-
PDF
- Split View
-
Views
-
Cite
Cite
P. A. Eliopoulos, D. C. Kontodimas, G. J. Stathas, Temperature-Dependent Development of Chilocorus bipustulatus (Coleoptera: Coccinellidae), Environmental Entomology, Volume 39, Issue 4, 1 August 2010, Pages 1352–1358, https://doi.org/10.1603/EN09364
Close - Share Icon Share
Abstract
The effect of temperature on development and survival of Chilocorus bipustulatus L. (Coleoptera: Coccinellidae), a predator of many scale insects, was studied under laboratory conditions. The duration of development of egg, first, second, third, and fourth larval instars, pupa, and preovioposition period at seven constant temperatures (15, 17.5, 20, 25, 30, 32.5, and 35°C) was measured. Development time decreased significantly with increasing temperature within the range 15–30°C. Survival was higher at medium temperatures (17.5–30οC) in comparison with that at more extreme temperature regimens (15 and >30οC). Egg and first larval instars were the stages where C. bipustulatus suffered the highest mortality levels at all temperatures. The highest survival was recorded when experimental individuals were older than the third larval instar. Thermal requirements of development (developmental thresholds, thermal constant, optimum temperature) of C. bipustulatus were estimated with application of linear and one nonlinear models (Logan I). Upper and lower developmental thresholds ranged between 35.2–37.9 and 11.1–13.0°C, respectively. The optimum temperature for development (where maximum rate of development occurs) was estimated at between 33.6 and 34.7°C. The thermal constant for total development was estimated 474.7 degree-days.
The armored-scale ladybeetle Chilocorus bipustulatus L. (Coleoptera: Coccinellidae) is one of the most important predators of diaspidids, with intense presence not only in Greece (Argyriou et al. 1976; Argyriou and Katsoyannos 1977; Stathas et al. 2003,2009) but in the whole Palearctic region (Bodenheimer 1951, Avidov and Harpaz 1969, Viggiani 1985). Apart from armored scales, it may alternatively feed on other scale families such as Coccidae, Asterolecaniidae (Yinon 1969), and Pseudococcidae (Avidov and Yinon 1969).
Prey species of C.bipustulatus are among the most important pests on perennials and ornamental plants, common in all regions in the world. The presence of armored scales on fruits and ornamentals affects their appearance, rendering them unmarketable. They may also cause discolorations, deformations, skin sclerification, and cavities on fruit surface. On the leaves, they cause chlorosis, reduce photosynthesis and transpiration, and encourage leaf senescence and abscission, followed by branch dieback, as a result of plant sap sucking through their long, filamentous mouthparts (Katsoyannos 1996, Gill 1997).
Although developmental thresholds and day-degree requirements for development of many aphid and mealybug coccinellid predators have been estimated, only a few data are available as far as diaspidid predators are concerned (Podoler and Henen 1983, Ponsonby and Copland 1996, Stathas 2000).
The lack of experimental data about the effect of temperature on the development of C. bipustulatus, its importance as a biocontrol agent against diaspidids, and the significance that the effect of temperature on insect development has for biological control made us determined to estimate the lower and upper developmental thresholds, as well as the optimum temperature and day-degree requirements, for development of this predator. Estimation of lower developmental threshold (t) and day-degree requirements for development (or thermal constant [K]) for each developmental stage was undertaken by fitting experimental data on a linear model. The upper developmental threshold (Tm) and optimum temperature for development (To) were calculated by application of a nonlinear model (Logan I). Most of these parameters are novel for C. bipustulatus.
Materials and Methods
Chilocorus bipustulatus was reared on the oleander scale Aspidiotus nerii Bouché (Hemiptera: Diaspididae) infesting potato tubes. The initial population of both predator and prey originated from olive orchards in Attiki, Greece. The duration of separate developmental stages (egg, first, second, third, and fourth larval instars, and pupa) and adult preoviposition period of C. bipustulatus was measured at seven constant temperatures (15 ± 1, 17.5 ± 1, 20 ± 1, 25 ± 1, 30 ± 1, 32.5 ± 1, and 35 ± 1°C). The relative humidity was 65 ± 5% and the photoperiod was 16-h light: 8-h darkness at all temperatures. All experimental individuals were kept in growth chambers with the desired temperature regimen according to experimental design. All dishes with individuals of the same temperature treatment were kept in the same chamber and were observed at the same time.
To get experimental individuals, mated females were transferred to oviposition cages and left undisturbed for 24 h to lay their eggs on A. nerii. Afterward, females were taken away, and eggs were collected and put individually on petri dishes (9 cm in diameter and 1.6 cm high). Special care was taken so that experimental individuals were always supplied with an excess of prey. Experimental individuals were checked daily (every 12 h for individuals kept at 32.5 and 35°C), and developmental stage was recorded. Data were taken from 25 individuals except those at 15, 32.5, and 35°C, where 50 individuals were studied (because of increased egg mortality).
For the preoviposition period study, newly emerged females were collected from the culture, paired with 7- to 10-d-old males, and put on petri dishes with excess of prey. Pairs were checked daily, and initiation of egg-laying was recorded. The study on the duration of preoviposition period was carried out on 12 females at 15 and 35°C, 14 females at 17.5°C, 15 females at 20, 30, and 32.5°C, and 16 females at 25°C.
Lower and Upper Developmental Thresholds.
The regression line was extrapolated back and met the absicca at the developmental zero t, which was calculated from t = -a/b. The total quantity of thermal energy required to complete development, the thermal constant K, was calculated from the reciprocal of the slope of the regression line (1/b) (Wigglesworth 1953, Campbell et al. 1974).
The base temperature Tb is the minimum temperature used in the experiments, is inserted as a fifth equation parameter, or can hypothesized as Τb = 0οC. As stated by Lactin et al. (1995) and Got et al. (1997), the model effectiveness is not affected by the hypothesis of zero Tb. Taking also into account that the model is simplified and easier for application, Tb was zero in this study. Parameters ψ, ρ, Τm, and ΔΤ are not measured directly but are estimated as parameters of nonlinear regression where the Logan I equation is used as model. This nonlinear regression was carried out with the statistical program JMP (SAS Institute 2007).
Statistical Analysis.
Data (duration of development) were subjected to analysis of variance (ANOVA) with α = 0.05. Means were separated using the Tukey-Kramer honestly significant difference (HSD) test (Sokal and Rohlf 1995). The differences in survival percentages during total development among treatments were determined by GLIMMIX procedure (SAS Institute 2008). Statistical analysis was performed using the statistical packages JMP (SAS Institute 2007) and SAS/STAT 9.2 (SAS Institute 2008).
Results
The effect of temperature on the development of C. bipustulatus is presented in Table 1 and shows that the developmental period decreased with increasing temperature, as expected, within the range of favorable temperatures. Duration of all developmental stages of C. bipustulatus differed significantly among various temperatures from 15 to 30οC (Table 1): egg (F = 437.65, df = 6,130, P < 0.0001), first larval instar (F = 421.94, df = 6,94, P < 0.0001), second larval instar (F = 224.98, df = 6,79, P < 0.0001), third larval instar (F = 244.39, df = 6,72, P < 0.0001), fourth larval instar (F = 345.50, df = 6,68, P < 0.0001), pupa (F = 265.02, df = 6,65, P < 0.0001), and total preimaginal period (F = 664.00, df = 6,65, P < 0.0001). The same pattern was recorded in the duration of preoviposition period of the adult female (F = 246.75, df = 6,92, P < 0.0001). No significant differences in duration of development were recorded among temperatures ≥30°C.
Duration of development (d) of C. bipustulatus (mean ± SE) fed on A. nerii at various constant temperatures

Values in the same row followed by the same letter are not significantly different, Tukey-Kramer HSD test, α = 0.05. Values in brackets are min-max.
n, no. of cohort adults.
Duration of development (d) of C. bipustulatus (mean ± SE) fed on A. nerii at various constant temperatures

Values in the same row followed by the same letter are not significantly different, Tukey-Kramer HSD test, α = 0.05. Values in brackets are min-max.
n, no. of cohort adults.
The linear regression equations and nonlinear model parameters that describe the relationship between temperature and developmental rate as well as upper (T m), lower (t), and optimum temperature threshold (To) and day-degree requirements (K) for each life stage and total immature development of C. bipustulatus are presented in Tables 2 and 3. The upper and lower threshold and optimum temperature for development varied with developmental stage. However, in most cases, the differences were minor. Eggs and preovipositing females were the most cold-tolerant stages, provided that their lower thresholds (11.1 and 11.4°C) were notably lower than those of the other developmental stages. Pupae and preovipositing females also proved to be the most tolerant stages at high temperatures (37.9 and 37.4°C). However, the rest of the preimaginal stages were more sensitive to high temperatures, showing identical upper developmental threshold (Tm = 35.2°C). The thermal constant reached 474.7 degree-days for overall (egg to adult) development (Table 2).
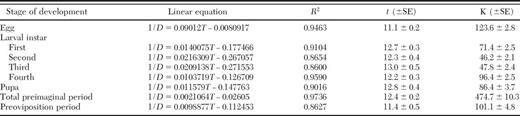
Data were obtained from five constant temperatures (15, 17.5, 20, 25, and 30°C).
D, duration of development (d); T, temperature (°C); R2 , coefficient of determination; t, lower developmental threshold (°C); K, thermal constant (degree-days).

Data were obtained from five constant temperatures (15, 17.5, 20, 25, and 30°C).
D, duration of development (d); T, temperature (°C); R2 , coefficient of determination; t, lower developmental threshold (°C); K, thermal constant (degree-days).
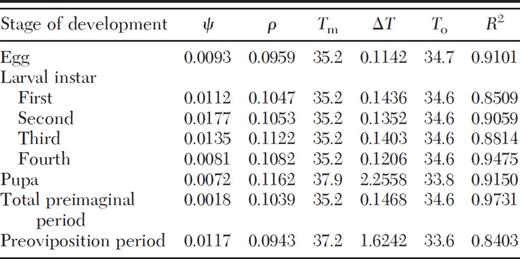
Data were obtained from seven constant temperatures (15, 17.5, 20, 25, 30, 32.5, and 35°C).
Ψ, ρ. Tm, Δ T, Logan equation parameters; To , optimum temperature for development; R2 , coefficient of determination for nonlinear regression.

Data were obtained from seven constant temperatures (15, 17.5, 20, 25, 30, 32.5, and 35°C).
Ψ, ρ. Tm, Δ T, Logan equation parameters; To , optimum temperature for development; R2 , coefficient of determination for nonlinear regression.
Survivorship (percentages) of immature stages of C. bipustulatus fed on A. nerii at various constant temperatures
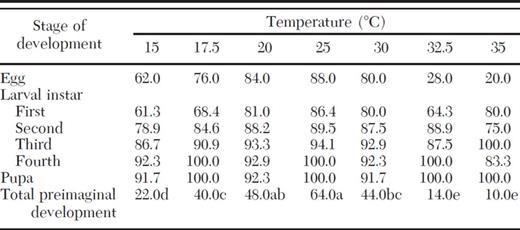
Values of total preimaginal development followed by the same letter are not significantly different (SAS/STAT 9.2, GLMMIX procedure, α = 0.05).
Survivorship (percentages) of immature stages of C. bipustulatus fed on A. nerii at various constant temperatures

Values of total preimaginal development followed by the same letter are not significantly different (SAS/STAT 9.2, GLMMIX procedure, α = 0.05).
The rate of development increased linearly with temperature within the temperature range 15-30°C, as indicated by the high values of coefficient of determination (R2 = 0.8600-0.9736; Table 2), and declined at higher temperatures (Fig. 1). Similarly, the nonlinear model (Logan I) proved to be very efficient in describing the relationship of rate of development with temperature (Table 3; Fig. 1), given that the regression coefficient valued from 0.8403 to 0.9731.
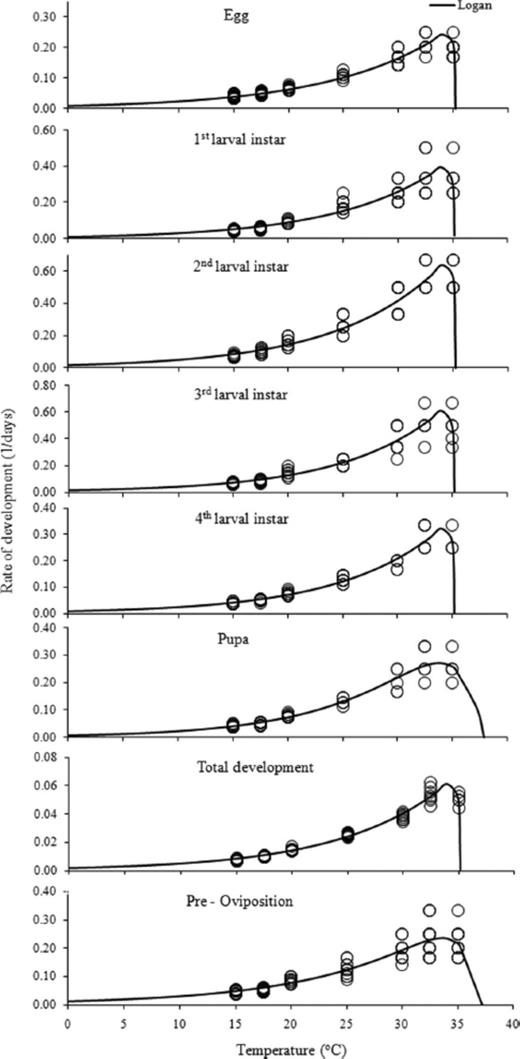
Relationship of rate of development of C. bipustulatus with temperature described by the Logan I model (white circles represent the observed data).
Experimental individuals showed significantly higher survival at medium temperatures (17.5-30°C; 40-64%) in comparison with those kept at more extreme temperature regimens (15 and >30°C; 10-22%). The egg was the stage where C. bipustulatus had the highest mortality levels at all temperatures, taking into account that 12-80% of individuals did not manage to hatch irrespective of temperature. On the contrary, the highest survival was recorded during the pupal stage (91.7-100% at all temperatures).
The proportion of the total development time spent in a particular developmental stage proved to be independent of temperature, provided that egg, larval, and pupal stage of C. bipustulatus ranged between 20.6-25.5, 55.9-60.0, and 18.8-20.7% of total preimaginal period, respectively, regardless of temperature.
Discussion
There is little information available on the effect of temperature on the development of C. bipustulatus. Podoler and Henen (1983) reported that the beetle completed its development (from egg to adult) in 80.5 d at 18°C, 53.9 d at 22°C, 38.2 d at 24°C, 27.8 d at 26°C, 27.8 d at 28°C, 31.2 d at 30°C, and 27.1 d at 32°C. They also estimated the lower developmental threshold and thermal constant for each developmental stage from egg to adult using the law of heat summation according to the Blünck-Bodenheimer, which is practically the same equation as the linear model used in this study. The lower developmental threshold provided by Podoler and Henen (1983) was 12.2, 8.5, 6.8, 3.0, 12.8, and 12.2°C and the thermal constant was 112.1, 104.4, 65.5, 107.2, 92.8 and 104.6 DD for egg, first, second, third, and fourth larval instar, and pupa, respectively.
It is obvious that there are many differences among the above-mentioned data with those of this study. This variation may be attributed to the different prey used in the earlier study, which was a mixture of diaspidids including Aonidiella aurantii (Mask.), Chrysomphalus aonidum L., and A. nerii (Hemiptera: Diaspididae). The type of prey has a significant effect on the duration of development of C. bipustulatus and its mortality (Uygun and Elekçioğlu 1998). This fact is also true for other coccinellids preying on aphids (Omkar and Srivastava 2003, Özder and Sağlam 2003, Ali and Rizvi 2007) and mealybugs (Chong et al. 2005). Another factor that may have caused this variation is the genetic differences among populations with different geographical origin, taking into consideration that our population originated from Attiki (Central Greece), whereas the Turkish population originated from Adana Turkey (east Mediterranean region). It is well known that intraspecific variation in developmental traits, especially in critical temperatures, is very common in Coccinellidae (Honĕk and Kocourek 1988, Lamana and Miller 1998). These differences in thermal requirements of populations from different origins within a species indicate the need to undertake relative studies on native coccinellids when trying to evaluate them as biological control agents.
Apart from that, data obtained from 32°C were included on the linear equation for the estimation of the lower developmental threshold and thermal constant. However, this temperature is extreme for C. bipustulatus, as has been shown by this and older studies, and lies within the range where the relationship of temperature and rate of development is nonlinear. Those values should not be included in the linear estimation of thermal values (Mills 1981). This is probably the reason for the extremely low value of many developmental thresholds of C. bipustulatus (8.5, 6.8, and 3.0°C) reported by Podoler and Henen (1983).
When the same prey (A. nerii) was used, development from egg to adult and preoviposition period lasted 26 and 9.6 d, respectively, at 25°C (Uygun and Elekçioğlu 1998). Similar values have been recorded in this study (29.4 and 8.3 d). In the same study, 16.6% of newly hatched larvae did not reach adult stage at 25°C, which agrees with our data (15.8%).
Another interesting finding of this study was the fact that the proportion of egg, larval, and pupal periods did not change notably, regardless of temperature. These results are consistent with the suggestion that the proportion of the development times of preimaginal stages for ladybird species is typical of each stage and independent of temperature (Hodek and Honĕk 1996, Dixon 2000).
Eggs and preovipositing females proved to be the most tolerant stages in low temperatures (11.1 and 11.4°C, respectively). This result comes in accordance with the fact that the latter is the hibernating stage of C. bipustulatus in nature (Bodenheimer 1951, Avidov and Yinon 1969, Katsoyannos 1996).
The Logan I model was very effective in describing the effect of temperature on the rate of development of C. bipustulatus as is clearly indicated by the high values of the nonlinear regression coefficient (R2 = 0.8403-0.9731). This model has been successfully used for describing the temperature-dependent development of coccinellids (Roy et al. 2002, Kontodimas et al. 2004).
This is the first time that upper developmental threshold and optimum temperature for development has been estimated for C. bipustulatus. Therefore, these results broadened our knowledge of the critical temperatures of this predator. All developmental stages of C. bipustulatus seem to be very tolerant of warm conditions, especially for the temperate climate of the Mediterranean region.
Among indigenous predators of armored scales, the coccinellids Rhyzobius lophanthae Blaisdell and C. bipustulatus (Coleoptera: Coccinellidae) and the nitidulid Cybocephalus fodori Endrödi-Younga (Coleoptera: Nitidulidae) are the most important in Greece (Katsoyannos 1984,1996; Stathas 1996,2000). Data regarding critical temperatures of the two above-mentioned competitors of C. bipustulatus are available only for R. lophanthae. This predator is more tolerant than C. bipustulatus in cool temperatures, given that the lower developmental threshold varies between 7.6 and 9.3°C according to developmental stage (Stathas 2000). This may explain the increased frequency of this coccinellid during winter in Greek olive and citrus orchards in comparison with C. bipustulatus (Katsoyannos 1984,1996; Stathas 1996,2000).
The importance of critical temperatures for understanding life histories has long been recognized. Lower developmental threshold and thermal constant are not only good predictors of the timing of life history events (Honĕk and Kocourek 1988), but also very useful indicators for an insect's potential distribution (Campbell et al. 1974). As far as biological control is concerned, the estimation of the performances of natural enemies (i.e., development time, critical temperatures, thermal constant), can contribute to the selection of the most suitable biological control agent to be used under different environmental conditions, as well as to set the best thermal condition for insect mass rearing (Perdikis and Lykouressis 2002).
In conclusion, the results of this study provide useful data for establishing or mass rearing C. bipustulatus (i.e., mass culture methods could be enhanced by setting temperature conditions for rapid development and maximum survival). Furthermore, the knowledge of the critical temperatures of C. bipustulatus is very useful for determining the climatic conditions affecting its efficacy against armored scales. Our findings indicate that C. bipustulatus could be a useful biological control agent against A. nerii and possibly other diaspidid pests, under a wide range of temperatures, especially in areas with a temperate climate. However, for a more accurate evaluation of the impact of this predator on its prey species, additional work on other biological parameters, apart from critical temperatures and survival, such as fecundity, population dynamics, searching efficiency, etc., is needed, especially under natural conditions where environmental heterogeneity may influence and perhaps alter predator and prey interactions.
We thank G. C. Fernandez and P. Shi for guidance with statistic analysis and the editor and anonymous reviewers for useful comments.
References



