)-6, 9-C27 were both prepared in high yield via Li2CuCl4-catalyzed Grignard coupling of heptylmagnesium bromide or nonylmagnesium bromide, respectively, with (Z, Z)-9, 12-octadecadien-1-ol tosylate (THF) that was prepared from tosylation of the alcohol produced from Red-Al reduction of methyl linoleate (Wong et al. 1985).
All synthetics were purified via flash chromatography, low-temperature crystallization, or both from acetone or hexane and acetone mixtures (Millar et al. 2009). Many compounds where necessary were subjected to Kugelrohr distillation. Alkenes were separated, purified, or both on silver nitrate impregnated silica gel (10% wt:wt) (Ginzel et al. 2006).
Identification of Cuticular Hydrocarbons.
The relative abundances of cuticular hydrocarbons were compared using solid phase microextraction (SPME) and GC/MS analysis. SPME was carried out by using a manual SPME assembly and a red 100-μm-polydimethylisiloxane fiber (Supelco, Bellafonte, PA). Insects were held with forceps and the fiber wiped across the elytra for ≈30 s while rotating but not bending it. A minimum of six individuals by species and sex were analyzed as composites and the percentage compositions and amounts estimated (Silk et al. 2009). Solvent extraction of whole insects was performed by immersing males or females in n-hexane for 2 min, combining the extracts from several individuals and concentrating under a gentle stream of nitrogen.
SPME samples (× 2 composites) were analyzed separately by GC/MS on a Hewlett–Packard 5890 GC and a 5971 mass selective detector in the electron impact (EI, 70 eV) mode. The column used for analysis was a Supelco SPB-5 capillary (30-m × 0.32-mm × 0.25-μm film) in the splitless mode with helium as carrier gas. The injection port was at 280°C for solvent extracts and 250°C for SPME fibers. The oven temperature was programmed from 70°C, held for 1 min, and then increased at 10°C/min to 240°C and held for 30 min. For DMDS derivatives, the same column was employed but the injection port temperature was 250°C; the initial column temperature was 100°C, held 1 min, and raised at 15°C/min to 300°C.
Hydrocarbons from insect derived material (hexane rinses) were identified by comparing mass spectra and retention times with those of standards with reference to the parent ion (M+) and molecular formulae. Diagnostic mass spectral fragments unequivocally demonstrated the position of methyl branches (Nelson 1993, Nelson and Blomquist 1995). Double bond positions in the mono-alkenes were determined by epoxide formation as follows: 500-μg m-chloroperbenzoic acid in 1:1 mixture methylene chloride/n-heptane was mixed with either 20 female equivalents or 40-μg synthetic compound and stirred at room temperature overnight. Subsequent GC/MS analysis revealed characteristic fragments by cleavage of the epoxide function (Ginzel et al. 2003a). To confirm double bond (E)- or (Z)- assignments, ≈10 mg of the (Z)-mono-ene was treated with 5.0 μl thiophenol in a sealed vial at 110°C for 1 h and, after work-up, was epoxidised as described above. The (E)-isomers eluted before the (Z)-isomers on an SPB-5 column (Ginzel et al. 2003a). Double bond positions in the dienes were inferred from the characteristic EI mass spectra and by direct comparison, by GC/MS, of the mass spectra of mixed mono-epoxides prepared as for monoenes (above) compared with authentic synthetic material (Ryall et al. 2010).
Bioassays – Response to Conspecific and Heterospecific Females.
Males of T. fuscum and T. cinnamopterum were exposed to conspecific and heterospecific females of four different treatments to assess the role of cuticular hydrocarbons as contact sex pheromones for conspecific mate recognition and elicitation of copulatory behavior. Individuals were chosen as available from rearings with all insects being no >2 wk from emergence from bolts. Females were subjected to the following treatments (duplicated for each species): 1) live untreated female, 2) the same female killed by freezing (−18°C for 1 h), 3) the same female rinsed twice in hexane for 3 min each (vials were gently agitated by hand), and 4) the same female with the corresponding rinsate concentrated under a nitrogen stream for ≈10 min and reapplied dropwise to the elytra (= reconstituted females). Treatments necessarily followed this order, but each male was exposed to the corresponding conspecific or heterospecific female of each treatment in random order. Thus each male was exposed to a total of eight different female treatments, four of which were conspecific females and four which were heterospecific. Each female was exposed to only one male of each species. Males were introduced into clean plastic petri dishes lined with filter paper and containing a female (secured on double-sided tape near the edge of the dish if dead). Males were allowed to come into physical contact with the female up to three times, with each encounter defined by contact of the male antennae with the female elytra. If the male showed no reaction by continuing past the female subsequent to each of the three encounters, the response was scored as zero and the bioassay was terminated. Alternatively, if the male displayed one of the following sequential behaviors to an encounter, it was recorded and the bioassay was subsequently terminated: 1) the male stopped and remained in contact with the female (including standing partially on the female body); 2) the male mounted the female (i.e., most of the male's legs were on the female and their bodies were aligned); and 3) the male attempted copulation (exhibited by curling of the male abdomen under and toward the female genitalia, regardless of actual coupling). Bioassays of all treatments were completed within the same day for each replicate male and were conducted in a temperature-controlled laboratory at 22°C. Twelve males of each species were tested. In most cases, males either displayed all three behaviors (stopping, mounting, and attempting copulation) in rapid succession or none at all, so only data for copulatory attempts were analyzed. The proportions of T. fuscum versus T. cinnamopterum males displaying copulatory behavior were compared separately for each of the eight different female treatments using a one-tailed Fisher-exact test for data in a 2 × 2 contingency table (Zar 1999). A one-tailed test was used because our hypothesis was that copulatory behavior would be displayed by a greater proportion (i.e., not simply a different proportion) of males in conspecific pairings than in heterospecific pairings.
Bioassays – Response to Synthetic Hydrocarbons.
Although all compounds identified were detected in both sexes of each species, the strategy for identifying the contact pheromones was to test the bioactivity of female-dominant compounds beginning with the most abundant.
Treatments, regardless of species tested, always included a positive (unrinsed) and a negative (hexane-rinsed) control. Females used as positive controls were freeze-killed as described above. Females used for the synthetic hydrocarbon treatments and negative controls were randomly selected from already frozen specimens in good condition and were rinsed en masse in 5 ml of fresh hexane four times for 3 min each and air dried. Depending on the test, we applied 10, 20, or 40 μg (i.e., two or 4 μl of a five or 10 μg/ μl solution) of synthetic hydrocarbons to the elytra of hexane-rinsed females using a syringe (10 μl Hamilton, VWR, Ontario); these lay within the range of concentrations naturally found on the female. The amounts of compounds on the elytra of females was estimated by reapplying 1.0-μl hexane solutions of relevant compounds onto freeze-killed rinsed females from hexane solutions at 0.1, 1.0, and 10.0 μg/μl and determining and comparing peak areas by resampling with SPME and GC/MS analysis.
In a given experiment, each female was used only once, i.e., presented to one male only. Individual males were exposed to each treatment, one at a time in random order without repetition (i.e., each male was tested with all treatments but only once per treatment) and their responses were scored as previously described. For example, test A (Table 3) used 20 males and 100 dead female T. fuscum. To compare the proportion of males responding to the different female treatments, data were analyzed by Cochran's Q test for dichotomous data from repeated measures or randomized blocks (Zar 1999), separately for each behavior, i.e., stopping, mounting, or attempting copulation. Each male beetle exposed to all treatments in random order served as a block replicate. If the Q statistic indicated significant differences (P < 0.05) among treatments in the proportion of males responding, Scheffé-like pairwise comparisons were used to compare appropriate synthetic treatments with both positive (unrinsed females) and negative (rinsed females) controls (Zar 1999).
Behavioral responses of male T. fuscum to freeze-killed females (unrinsed controls), hexane-washed freeze-killed females (rinsed controls) and hexane-washed females treated with synthetic cuticular hydrocarbons
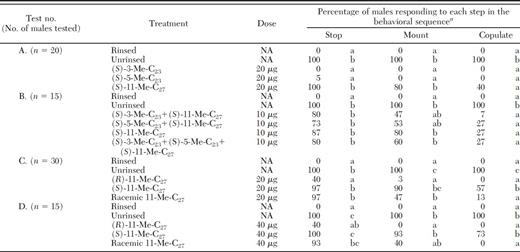
Within each test and step in the behavioral sequence, different letters indicate a significant difference in the proportion of males responding (Cochran's Q test for dichotomous data from a repeated measures exp followed by pairwise comparisons, P < 0.05). The proportion of males stopping, mounting or copulating differed significantly (P < 0.0001) among treatments in each test, with respective Q values as follows: A) Q = 465, 397, 307; B) Q = 177,162, 179; C) Q = 726, 789, 685; and D) Q = 182, 212, 211.
Behavioral responses of male T. fuscum to freeze-killed females (unrinsed controls), hexane-washed freeze-killed females (rinsed controls) and hexane-washed females treated with synthetic cuticular hydrocarbons

Within each test and step in the behavioral sequence, different letters indicate a significant difference in the proportion of males responding (Cochran's Q test for dichotomous data from a repeated measures exp followed by pairwise comparisons, P < 0.05). The proportion of males stopping, mounting or copulating differed significantly (P < 0.0001) among treatments in each test, with respective Q values as follows: A) Q = 465, 397, 307; B) Q = 177,162, 179; C) Q = 726, 789, 685; and D) Q = 182, 212, 211.
Results
Identification of Cuticular Hydrocarbons.
GC/MS analyses of SPME samples of elytra of female and male T. fuscum and T. cinnamopterum are shown in Fig. 2 a, b respectively. The identity, relative abundance and EI-diagnostic ions of the cuticular hydrocarbons associated with each peak number in Fig. 2 a, b are listed in Table 1 (T. fuscum) and Table 2 (T. cinnamopterum), respectively.
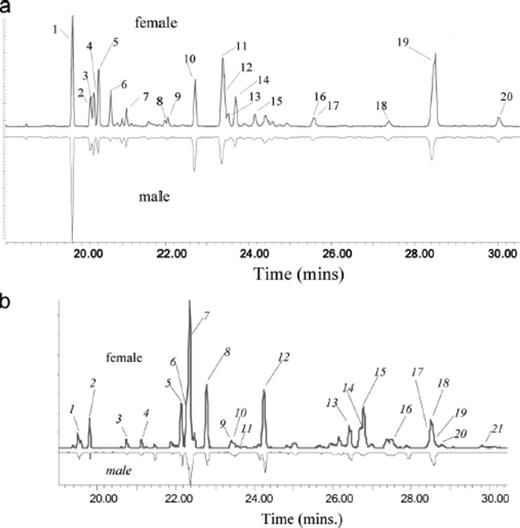
a, GC-MS chromatogram of SPME sample of the cuticular hydrocarbons from the elytra of 5-d-old female (upper) and male (inverted lower) Tetropium fuscum (composite of six individuals of each sex). Analysis conditions are described in the text. Numbers on peaks refer to compounds in Table 1 . b, GC-MS chromatogram of SPME sample of the cuticular hydrocarbons from the elytra of 5-d-old female (upper) and male (inverted lower) Tetropium cinnamopterum (composite of six individuals of each sex). Analysis conditions are described in the text. Numbers (italics) on peaks refer to compounds in Table 2 .
GC/MS analysis of cuticular hydrocarbons of mature male and female T. fuscum sampled using solid-phase microextraction (SPME)

Peak numbers correspond with those in Figure 2a.; + indicates compound was detected, + + + + indicates quantitatively dominant in that sex.
+ Peaks contributing < 5% of total area; ++, 5-30%; + + + +, > 50%.
GC/MS analysis of cuticular hydrocarbons of mature male and female T. fuscum sampled using solid-phase microextraction (SPME)

Peak numbers correspond with those in Figure 2a.; + indicates compound was detected, + + + + indicates quantitatively dominant in that sex.
+ Peaks contributing < 5% of total area; ++, 5-30%; + + + +, > 50%.
GC/MS analysis of cuticular hydrocarbons of mature male and female T. cinnamopterum sampled using solid-phase microextraction (SPME)
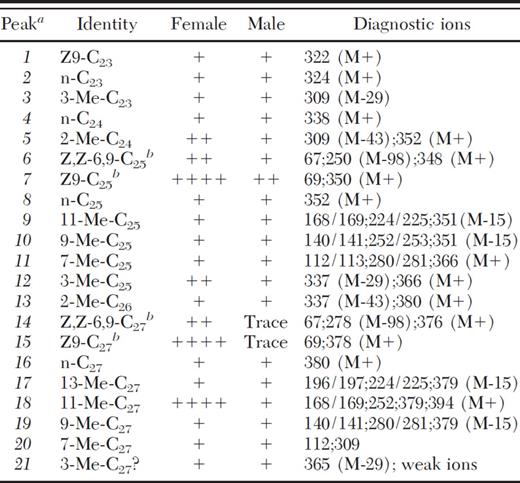
Peak numbers (italics) correspond with those in Figure 2b; + indicates compound was detected, + + + + indicates dominant in this sex.
Double bond positions and stereochemistry inferred from EI mass spectra and retention times compared with authentic synthetic material: see Methods section.
+ Peaks contributing < 5% of total area; ++, 5-30%; + + + +, > 50%.
GC/MS analysis of cuticular hydrocarbons of mature male and female T. cinnamopterum sampled using solid-phase microextraction (SPME)

Peak numbers (italics) correspond with those in Figure 2b; + indicates compound was detected, + + + + indicates dominant in this sex.
Double bond positions and stereochemistry inferred from EI mass spectra and retention times compared with authentic synthetic material: see Methods section.
+ Peaks contributing < 5% of total area; ++, 5-30%; + + + +, > 50%.
In male T. fuscum, chromatograms of SPME samples consistently showed nC23 and nC25 interspersed with methyl-branched compounds with single methyl groups (Fig. 2 a; Table 1). Analysis of female T. fuscum showed very similar patterns except that 3-Me-C23, 5-Me-C23, 3-Me-C25, 5-Me-C25, and, especially, 11-Me-C27, was much more abundant at ≈40 μg/ female. No alkenes were detected in this species.
In female T. cinnamopterum, a different picture emerged with the n-alkanes, nC23, nC25, and nC27 interspersed with methyl-branched compounds that appeared similarly in males (Fig. 2 b). In contrast to T. fuscum, however, the n-alkenes, Z9-C25 (≈10 μg/ female) and Z9-C27 and the alkadienes, (Z, Z)-6, 9-C25 and (Z, Z)-6, 9-C27 were female dominant along with 11-Me-C27 (≈10 μg/female) (Table 2).
Double bond positions in the alkadienes were inferred from a diagnostic fragment that originates from neutral olefin loss after a hydrogen shift (M-98) and mass spectral and retention time information compared with authentic synthetic compounds (see Millar 2000 and references therein). The retention time of each diene was less than the corresponding saturated n-alkane and, therefore, the double bonds in both cases were not conjugated. The double bond positions were confirmed by epoxide formation (mixed mono-epoxides are easily GC-separated and identified) or DMDS adduct formation with mono-alkenes only and GC/mass spectrometry (Dunkelblum et al. 1985, Millar 2000). The retention times for the DMDS derivatives for Z9-C25 and Z9-C27 were 28.7 and 31.0 min, respectively, clearly showing diagnostic fragments. The addition of DMDS to alkenes is also a stereospecific process with the (E)-isomer giving the erythro-bis(methylthio) adduct and the (Z)-isomer giving the threo-adduct (Caserio et al. 1985) with both adducts resolving well on the DB-5 column. The (E)- and (Z)-stereochemistry was also inferred from retention time and mass spectral data of samples against authentic materials.
Bioassay - Response to Conspecific and Heterospecific Females.
Males of both species showed a clear succession of behaviors similar to those reported for other cerambycid species (Ginzel et al. 2003a, b ; 2006). Upon contacting an acceptable female with his antennae, the male would stop crawling, align his body with that of the female, mount, and attempt copulation, usually in rapid succession. Males showed a significant preference for live conspecific females (Fig. 3 a) but heterospecific copulations occurred in two of 12 (17%) pairings with each species of female. This was reflected in an analogous manner in interactions of males with freeze-killed females (Fig. 3 b), showing that the behavior of the female did not appear important to male sexual behavior. Almost total activity was removed on hexane-rinsing of the females (Fig. 3 c) demonstrating that the recognition signal had been removed by solvent extraction. Because a small proportion of males (10%) still responded to rinsed females, we doubled the number of hexane rinses from two to four for the bioassays of synthetic compounds and observed zero males responding to rinsed females (see below). These results point to a contact sex pheromone that was removed by solvent extraction and that was different and specific to each species. However, male sexual behavior could not be demonstrated on reapplication of solvent washes to freeze-killed females (Fig. 3 d).
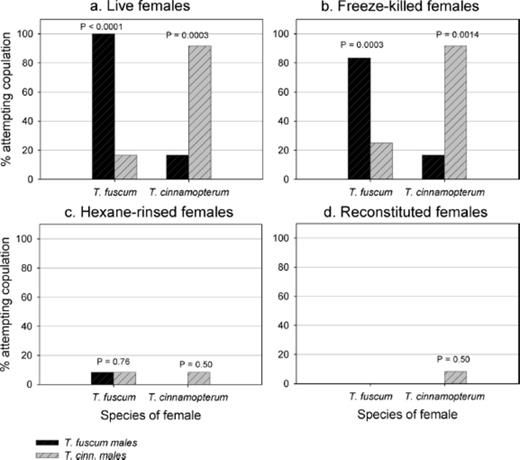
Percentage of Tetropium fuscum and T. cinnamopterum males (n=12 per species) attempting to copulate with conspecific and heterospecific females subjected to four different treatments: a) live females; b) freeze-killed females; c) hexane-rinsed females; and d) freeze-killed females to which the hexane rinse from females was reapplied. For each of the four treatments for each female species, a one-tailed Fisher exact test was used to test the null hypothesis that the proportion of conspecific males attempting to copulate with a given female was not greater than the proportion of heterospecific males attempting to copulate. Comparisons with associated P values (probability the null hypothesis was true) < 0.05 are significantly different.
Bioassays – Response to Synthetic Hydrocarbons.
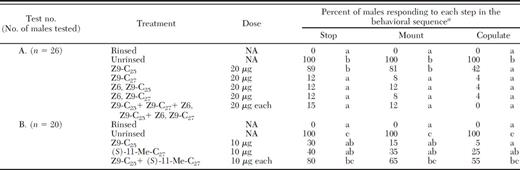
GC/MS analysis of SPME samples of female elytra of T. fuscum showed the presence of n-alkanes (nC23, nC25, nC27) and mono-methyl branched alkanes of which 11-Me-C27 was always at 4–5 × higher levels in females than males. In preliminary screening bioassays, none of the n-alkanes (nC23, nC25, or nC27) or racemic methyl-branched compounds (11, 5, or 3-Me-C23, 11, 5, or 3-Me-C25) and 11-Me-C27, singly or blends thereof in a variety of ratios approximating female proportions (data not shown), restored contact sexual behavior in T. fuscum at estimated female equivalent dosages except for 11-Me-C27, the dominant female elytral hydrocarbon. Thus in the subsequent bioassays we focused on measuring the activity of 11-Me-C27. Both chirality and dosage of 11-Me-C27 significantly affected response of male T. fuscum (Table 3). Most T. fuscum males stopped and mounted rinsed females that had been treated with (S)-11-Me-C27 at 10-μg, 20-μg, or 40-μg dosages, but only the 40-μg dosage (female equivalent) elicited copulatory attempts at levels similar to that elicited by unrinsed female (Table 3, test D). Conversely, no males attempted to copulate with females that were treated with the unnatural (R)-11-Me-C27 at either 20-μg or 40-μg dosages and did not differ from that to rinsed females (Table 3, tests C, D). The racemate caused most males to stop but fewer than half of males to mount and almost none to copulate (Table 3, tests C, D). (S)-3- and (S)-5-Me-C23 were inactive alone (Table 3, test A). The (R)-enantiomers and racemates of 3-Me-C23 and 5-Me-C23 were tested separately and elicited no mating behavior (10% stopped, 0% mounted, and 0% copulation) in males compared with 70% stopping, 60% mounting, and 50% copulatory attempts (n=10 reps) elicited by unrinsed conspecific females. Admixture of the 3- and 5-Me-C27 analogues with (S)-11-Me-C27 or its racemate did not show any synergistic activity (Table 3, test B) but it is possible that behavioral responses may have been greater if the synthetic blend was presented at relative ratios observed on females rather than the equal proportions as tested.
For T. cinnamopterum, screening of individual alkenes and alkadienes at 2 × female equivalent dosages showed activity only for Z9-C25 which elicited stopping and mounting of most males but significantly fewer copulatory attempts than that elicited by unrinsed females (Table 4, test A). Neither the Z9-C27 nor the dienes were behaviorally active when applied on their own but when all three compounds (Z9-C27, Z, Z-6, 9-C25 and Z, Z-6, 9-C27) plus Z9-C25 were applied to rinsed females, the proportion of males stopping and mounting decreased significantly compared with the Z9-C25 treatment alone and no males attempted copulation (Table 4, test A). At dosages of 10 μg per female (one female equivalent), neither Z9-C25 nor (S)-11-Me-C27, by itself, differed significantly from rinsed females in eliciting male mating behaviors but when both compounds were applied to individual females, males stopped, mounted and copulated in proportions significantly greater than those to rinsed females and not significantly different from those to unrinsed females (Table 4, test B). These results suggest that both Z9-C25 and (S)-11-Me-C27 elicit contact sex pheromone-mediated behavior in T. cinnamopterum and that at female equivalent dosages, both compounds are necessary to elicit mating behaviors. We have assumed that the (R)-11 methyl-C27 is not behaviorally active in T. cinnamopterum but this remains to be tested. Statistical equivalence aside, the combination of both Z9-C25 and (S)-11-Me-C27 elicited copulation in only 55% of males compared with 100% in response to unrinsed females, suggesting that complete mating behavioral response may involve additional cuticular hydrocarbons.
Behavioral responses of male T. cinnamopterum to freeze-killed females (unrinsed controls), hexane-washed freeze-killed females (rinsed controls) and hexane-washed females treated with synthetic contact pheromone
Within each test and step in the behavioral sequence, different letters indicate a significant difference in the proportion of males responding (Cochran's Q test for dichotomous data from a repeated measures exp followed by pairwise comparisons, P < 0.05). The proportion of males stopping, mounting or copulating differed significantly (P < 0.0001) among treatments in each test, with respective Q values as follows: A) Q = 1048, 1018, 857; B) Q = 47, 48, 54.
Behavioral responses of male T. cinnamopterum to freeze-killed females (unrinsed controls), hexane-washed freeze-killed females (rinsed controls) and hexane-washed females treated with synthetic contact pheromone

Within each test and step in the behavioral sequence, different letters indicate a significant difference in the proportion of males responding (Cochran's Q test for dichotomous data from a repeated measures exp followed by pairwise comparisons, P < 0.05). The proportion of males stopping, mounting or copulating differed significantly (P < 0.0001) among treatments in each test, with respective Q values as follows: A) Q = 1048, 1018, 857; B) Q = 47, 48, 54.
Discussion
Our results indicate that the mating responses of both T. fuscum and T. cinnamopterum are mediated by contact sex pheromones that are contained in the cuticular hydrocarbons on the surface of females. Furthermore, we provide evidence that chirality and dosage of the dominant cuticular hydrocarbon on T. fuscum females, 11-Me-C27, significantly affect mating behavior of conspecific males. It is clear that (S)-11-methyl-C27 is the key contact pheromone component in T. fuscum. The cuticular hydrocarbons on T. fuscum contained n-alkanes (nC23, nC25, nC27) and mono-methyl branched hydrocarbons of which 11-Me-C27 dominated in females. Only (S)-11-Me-C27 restored contact behavior at dosages estimated to be found naturally on female T. fuscum. At lower dosages (one half or one quarter a female equivalent), (S)-11-Me-C27 elicited stopping and mounting of most males but copulation was elicited at lower levels than that in response to unrinsed females. Males did not respond to (R)-11-Me-C27 at full or half female equivalent dosage. The racemate, tested at both one half and full female equivalent dosage (i.e., respectively, one quarter and one half the natural female dosage of (S)-11-Me-C27) caused most males to stop but fewer than half to mount females and almost none to attempt copulation.
Enantiomer-specific olfactory responses to long-range sex and aggregation pheromones are not uncommon in insects (Borden et al. 1976, Tumlinson et al. 1977, Silverstein 1979, Mori 1998) including T. fuscum (Sweeney et al. 2010) but fewer studies have reported chiral effects in contact pheromones (e.g., Eliyahu et al. 2004, Mori 2007) and the effects of chirality on activity of contact pheromones has rarely been investigated in the Cerambycidae. Our study is only the second to demonstrate chiral specificity in a longhorn beetle contact pheromone. Mori (2007) reported enantiomer-specific behavior of the white-spotted longicorn beetle Anoplophora malasiaca (Thomson) [=Anoplophora chinensis (Forster), synonymized by Lingafelter and Hoebeke (2002) ] in response to female-produced gomadolactones. However, the chirality of contact pheromones did not affect the behavior of the yellow spotted longhorn beetle, Psacothea hilaris (Pascoe) (Fukaya et al. 1997, Fukusaki et al. 1998). Eliyahu et al. (2004) showed that dose and chirality affected activity of contact pheromones in the German cockroach Blatella germanica (L.): high doses of all four synthetic stereoisomers of the most abundant contact pheromone component, 3, 11-dimethylnonacosan-2-one, elicited response in 100% of males, but at female equivalent dosages, the naturally occurring stereoisomer, (3S, 11S)-dimethylnonacosan-2-one, was actually the least effective.
Biosynthesis of methyl-branched hydrocarbons such as 11-Me-C27 likely occurs through elongation of fatty acid CoA's, with the methyl branch arising from methyl malonyl CoA in place of malonyl CoA at a specific point during chain elongation (Nelson and Blomquist 1995). Little is known, however, about the stereochemistry of methyl branches, but our investigation indicates that chiral specificity and discrimination are important, albeit current technology does not allow separation of the two possible enantiomers and/or recognition of nonracemic blends. Clearly, (S)-11-methylheptacosane is active while the (R)-enantiomer has much reduced activity and did not induce copulatory behavior.
In T. cinnamopterum, our data indicate that the mating response is mediated by both Z9-C25 and (S)-11-methylheptacosane and that dosage and blend are important. When Z9-C25 was applied alone at 2 × female equivalent dosage (20 μg) to freeze-killed females, it restored much of the contact sex pheromone behavior observed in response to unrinsed females (e.g., stopping, mounting), but induced copulatory attempts in fewer than half the males. Z9-C27 and the dienes, Z, Z-6, 9-C25 and Z, Z-6, 9-C27, were inactive when applied alone and, furthermore, significantly reduced male response when combined with Z9-C25, suggesting that mate recognition was affected by the blend of cuticular hydrocarbons, and not simply by the presence of Z9-C25. (S)-11-methylheptacosane did not significantly affect male behavior when applied alone but when combined with Z9-C25 at female dosages (10 μg of each), male behavioral responses were approximately doubled. Although statistically similar to unrinsed females, the combination of Z9-C25 and (S)-11-methylheptacosane elicited copulation in only 55% of males compared with 100% that responded to unrinsed females, suggesting the complete contact sex pheromone of T. cinnamopterum may include additional cuticular hydrocarbons, and this idea should be further investigated. The low level of heterospecific mating behavior observed in crosses between T. fuscum and T. cinnamopterum is likely because of the presence of 11-methylheptacosane found on females of both species.
The inability to reconstitute contact pheromonal activity on reapplication of hexane washes to extracted freeze-killed females in both species was unexpected. It is possible that the hexane rinses of female beetles contained additional compounds dissolved from internal body tissues such that the reconstituted blend of hydrocarbons that we placed back on rinsed females differed from that found on the cuticle of natural females.
Mono-alkenes similar to those found in T. cinnamopterum have been reported in other cerambycid cuticular hydrocarbon profiles and as contact pheromone components (Ginzel et al. 2003a, 2006). Monoalkenes have also been reported in the woodwasp Sirex noctilio (F.) as contact sex pheromones (Hymenoptera: Siricidae) (Böröszky et al. 2009). The alkadienes are quite common in insects (Blomquist and Bagnerès 2010) in, for example, the cuticular hydrocarbons of Hawaiian swordtailed crickets (Gryllidae: Trigonidiinae: Laupala) (Mullen et al. 2008) and as sex pheromone components of the almond seed wasp Eurytoma amygdale Enderlein (Hymenoptera: Eurytomidae). To our knowledge, none have been reported as contact sex pheromone components. The biochemical origin of these mono-alkenes and alkadienes is likely through oleic and linoleic acids, respectively, followed by chain elongation and decarboxylation (Howard and Blomquist 2005).
Methyl-branched hydrocarbon contact sex pheromones have been reported in several species of cerambycids in the subfamily, Cerambycinae, e.g., Xylotrechus colonus (F.) (3-Me-C25 and 9-Me-C25,Ginzel et al. 2003b) and Neoclytus acuminatus acuminatus (F.) (7-Me-C25,Lacey et al. 2008), as well as in the buprestid emerald ash borer, Agrilus planipennis Fairmaire, which uses 9-Me-C25 (Silk et al. 2009). A dimethyl-branched hydrocarbon, 5, 17-dimethyl-nonacosane, has recently been identified as an important contact pheromone component in the cerambycid, Callidiellum rufipenne (Mostchulsky) (subfamily Cerambycinae) (Rutledge et al. 2009). Host and mate location by T. fuscum and T. cinnamopterum (subfamily Spondylidinae) appears to be very similar to these behaviors in the subfamily Cerambycinae (Ginzel and Hanks 2003, Ginzel et al. 2003a, b ; 2006) in that both sexes of each species are attracted by host volatiles (Sweeney et al. 2004) and male-produced sex pheromones (Silk et al. 2007, Lemay et al. 2010, Sweeney et al. 2010) with males recognizing conspecific females by contact chemoreception. The latter appear to be species-specific and likely play an important role in the reproductive isolation of these now sympatric species because both use the identical male-produced sex pheromone, (S)-fuscumol. The fact that T. fuscum and T. cinnamopterum use similar volatile cues to locate host plants (Sweeney et al. 2004, 2006), identical long-range sex pheromone (Silk et al. 2007, Sweeney et al. 2010) and share a common component of their respective contact sex pheromones, 11-methyl-heptacosane, suggest they are phylogenetically closely related. Knowledge of the contact pheromones in the longhorn beetles, in particular commonalities and differences among the subfamilies, will lend insight into the evolution of mating systems in the Cerambycidae. This present work provides further evidence of the critical role of contact pheromones in the reproductive behavior of longhorned beetles.
We thank the Canadian Food Inspection Agency, Natural Resources Canada - Canadian Forest Service Forest (Forest Invasive Alien Species Fund), and (through the auspices of the Spray Efficacy Research Group International) Forest Protection Limited (NB), Ontario Ministry of Natural Resources, and the Nova Scotia Ministry of Natural Resources for generous funding and in-kind support. We thank J. Price, N. Brawn, K. Burgess, A. Doane, C. MacKay, N. Harn, W. MacKay, and X. Sun for technical assistance and support; E. Kettela and E. Hurley for advice and support; and our thanks to two anonymous reviewers. All experiments reported here comply with the laws of Canada.
References



