-
PDF
- Split View
-
Views
-
Cite
Cite
Victoria Y. Yokoyama, Pedro A. Rendón, Xin-Geng Wang, Susan B. Opp, Marshall W. Johnson, Kent M. Daane, Response of Psyttalia humilis (Hymenoptera: Braconidae) to Olive Fruit Fly (Diptera: Tephritidae) and Conditions in California Olive Orchards, Environmental Entomology, Volume 40, Issue 2, 1 April 2011, Pages 315–323, https://doi.org/10.1603/EN10186
Close - Share Icon Share
Abstract
The larval parasitoid, Psyttalia humilis (Silvestri), reared on Mediterranean fruit fly, Ceratitis capitata (Weidemann), by USDA-APHIS-PPQ, San Miguel Petapa, Guatemala was imported into California for biological control of olive fruit fly, Bactrocera oleae (Rossi). This study reports the results of field releases and recovery of P. humilis in California, and laboratory investigations to determine the effects of food provision, high temperature, and insecticidal bait spray on the parasitoid's survival and fecundity. Parasitoids (3,613-7,823) were released in Orland, San Juan Bautista, Cayucos, Sylmar, Santa Barbara, and San Diego during October through December 2006. Mean daily temperatures at the release sites ranged from 10.7°C in Orland to 20.9°C in San Juan Bautista. The lowest (0.5) and highest (29.7) mean number of adult B. oleae per day per trap was captured in Orland and Sylmar, while the lowest (0.01) and highest (2.21) number of third instar larvae per fruit was collected on 11 December in Orland and on 5 October in San Diego in prerelease samples, respectively. Parasitoids were recovered from all release sites, the lowest (0.3%) and highest (100%) parasitism occurred on 25 January in Sylmar and on 26 October in Cayucos, respectively. At 24°C, parasitoids reared from B. oleae larvae survived 36 d on honey, 31 d on orange juice, and 28 d on honeydew, which was significantly longer than on cut olive fruit (8 d) or without food (11 d), but was similar to those reared from C. capitata larvae under the same food conditions. Under a high diurnal temperature regime (18.3-35°C) reflecting the summer olive growing conditions in the California Central Valley, the parasitoids survived <5 d when no food or only water was provided. Its longevity and life-time fecundity significantly increased by provision of honey or honeydew. There was no difference in the parasitoid's longevity between females and males or between food and sexes. Percent mortality of parasitoid adults was not significantly affected by the exposure to insecticidal fruit fly bait (GF-120) in four different types of choice tests with artificial honeydew and GF-120.
Olive fruit fly, Bactrocera oleae (Rossi), has created a serious economic threat to the California olive industry since it was first detected in 1998 (Rice et al. 2003). High numbers of olive fruit fly occur in the coastal areas, where nearly every fruit can be infested on untreated trees, while lower numbers of the pest are found on olives in the warmer interior valleys (Yokoyama et al. 2006, Yokoyama 2011). Current olive fruit fly management strategies are based on spray applications of spinosad-formulated fruit fly bait (GF-120) (Johnson et al. 2006) that are timed using pheromone-based trapping programs (Yokoyama et al. 2006, Burrack et al. 2008), information on pest and olive cultivar associations (Burrack and Zalom 2008), and cultivation practices (Yokoyama and Miller 2007). Bait sprays may have limited future use because olive fruit fly resistance to spinosad has been recently reported (Kakani et al. 2010). Although postharvest treatments for fruit have been developed (Yokoyama and Miller 2004), the market for table olives has a very low tolerance for infested fruit and researchers have shown olive fruit fly can negatively impact olive oil quality (Gomez-Caravaca et al. 2008). Therefore, additional control tools are needed to suppress olive fruit fly infestations.
Natural enemies have the potential to reduce olive fruit fly populations, especially in untreated ornamental olive trees and un-harvested orchards that serve as a reservoir for fly populations (Collier and van Steenwyk 2003). Reports of classical biological control programs against the olive fruit fly have provided mixed results (Tzanakakis 2006, Daane and Johnson 2010). The most commonly used parasitoid species, in past olive fruit fly programs, has been Psyttalia concolor (Szépligeti). Results from early European and Mideast importation programs suggest this parasitoid has established in some regions (Neuenschwander 1982, El-Heneidy et al. 2001), but rarely contributes to olive fruit fly control without large inundative releases (Monastero and Delanoue 1966, Kapatos et al. 1977, Liaropoulos et al. 1977). More recently, P. concolor has been reported to provide a seasonal average parasitism >20% in Majorca (Balearic Islands) in organically managed orchards without augmenting parasitoid populations (Miranda et al. 2008).
Reported differences in P. concolor effectiveness may result from the parasitoid's environmental tolerance to each region as olives are grown in climatic zones ranging from arid-desert to coastal-Mediterranean. Different pest complexes are also found in olive orchards (Tzanakakis 2006) and insecticide treatments against these pests may be incompatible with the survival or performance of some natural enemy species (Nadel et al. 2007). Most important may be differences among populations of the P. concolor complex. Sime et al. (2006) showed slight biological differences between two P. concolor populations that originated from the same stock of north Africa material, but had been reared for years under different conditions. Greater differences may exist among geographically distinct natural populations within the P. concolor complex, which are widely distributed in Africa (Wharton et al. 2000, Mkize et al. 2008). Recent molecular analyses showed clear separation of the northern Africa and sub-Saharan populations (Karam et al. 2008, Rugman-Jones et al. 2009). Most of the early European programs used P. concolor from northern Africa, specifically Tunisia, Libya, and Morocco (Clausen 1978, Wharton 1989a). In contrast, parasitoid material used in this study was collected in Kenya, which we henceforth refer to as P. humilis (Silvestri) as this is an available name for sub-Saharan populations (Rugman-Jones et al. 2009) previously referred to as P. concolor (Wharton 1989b, Wang et al. 2009c) or P. cf. concolor (Kimani-Njogu et al. 2001, Yokoyama et al. 2008a).
Yokoyama et al. (2004) first used P. humilis for biological control of olive fruit fly in 2002 in California and later mass-produced the parasitoid in Guatemala on irradiated Mediterranean fruit fly (Medfly), Ceratitis capitata (Wiedemann) and the parasitoid adults were shipped to California for field releases (Yokoyama et al. 2010). In this study, we used P. humilis reared on fertile Medfly. We first sought to determine the ability of P. humilis to be field-released and consistently recovered in California olive orchards that used standard management practices, with the exception of insecticide sprays. The seasonal abundance of olive fruit fly in different regions of California is well known (Yokoyama et al. 2006, 2010), and field releases were concentrated in those California regions where high densities of olive fruit fly populations are commonly found. Fly densities and climate were monitored during pre- and postrelease samples at the release sites. We then conducted a series of laboratory studies to assess the potential effects of various conditions found in California olive orchards on the parasitoid's survival and/or fecundity, to better understand the field conditions that would favor survival and possible establishment of the parasitoids after being released. Specifically, we investigated the effects of (1) presence or absence of foods that may be found in the vicinity of olive groves on the parasitoid's survival; (2) weather extremes found in the inland olive growing regions on the parasitoid's survival as well as fecundity; and (3) GF-120 bait spray on the parasitoid's survival.
Materials and Methods
Field Release and Recovery of P. humilis.
Six sites were selected for field releases of P. humilis in California (Fig. 1). The olive trees in each site were not sprayed with insecticides or harvested for fruit, which was desired for postrelease sampling and parasitoid establishment.
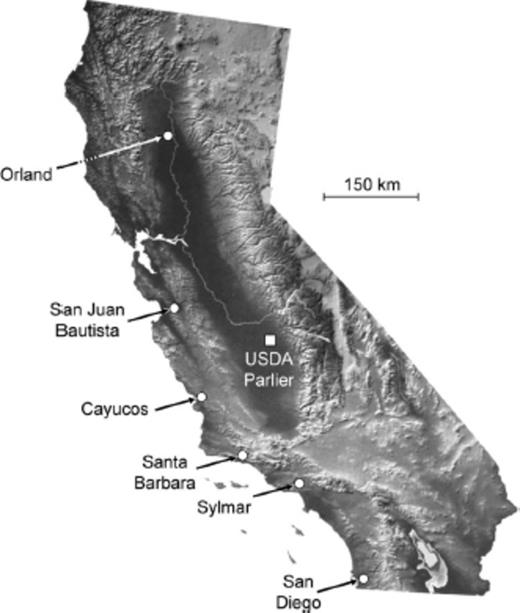
Adult P. humilis were reared from third instar Medfly (Antigua strain) in the Moscamed biological control laboratory, San Miguel Petapa, Guatemala, as described by Yokoyama et al. (2008b). Adult parasitoids were packaged in waxed paper cups (0.275 liters), with ≈400 parasitoids, ≈50% females per cup, and shipped to the USDA-ARS in Parlier, CA (Fig. 1), as described by Yokoyama et al. (2010). On arrival, 3–4 waxed paper cups each were placed in aluminum-framed, organdy-screened cages (30.5 × 30.5 × 30.5 cm, BioQuip Products, Rancho Dominguez, CA). The cups were opened in the cages, and the parasitoids provided with water and honey for food, allowed to mate, and observed within 1 d. The parasitoids were then transported to release sites where the cages were placed beneath trees with fruit visibly infested with olive fruit fly larvae, and the parasitoids released directly from the cages. The number of dead parasitoids were counted inside each paper cup and cage and subtracted from known number in each shipment to determine the number of parasitoids released.
Releases were made from 5 October to 15 December 2006, during the period when olive fruit can be heavily infested. The number of parasitoids released was based on the quantity received in each shipment. The number of trees and fruit per tree sampled was based primarily on availability at each site, with a goal of sampling and releasing in four trees per site, and collections of >100 fruit per tree. Postrelease samples were made from those trees receiving parasitoids the previous sample date, and typically ranged from 4 to 8 d after the release. Information in Table 1 shows the region and specific location of parasitoid releases, description of the olive trees, and fruit sample dates and trees sampled at each release site.
Region and specific location, description of the olive trees, and fruit sample dates from Oct. 2006 to Jan. 2007 in parasitoid release sites
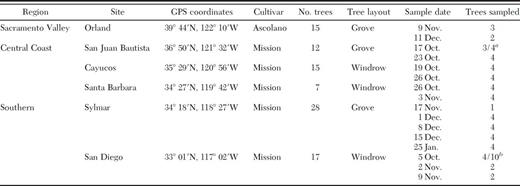
Three prerelease trees sampled, and parasitoids released on four trees.
Four prerelease trees sampled, and parasitoids released on 10 trees.
Region and specific location, description of the olive trees, and fruit sample dates from Oct. 2006 to Jan. 2007 in parasitoid release sites

Three prerelease trees sampled, and parasitoids released on four trees.
Four prerelease trees sampled, and parasitoids released on 10 trees.
Temperature loggers (model XTI08–5+37) with external thermistors (model TMC6–1T) and humidity loggers (model SRHA08, Onset Computer, Bourne, MA) were used to record temperature and relative humidity at each site. The data loggers (2–4 per site) were placed in the canopy of sampled trees, and programmed to record every hour. During the study, two temperature loggers failed in San Juan Bautista, and the hourly temperatures were obtained from the California Irrigation Management Information System (CIMIS) weather station No. 143, San Juan Valley (36° 49′N, 121° 28′W), ≈6.6 km southeast. The temperature logger for 17 November through 1 December was lost at the Sylmar location, and the daily temperatures were obtained from the Weather Underground station KCASYLMA2 (34° 18′N, 118° 27′W) in Sylmar.
To estimate olive fruit fly populations, adults were trapped using four to six yellow panel Pherocon AM traps (Trécé Inc., Adair, OK), with a clear packet of ammonium bicarbonate bait (15–20 g), and a plastic dispenser (1.7 cm wide × 4.8 cm long) containing pheromone (1,7-dioxaspiro[5,5]undecane, 80 mg). Traps were placed at mid-canopy height (≈2.4 m) in a shaded area near fruit, with each trap in a separate tree and traps spaced one to several trees apart. At the end of the exposure period, the total number of females and males in each trap were counted, and the results reported as mean (±SEM) per day per trap and percentage females.
To determine parasitism levels, samples of olive fruit were randomly sampled from trees before and after parasitoid releases to record emerging numbers of olive fruit fly pupae and adults or P. humilis adults. Fruit samples were placed in plastic containers (22 cm wide × 32 cm long × 13 cm high), covered with organdy cloth, and held in the laboratory at 23 ± 2°C. After all insects had emerged, rates of parasitism were calculated for each site and sample date, based on previously published procedures (Yokoyama et al. 2008, 2010). Percentage parasitism was based on the number of available third instar, rather than the total number olive fruit fly larvae present, as eggs and young instar olive fruit fly are unsuitable oviposition of the P. concolor species complex (Sime et al. 2006) and Yokoyama et al. (2008) reported that P. humilis caused mortality of the first and second instars. Thus, the number of third instars available to the parasitoids on each release date was calculated from the number of olive fruit fly larvae and pupae that emerged for 4 d in prerelease fruit samples. The number of available third instars was multiplied times the number of fruit in each postrelease fruit sample, and reported as the mean (±SEM) of the replicates. Percentage parasitism was calculated by dividing the number of parasitoids that emerged in the postrelease fruit samples by the estimated number of available third instars and reported as the mean (±SEM) of the replicates.
Laboratory Studies and Insect Colonies.
A series of three laboratory studies were conducted to determine the effect of limited food resources, temperature, and insecticidal bait use on longevity and/or fecundity of P. humilis at the University of California's Kearney Research and Education Center (KREC) in Parlier, CA; USDA-ARS in Parlier; and California State University, East Bay, CA, respectively.
At KREC, colonies of olive fruit fly and P. humlis were maintained on olives in an insectary room at 25 ± 2°C, 16L:8D, and 40–60% RH. The olive fruit fly colony had been maintained since 2003, with periodic additions of field-collected insects into the colonies. Fruit were collected from an insecticide-free KREC orchard. The fruit were placed in organdy-screened cages (61 × 61 × 61 cm, Bug Dorm2, BioQuip, Rancho Dominguez, CA). Each cage had 100–200 mature (1–2 wk old) olive fruit fly females, which were supplied with honey, water, and hydrolyzed yeast (FisherBiotech, Fairlawn, NJ). The fruit were left in the cage until each fruit had 3–5 ovipositional sites. Exposed fruit were removed from the cage and placed on a raised metal screen, held 2 cm above a plastic tray. After 10–12 d olive fruit fly larvae exited the fruit and dropped into the tray, where puparia were collected and placed into holding cages for adult emergence. A P. humilis colony was initiated with ≈400 adults from Guatemala in 2007, and the parasitoid had been maintained on olive fruit fly. Adult parasitoids were held in clear acrylic cages (30 × 30 × 30 cm) that had organdy screen on three sides for ventilation and provisioned with water and honey. Between 50–100 olives, containing second and third instar olive fruit fly, were placed in the cages for a 1–3 d exposure period, depending on parasitoid density. After exposure, the olives were placed on the metal grids above the plastic trays to collect the parasitized olive fruit fly larvae and pupae as previously described (Wang et al. 2009c).
At the USDA-ARS in Parlier, a colony of P. humilis was also reared from olive fruit fly in a manner similar to the KREC colony (Yokoyama et al. 2008). The parasitoids used in all laboratory studies were collected from the KREC or USDA-ARS P. humilis colonies, or directly imported from the Guatemala reared on Medfly.
Parasitoid Longevity in Response to Food Source.
The effect of food source on the longevity of adult P. humilis was tested under a laboratory condition (24.0 ± 1.5°C, 63 ± 3% RH and a photoperiod of 12L:12D) at the USDA-ARS in Parlier, using potentially available food sources in or near olive orchards. Natural food sources may include honeydew, excreted by the black scale, Saissetia oleae (Olivier) or brown soft scale, Coccus hesperidum L., (Hemiptera: Coccidae), common olive or citrus pests in California (Ewart and Metcalf 1956, Daane et al. 1991); fruit juices (Sivinski et al. 2006) such as orange commonly found in nearby citrus orchards; or damaged olive fruit.
Food treatments were (1) damaged green olive fruit manually cut to the pit with a knife; (2) 13–16 drops of frozen orange juice concentrate that was diluted with water (1:1); (3)13–16 drops of bee honey; (4) drops of brown soft scale honeydew presented on 3–4 leaves of a ficus (Ficus benjamina L.); and (5) no food or water. For each treatment, 13–15 newly emerged wasps (<1d old) collected from the USDA-ARS colony were placed in an aluminum frame and screened cage (20.3 × 20.3 × 20.3 cm) (model 1450AS, BioQuip Products) with water in a shell vial at the top of the cage. Food provisions were placed on the bottom of the cage. Every 1–3 d thereafter, parasitoid survival was evaluated in each cage and food treatments were renewed. There were four or five replicate for each food treatment.
The tests were repeated using 3–5 d-old parasitoids (22–30 wasps per cage) imported from the Guatemala. Food treatments were similar except that honeydew from the black scale on olive leaves was used instead of honeydew from brown soft scale. Results were reported as the survival time mean (±SEM) of the replicates and compared among the different foods using a one-way analysis of variance (ANOVA), and Tukey's test (GraphPadSoftware 2007).
Parasitoid Longevity and Fecundity in Response to a High Diurnal Temperature Regime and Food Source.
The effects of high temperature and food source on the survival and fecundity of P. humilis were evaluated at KREC in an incubator under a high diurnal temperature regime of 35°C from 1200 hours to 1900 hours, 18.3°C from 1900 hours to 1200 hours, and photophase from 0500 hours through 1900 hours. This temperature regime reflects common daily summer conditions in the inland valleys of California (Wang et al. 2009a). Chamber temperature was monitored with a data logger (HOBO, Onset Computer, Bourne, MA). Food provision treatments were (1) no food or water; (2) water only; (3) water and black scale honeydew; and (4) water and honey. Fresh black scale honeydew was collected on olive leaves from an untreated olive orchard at KREC. The availability of water and food were continuously monitored and replenished as needed throughout each test.
Infested fruit containing olive fruit fly early third instars were obtained using similar methods as described above for insect rearing. For each food treatment, a pair of newly emerged parasitoids (<1-d old) was released into a cylindrical acrylic cage (20 × 15 × 15 cm) that had three organdy screen holes for ventilation. Each female parasitoid was provided with five infested fruit every 2 d until the female died. The longevity of both male and female parasitoids was evaluated in the morning and afternoon each day after the beginning of the test. Life-time parasitoid fecundity was estimated based on the number of F1 parasitoids that emerged from the exposed olives. Twenty replicates were used for each treatment. Data were compared using two-way or one-way ANOVA and Tukey's honestly significant difference test among different treatments (JMP, V. 6.0.3, SAS 2006, Cary, NC).
Parasitoid Response to GF-120 Fruit Fly Baiting.
The potential effect of fruit fly baiting using GF-120 on P. humilis was evaluated under laboratory conditions at California State University in East Bay, CA, using female parasitoids directly imported from Guatemala. Parasitoids were maintained in the laboratory and provided with honey and water but the honey was removed 20 h before the tests. Five to 10 female parasitoids were placed in plastic and screen testing containers (23 cm2) and exposed to test substances for 2 h. Test substances were artificial honeydew (0.49 g fructose, 0.42 g sucrose, 0.09 g glucose in 10 ml distilled water, following the description by Byrne et al. 2003) and GF-120, Naturalyte (Dow AgroSciences, Indianapolis, IN) fruit fly bait (diluted 1:4 with distilled water). Four treatments were (1) no-choice, 30 2-μl drops of artificial honeydew; (2) no-choice, 30 2-μl drops of GF-120; (3) separated-choice, 15 2-μl drops of artificial honeydew and 15 2-μl drops of GF-120 physically separated; and (4) mixed-choice of 15 2-μl drops of artificial honeydew interspersed with 15 2-μl drops of GF-120 in the test container. Eight to nine replicates were used for each treatment.
After exposure, test substances were removed, and the parasitoids were allowed to feed on honey and water. Numbers of dead parasitoids were counted and removed for 4 d after each test. The results were reported as the mean (±SEM) percentage mortality. Mortality was arcsine transformed and compared among the different treatments using a one-way ANOVA, and Tukey's test (GraphPad Software 2007).
Results
Field Release and Recovery of P. humilis.
Olive fruit fly adult trap captures at six release sites in California are shown in Table 2. The mean number of adults captured per trap per day ranged from 0.5 in Orland (9 November through 11 December) to 29.7 in Sylmar (1–8 December). Mean percentage of females per trap ranged from 19% in Sylmar (8–15 December) to 61% in San Diego (2–9 November). Mean daily temperatures at the locations ranged from 10.7°C in Orland (9 November through 11 December) to 19.9°C in Santa Barbara (26 October through 3 November). Mean daily relative humidity ranged from 20.4% in Santa Barbara (26 October through 3 November) to 77.8% in San Diego (2–9 November). The lowest temperature (1.6°C) was recorded in Sylmar (21 January 2007) and on a subsequent visit to the site (25 January) most of the fruit had fallen from the trees.
Mean (±SEM) trap captures of olive fruit fly adults, and daily temp and relative humidity from Oct. 2006 to Jan. 2007 at parasitoid release sites
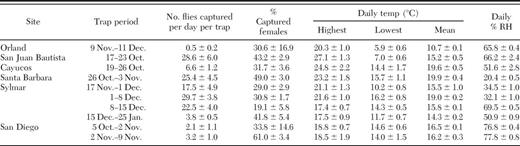
Mean (±SEM) trap captures of olive fruit fly adults, and daily temp and relative humidity from Oct. 2006 to Jan. 2007 at parasitoid release sites

The mean number of olive fruit fly third instars per fruit and percentage parasitism by P. humilis is shown in Table 3. In the Orland location, olive fruit fly third instars did not emerge from prerelease fruit samples and the number of third instars per fruit and the rate of parasitism were calculated from the postrelease fruit. Numbers of third instars emerging from fruit samples was lowest (0.01 per fruit) in Orland (11 December) and highest (2.21 per fruit) in San Diego (5 October). Parasitism was lowest (0.3%) in postrelease fruit collected in Sylmar (25 January). In Cayucos, a total of 350 parasitoids were collected from postrelease fruit collected on 26 October exceeding the calculated number of third instars per fruit and parasitism was reported as 100% in all replicate fruit samples.
Number and dates P. humilis released, mean (±SEM) no. of fruit and olive fruit fly emerged, estimated third instars exposed to parasitoids, and percentage parasitism of third instars at six sites from Oct. 2006 to Jan. 2007

Number and dates P. humilis released, mean (±SEM) no. of fruit and olive fruit fly emerged, estimated third instars exposed to parasitoids, and percentage parasitism of third instars at six sites from Oct. 2006 to Jan. 2007

Parasitoid Longevity in Response to Food Source.
At 24°C, parasitoids reared from olive fruit fly and provided with water survived the longest (36 d) on honey for food (Table 4) under constant laboratory temperatures. Mean survival on honey was similar to orange juice (31 d) and honeydew (28 d) and significantly higher than on cut olive fruit and no food or water (F=18.09; df=4, 17; P=< 0.0001). Survival of parasitoids reared on Medfly ranged from 31 d on orange juice to 34 d on honey and honeydew.
Comparison of mean (±SEM) survival (d) of P. humilis adults reared from olive fruit fly or Medfly under various food conditions
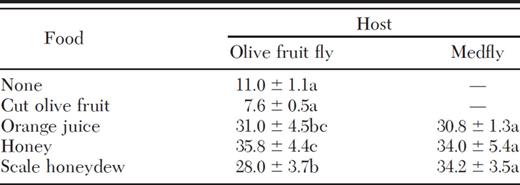
Means within a column followed by the same letter are not signif-icantly different (P > 0.05, Tukey's test).
Comparison of mean (±SEM) survival (d) of P. humilis adults reared from olive fruit fly or Medfly under various food conditions

Means within a column followed by the same letter are not signif-icantly different (P > 0.05, Tukey's test).
Parasitoid Longevity and Fecundity in Response to a High Diurnal Temperature Regime and Food Source.
At a high diurnal temperature regime (18.3–35°C), longevity of P. humilis was significantly different among different food or water provision treatments (F=39.70; df=7, 152; P < 0.001), but not between sexes (F=3.35; df=7, 152; P=0.069) or between food source and sexes (F=0.45; df=7, 152; P=0.713) (Fig. 2 A). The parasitoids survived <5 d when no food or only water was provided. Provision of honey and honeydew significantly increased the longevity of females (F=24.79; df=3, 76; P < 0.001) and males (F=15.67; df=3, 76; P < 0.01). No difference was observed in parasitoid longevity between no food versus water only, or between honeydew versus honey.

Longevity (A) and fecundity (B) of P. humilis under a high diurnal temperature regime (18.5–35°C) and different water and food sources. Bars are mean (±SEM) and different letters over the bars indicate significant differences (P < 0.05, Tukey's test).
The life-time fecundity of P. humilis was significantly different among water and food sources (F=20.40; df=3,76; P < 0.001) (Fig. 2 B). Parasitoid fecundity was increased when females were provided with water, honeydew and honey versus no food, and with honey versus honeydew treatments.
Parasitoid Response to GF-120 Fruit Fly Baiting.
Percent mortality of P. humilis adults was not significantly different (F=1.94; df=3, 31; P=0.144) among the four choice tests with artificial honeydew and GF-120 (Table 5).
Mean (±SEM) mortality of female parasitoids exposed to artificial honeydew or GF-120 bait spray in four types of choice tests
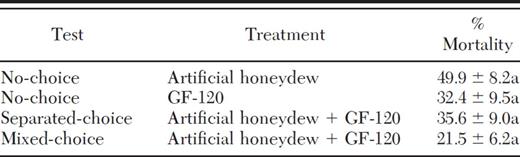
Means within a column followed by the same letter are not signif-icantly different (P > 0.05).
Mean (±SEM) mortality of female parasitoids exposed to artificial honeydew or GF-120 bait spray in four types of choice tests

Means within a column followed by the same letter are not signif-icantly different (P > 0.05).
Discussion
Our results showed highly variable captures of adult olive fruit flies at six different field release sites. In some sites a higher number of captures seemed to be related to favorable temperatures but not so in other sites. For example, 20–25 flies were captured per day per trap when temperature ranged from 19 to 21°C in San Juan Bautista, Sylmar, and Santa Barbara. However, in Cayucos only 6.6 flies were captured even under warm temperatures and moderate humidities. The high variability in adult captures may be affected by many factors, and each release is unique in terms of geography, climatic conditions, and habitat types. Thus, the six locations we selected for field releases of P. humilis cover the diverse olive growing regions in California where olive fruit are produced either primarily for oil in coastal areas (San Juan Bautista, Cayucos, Santa Barbara, and San Diego), or canned fruit in inland valleys (Orland and Sylmar).
Although the difference in habitat types among these release sites, P. humilis were consistently recovered within the same fruit season after the release of the parasitoid, with the exception of one release in San Diego. Inability to recover the parasitoid in San Diego after the 5 October release was likely related to the low number of parasitoids being released at this site and the relatively large release area (10 trees) when compared with other locations. In addition, the postrelease sample was collected on 2 November, that is, ∼1 mo later when the parasitized larvae could have exited from the fruit for pupation, or the second generation had not emerged in the field. In Orland, the parasitoids were recovered even at an extremely low host density and low temperatures. Furthermore, the olive cultivar in Orland was Ascolano that is a large-size fruit and reported by Wang et al. (2009c, d) to impede parasitism. These results suggest that P. humilis can be used for biological control of the pest in many diverse olive growing regions in California.
P. humilis had been released in California in 2002 (Yokoyama et al. 2008a) and in 2005 (Yokoyama et al. 2008b) for control of olive fruit fly. In general, more parasitoids were released on each release date in this study than previously done before 2006 at various locations in California. From 2008–2009, P. humilis rearing procedures were changed in the biological control laboratory in Guatemala. The parasitoid was mass reared on irradiated Medfly larvae to reduce the risk of fertile adults in shipments, and imported and released in high numbers (Yokoyama et al. 2010). In the same study, rearing P. humilis on irradiated Medfly did not affect parasitoid fitness based on fecundity and flight tests. Although fruit infested with olive fruit fly was available in the release years of October 2006 to January 2007 (Table 3) and 2008–2009 in the Orland, Cayucos, and San Diego release sites, rates of parasitism in the latter study were lower and ranged from 0 in Orland to 7.6 in Cayucos. Releasing greater numbers of parasitoids in 2008–2009 did not improve calculated rates of parasitism. However, the only report of recaptures of P. humilis that may have resulted in prior year releases was by Yokoyama et al. (2009). One adult of P. humilis was recovered from fruit infested with olive fruit fly in October 2006 in San Jose after multiple releases of 2,587; 3,600; 4,630; 3,655; and 5,089 parasitoids and subsequent rates of 0.6, 0.5, 0.7, 3.6% parasitism in November to December 2005.
Multiple parasitoid releases were conducted in Sylmar (Table 3) with the intent to permanently establish P. humilis on olive fruit fly at this location. Low numbers of olive fruit fly persisted in the fruit from November to December and the parasitoid was recaptured in postrelease fruit samples during this period. However, the work was discontinued in January because mid-month freezing conditions throughout the state caused the fruit to drop from the trees. Freezing conditions may have caused high mortality of immature parasitoids developing in the host resulting in low calculated rates of parasitism on the last postrelease sample date.
As previous studies, a relationship was not observed between host fly density per fruit and percentage parasitism (Table 3). For example, in Cayucos and San Diego (5 October) the mean numbers of third instars per fruit was 0.08 and 2.21, but the mean rates of parasitism were 100 and 0%, respectively. Many factors, other than host fly density, could have affected the observed parasitism. These factors may include release site size, tree size, fruit size (varied even within the same cultivar, Wang et al. 2009d), fruit load, number of parasitoids released, sample size, and microclimates in the vicinity of trees where parasitoids have been released. In this study, the parasitoids were released in different types of olive trees, at different times in different sites that varied in location size, tree size, fruit load, fruit maturity, and other factors. Numbers of P. humilis released was based on parasitoids shipped and mortality after arrival. The complexity of parasitoid-host-weather field relationships in this study lacks predictability and in the absence of independent measurements the data does not lend itself to further analysis especially in relation to laboratory test results under controlled conditions. Analysis of possible relationships between host abundance or parasitism and environmental factors may be more appropriate for studies conducted in a relatively controlled agricultural crop system. However, quantitative studies were only possible with cage tests and this has been done (Wang et al. 2010) showing parasitism was lower in large than small fruit, and high temperature and low humidity in the summer reduced parasitism.
Parasitoids rely on food for survival (Yokoyama et al. 2008). As expected, orange juice and scale honeydew could sustain the longevity of P. humilis and longevity of the parasitoid feeding on these two food sources was similar to those feeding on honey, regardless if the parasitoids were reared from Medfly or olive fruit fly. Two different honeydews, produced by either soft brown scale or black scale, respectively, were tested in this study. Ewart and Metcalf (1956) compared these honeydews between the species and found no differences in the composition of sugars or amino acids. The species of leaf on which the honeydew was presented, F. benjamina versus O. europaea, probably did not affect the behavior of the parasitoid because survival on honeydew was comparable to honey in all tests. Sivinski et al. (2006) found that another fruit fly parasitoid Diachasminorpha longicaudata (Ashmead) had similar longevities on homopteran honeydew, extrafloral nectary secretions, and honey, and was able to sustain their longevity by feeding on fruit juice or pulp. In related studies, Bautista et al. (2001) found that females of the egg parasitoid Fopius arisanus (Sonan) lived longer when fed honey, and discussed the use of sugars as potential food supplements in the field to sustain parasitoid effectiveness. In California, olives may be grown in proximity to citrus orchards. The black scale is common throughout the coastal and Central Valley of California (Daane et al. 1991). The current study suggests that those natural food sources may improve the parasitoid survival in the field after being released.
Our laboratory studies also showed that high summer temperatures that reflect the San Joaquin Valley summer conditions (Johnson et al. 2006) could significantly reduce the longevity of P. humilis without water or food. However, when adequate food sources are available, the parasitoid could still produce F1 progeny. Therefore, the availability of food is key for survival and reproduction of the parasitoids under adverse climate conditions, and also applies to the host fly (Wang et al. 2009a, b). Therefore, our laboratory studies suggest that parasitism or recovery of the parasitoid could be affected not only by temperature condition but also the availability of food sources in the field. However, the availability of food sources could be quite different at different release sites, and this may partly explain the large variation of parasitism among different sites. For example, the highest percentage parasitism in Cayucos could contribute to the most suitable temperature condition but also the possibility that the parasitoids may have readily located food and survived throughout the exposure period. This further limits our ability to predict field parasitism.
The current study shows that a brief exposure to GF-120 for 2 h did not cause a significantly higher mortality of P. humilis when compared with artificial honeydew. However, several other laboratory studies showed low adult survival after direct contact to GF-120 for the fruit fly parasitoids Psyttalia fletcheri Silvestri, Fopius arisanus (Sonan), and Diachasmimorpha tryoni (Cameron) (Wang et al. 2005), and D. longicaudata (Ruiz et al. 2008). Parasitoids are not attracted to GF-120 or directly fed on GF-120, but are observed to be trapped in the bait solution that leads to a direct body contact with GF-120 and consequently mortality (Wang et al. 2005). The current study further suggests that P. humilis does not feed on GF-120 that supports use of the parasitoid in olive integrated pest management programs that recommend the use of GF-120 for control of olive fruit fly, although repeat application of GF-120 in orchards may contaminate honeydew; reducing the availability of natural food sources for natural enemies in ecosystems.
We are grateful to Gina T. Miller and Gail E. Sergent, USDA-ARS, San Joaquin Valley Agricultural Sciences Center, Parlier, CA; Miguel Lopez and Alicia Aldana, USDA-APHIS-PPQ, Moscamed Program, Guatemala City, Guatemala; Martha Gerik, University of California Kearney Agricultural Center, Parlier, CA; Melanie Durbin, California State University, East Bay, Hayward, CA; and Barat Bisabri, Dow AgroSciences, Orinda, CA, for their assistance; Bill Krueger, Orland, CA; Mission San Juan Batista, CA; Steve Martin, Cayucos, CA; Mike Fusano, Sylmar, CA; Gebb Turpin, Santa Barbara, CA; and Ross Rizzo, Rancho Bernardo Winery, Rancho Bernardo, California for use of their olive orchards for this project. This research was funded in part by the California Olive Committee, Fresno, CA, and the USDA CSREES Special Grants Program: Pest Management Alternatives.
References
Author notes
This article reports the results of research only. Mention of a proprietary product does not constitute an endorsement or recommendation for its use by the USDA-ARS or USDA-APHIS-PPQ.



