 The Oxford Handbook of Mood Disorders
The Oxford Handbook of Mood Disorders
Contents
-
-
-
-
-
-
-
-
Introduction Introduction
-
Adaptation and Disorder Adaptation and Disorder
-
Depressive Adaptations Depressive Adaptations
-
Sickness Behavior Sickness Behavior
-
Starvation Depression Starvation Depression
-
A Generalized Hypothesis A Generalized Hypothesis
-
-
The Evolution of Melancholic Depression The Evolution of Melancholic Depression
-
Analytical Processing is Promoted Throughout the Continuum of Melancholic Symptoms Analytical Processing is Promoted Throughout the Continuum of Melancholic Symptoms
-
Depression Is Triggered by Complex Problems Depression Is Triggered by Complex Problems
-
Analytical Rumination Helps People Solve, Manage, or Cope with the Triggering Problem Analytical Rumination Helps People Solve, Manage, or Cope with the Triggering Problem
-
Analytical Rumination Is Energetically Expensive Analytical Rumination Is Energetically Expensive
-
Other Energetically Expensive Processes Are Downregulated Other Energetically Expensive Processes Are Downregulated
-
-
Other Depressive Adaptations? Other Depressive Adaptations?
-
Conclusions Conclusions
-
References References
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
3 The Evolution of Depressive Phenotypes Purchased
Evolutionary Ecology of Health Research Labs, Department of Psychology, Neuroscience and Behaviour, McMaster University, Hamilton, Ontario, Canada
Evolutionary Ecology of Health Research Labs, Department of Psychology, Neuroscience and Behaviour, McMaster University, Hamilton, Ontario, Canada and Social Aetiology of Mental Illness (SAMI) CIHR Training Program, Centre for Addiction and Mental Health, Toronto, Ontario, Canada
-
Published:19 January 2016
Cite
Abstract
Depression is a heterogeneous collection of phenotypes sharing partially overlapping genes, neurobiology, and symptoms. This chapter applies an evolutionary perspective to the distinct etiologies and functions of three reliably identified depressive phenotypes: sickness behavior, starvation depression, and melancholia (i.e., depression with melancholic features). Infection and food shortage are evolutionarily ancient problems, and so sickness behavior and starvation depression probably evolved first. Melancholia probably evolved more recently and shows signs of an evolutionary design for a cognitive function. More specifically, evidence suggests that melancholia is an adaptation for promoting analytical reasoning, and probably evolved as an adaptive response to complex problems involving resource management or conflicts with close social partners. These depressive phenotypes, although distinct, are functionally similar, which explains the overlapping genetics, neurobiology, and symptomatology. In all three, depressive symptoms help the body coordinate the reallocation of limited energy resources in response to persistent threats.
Introduction
Depressive disorders, as they are currently diagnosed, encompass a suite of behavioral syndromes defined by sad mood and anhedonia that are otherwise heterogeneous in symptom and cause (Akiskal, 2008). Different depressive disorders are distinguished by the presence or absence of other behavioral symptoms. There are many diagnostic categories, and not all of them are reliable, so it is not clear that they all “carve nature at its joints.” Moreover, many etiological pathways can lead to clusters of depressive symptoms, including some not typically classified as psychiatric disorder (Durisko, Mulsant, & Andrews, 2015). In this chapter, we focus on the evolution of three reliably identified depressive phenotypes with overlapping symptoms: sickness behavior, starvation depression, and melancholic depression. The symptoms of each are listed in Table 3.1.
Sickness behavior is induced following the onset of a challenge to the immune system and is thought to promote recovery. Starvation depression is caused by a persistent lack of food and is thought to promote survival during periods of energy shortage. Of clinically diagnosed episodes of major depression, melancholia is the most reliably diagnosed and most common subtype, often accounting for 50% or more of episodes in community or patient samples (Angst, Gamma, Benazzi, Ajdacic, & Rössler, 2007; Xiang et al., 2012). Some episodes of melancholic depression used to be termed endogenous to refer to the lack of an obvious environmental trigger. However, these causes are sometimes underreported due to their severe, personal, or embarrassing nature (Leff, Roatch, & Bunney, 1970; Mundt, Reck, Backenstrass, Kronmüller, & Fiedler, 2000). In fact, melancholia is highly associated with adverse events and life stressors (Taylor & Fink, 2008). Although melancholia is not a separate diagnostic category in the Diagnostic and Statistical Manual for Mental Disorders (DSM), a recent post for The New Yorker revealed that the writers of the latest edition did not create a separate category because its reliability would have drawn attention to the subjectivity and imprecision associated with other DSM categories (Greenberg, 2013).
| Symptoms . | Sickness Behavior . | Starvation . | Melancholia . | Inescapable Shock (Rats) . |
|---|---|---|---|---|
Anhedonia | ↑9 | ↑21 | ↑22 | |
Weight | ↓9 | ↓21 | ↓22 | |
Sexual behavior | ↓9 | ↓21 | ↓22 | |
HPA axis activity | ↑3 | ↑20 | ↑21 | ↑22 |
Altered focus of attention | Yes10 | Yes9 | Yes1 | |
Complex information processing | ? | Yes1 | ||
Sleep duration | ↑7 | —13 | ↓21 | ↓16 |
REM sleep | ↓11 | ↓13 | ↑21 | ↑16 |
Slow wave sleep | ↑11 | ↑13 | ↓21 | ↓16 |
Passive coping | Yes3 | ? | Yes15 | Yes22 |
Motor activity | ↑6 | ↓21 | ↓8 | |
Body temperature | ↓18 | ↑19 | ↑4 | |
Preference for carbohydrate over protein | ↑3 | ↓17 | ↑2 | ↑5 |
| Symptoms . | Sickness Behavior . | Starvation . | Melancholia . | Inescapable Shock (Rats) . |
|---|---|---|---|---|
Anhedonia | ↑9 | ↑21 | ↑22 | |
Weight | ↓9 | ↓21 | ↓22 | |
Sexual behavior | ↓9 | ↓21 | ↓22 | |
HPA axis activity | ↑3 | ↑20 | ↑21 | ↑22 |
Altered focus of attention | Yes10 | Yes9 | Yes1 | |
Complex information processing | ? | Yes1 | ||
Sleep duration | ↑7 | —13 | ↓21 | ↓16 |
REM sleep | ↓11 | ↓13 | ↑21 | ↑16 |
Slow wave sleep | ↑11 | ↑13 | ↓21 | ↓16 |
Passive coping | Yes3 | ? | Yes15 | Yes22 |
Motor activity | ↑6 | ↓21 | ↓8 | |
Body temperature | ↓18 | ↑19 | ↑4 | |
Preference for carbohydrate over protein | ↑3 | ↓17 | ↑2 | ↑5 |
“?” indicates data are not available. “—” indicates no significant change in the symptom. Symptoms shaded in gray are shared across phenotypes.
The multiple pathways for producing depressive syndromes probably require multiple evolutionary explanations. There are many such hypotheses (Durisko et al., 2015; Hagen, 2011), although most have not been rigorously tested and many are undoubtedly incorrect. This chapter focuses on hypotheses that offer insights into the symptomatic similarity between sickness, starvation, and melancholic depressions. We first define relevant evolutionary concepts.
Adaptation and Disorder
An important evolutionary insight into mood disorders is that some may not really be “disorders” at all. The current DSM acknowledges the inability to “completely separate normal and pathological symptom expressions” in its diagnostic system (APA, 2013, p. 21). In this volume, Jerome Wakefield argues that all unambiguous cases of disorder involve an adaptation that is not performing its evolved function, and we adopt this definition in this chapter. An adaptation is a trait that has been modified by natural selection over evolutionary time for a unique gene-propagating effect (Andrews, Gangestad, & Matthews, 2002). That gene-propagating effect is then called the function of the trait. Like man-made machinery, adaptations can break down abruptly or gradually decline in function, and these events can be termed malfunctions or dysfunctions. The vertebrate eye is a classic example of an evolved adaptation, yet it is susceptible to many known malfunctions (or disorders). Under this evolutionary definition of disorder, a properly functioning adaptation cannot be disordered.
As we show in this chapter, all the purportedly pathological symptoms of depression, including anhedonia, reduced sexual function and appetite, altered cognition, and suicidal behavior, can be produced by properly functioning adaptations. This makes it difficult to distinguish true instances of disorder and “normal” responses to stressors by such criteria. Importantly, the claim that many serious episodes of depression may be adaptive does not imply that they should not be treated. Childbirth is also a normal adaptation with an evolutionarily ancient history, but it often requires medical intervention because it can be dangerous in humans. Nevertheless, it is a cardinal rule of medicine that appropriate treatment depends on an accurate understanding of etiology. Determining an appropriate treatment for depression depends on determining whether it is a properly functioning adaptation or a malfunctioning adaptation.
How do we recognize adaptations and determine whether they are functioning properly? This cannot be done reliably by measuring the reproductive success associated with the trait in the modern environment (Andrews et al., 2002). Claims about adaptations are historical and refer to the ancestral selection pressures that shaped the trait in question. Reproductive success associated with an adaptation may be different in modern and ancestral environments. Adaptations associated with lower reproductive success in modern environments exist because they conferred an advantage in ancestral environments. Our preference for sugar was undoubtedly adaptive in ancestral environments where refined carbohydrates were not freely available. Although this preference can cause disorders and reduce reproductive success in modern environments (diabetes, heart disease, etc.), the preference is not itself a disorder. Rather, it is an adaptation that was forged in ancestral environments, and it is still operating as it evolved to operate even though the environment has changed.
The only reliable method for identifying adaptations is to decompose all the working parts of a trait and figure out how they interact together. This process has been called “reverse engineering” (Andrews et al., 2002; Tooby & Cosmides, 2000). Biological organisms are highly organized, well-coordinated assemblages of matter, and evolution by natural selection is the only known natural process that can generate nonrandom biological organization or coordination. If a trait exhibits a highly nonrandom organization or coordination for promoting a particular effect, so that the only plausible explanation is that natural selection shaped the trait for the effect, then the trait is an adaptation and the promoted effect is its function. Thousands of years of reverse engineering research, for instance, has revealed that the eye is composed of multiple highly organized structures (retina, lens, pupil, iris, etc.). These components operate together in a highly nonrandom, coordinated fashion to promote vision. The only plausible scientific account for this design is that natural selection shaped the eye over evolutionary time to promote vision.
During the reverse engineering process, researchers develop a conceptual “blueprint” of the structure and operation of the adaptation. This blueprint also provides a natural understanding of the ways in which the adaptation can malfunction and cause disorder. For instance, the conceptual blueprint of the eye allows us to understand many specific disorders (e.g., hardening of the lens, detachment of the retina from the choroid, increased intraocular pressure due to degradation of the trabecular meshwork).
Depressive Adaptations
Depressive syndromes are almost certainly not a hodgepodge of symptoms without causal ordering. Evolutionary theory suggests that they can be usefully organized around the emotion of sadness (Figure 3.1).
Emotions are ancient adaptations that evolved to coordinate body systems to promote adaptive responses to problems in the environment (Tooby & Cosmides, 1990). The behavioral outputs of emotions are not fixed, so the evolved function of emotions must lie upstream, at the level of information processing, where the body can assess the situation and make decisions about how best to respond. Thus, emotions such as sadness are thought to be an integral part of the causal pathway by which the body produces adaptive responses to the environment. They are reliably triggered by specific situations in the environment, and they coordinate various systems of the body to promote an information-processing function. Because depressive phenotypes are really clusters of symptoms, of which sadness is one, some symptoms may be part of the triggering process, whereas others are part of the subsequent coordination process. We discuss sickness behavior and starvation depression in terms of this general causal model.
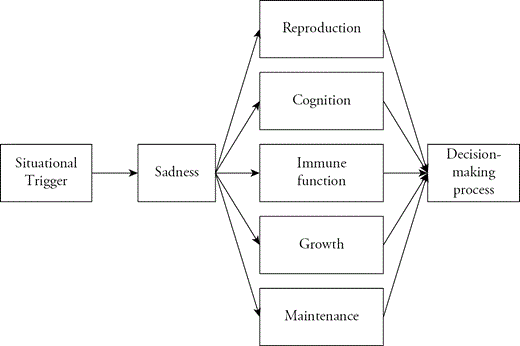
Hypothetical schematic of the causal structure of depressive adaptations.
Sickness Behavior
Infection is a problem facing all organisms (Fuhrman, 1999), and sick organisms exhibit an adaptive depressive phenotype that is evolutionarily ancient (Dantzer, 2001; Hart, 1988). Sickness behavior is present across mammals (Hart, 1988), and aspects can even be found in insects (Chambers & Schneider, 2012). Obviously, eliminating a pathogen from the body requires a response from the immune system. This response is not fixed, but rather is tailored to the pathogen, so pathogen-specific information must be processed to create the appropriate response. Generating an appropriate immune response requires massive amounts of energy, so the body reallocates energy to immune function while downregulating other energetically expensive processes, including growth, reproduction, and physical activity (Lochmiller & Deerenberg, 2000) (Figure 3.2). General cognitive functioning declines during infection, because attention is drawn to painful sensations (Kaplan, Trevino, Johnson, & Levy, 2003; Kramer et al., 2002), which promotes lethargy and rest. Depressive symptoms play a role in coordinating these trade-offs (Dantzer, 2001; Hart, 1988). Lethargy and anhedonia reduce energy spent on normally rewarding activities such as sex and play. Appetite also decreases, which may seem puzzling given the energetic demands of the immune system. But organisms must often expend considerable energy to acquire food and may be better off conserving energy (Hart, 1988). Moreover, the consumption of foods high in iron (e.g., animal protein) can promote pathogen replication (Hart, 1988), whereas the immune system preferentially runs on carbohydrates (Wolowczuk et al., 2008). Thus, although appetite and overall caloric intake are reduced during infection, the proportion of carbohydrates in the diet increases and the proportion of protein decreases (Dantzer, 2001).
Starvation Depression
Starvation is also a problem that all organisms face, and it can trigger an adaptive depressive phenotype (Keys, Brozek, & Henschel, 1950). To outlast food shortages, the body must monitor systems crucial to maintenance and carefully triage the allocation of energy stored in tissues (adipose tissue, muscle) to maintenance functions (McCue, 2012; Prentice, 2005) (Figure 3.2). The brain in particular is preserved, and other tissues are sacrificed (Ruiz-Núñez, Pruimboom, Dijck-Brouwer, & Muskiet, 2013). Growth, reproduction, and immune function are downregulated to reduce metabolism (Prentice, 2005; Prentice & Keneba, 2007). The symptoms of depression triggered during starvation coordinate these trade-offs (Engel & Schmale, 1972). For instance, many hedonic activities (e.g., sex, social interaction) are energetically expensive, so interest in such activities is reduced.
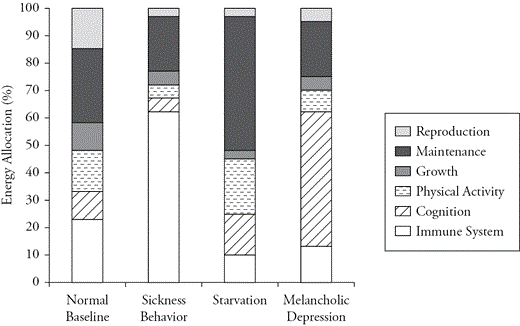
Hypothetical energy allocation during sickness, starvation, and depression, relative to normal baseline.
A Generalized Hypothesis
Sickness behavior and starvation depression share a functional similarity. In both, depressive symptoms help coordinate energetic trade-offs in response to persistent threats—infection and starvation. In sickness behavior, immune function gets prioritized access to resources while growth and reproduction are reduced. In starvation, maintenance functions get prioritized access to resources while growth, reproduction, and immune function are reduced.
This suggests that natural selection may favor the evolution of depressive phenotypes in response to any persistent threat in which the body must make sustained trade-offs in the allocation of limited resources. This generalized resource allocation hypothesis suggests that any specific hypothesis for depression that is structured around resource allocation logic warrants greater attention and should be rigorously tested. In the next section, we focus in detail on one hypothesis for melancholic depression.
The Evolution of Melancholic Depression
The symptoms of sickness behavior and melancholic depression are strikingly similar (Table 3.1), and they share common genes and neurobiology (Dantzer, O’Connor, Freund, Johnson, & Kelly, 2008; Raison & Miller, 2013). This similarity has spurred hypotheses characterizing depression as a disorder of the immune response (Dantzer et al., 2008) or as an adaptive response to social stressors that predict the risk of infection (Raison & Miller, 2013). However, these hypotheses are inconsistent with the fact that sickness behavior and melancholia have key symptomatic differences. Cognition is generally impaired during sickness, and more time is spent in slow wave sleep (Dantzer, 2001; Larson & Dunn, 2001). In contrast, melancholic depression is associated with an increase in rapid eye movement (REM) sleep (Taylor & Fink, 2008) and rumination (Nelson & Mazure, 1985). Rumination is a cognitive symptom referring to intrusive, distraction-resistant thoughts focused on the circumstances surrounding the episode (Andrews & Thomson, 2009).
We can now draw powerful inferences about the origin and function of melancholia. Although sickness, starvation, and melancholic depressions are all expressed in humans, the cognitive nature of melancholia suggests that it evolved more recently (Figure 3.3). The neurobiology they share in common likely first evolved to promote sickness behavior or starvation depression. Later, it was coopted and modified to promote melancholia. The evidence of cooption lies in the overlapping symptoms, genes, and neurobiology. The evidence of modification comes from the symptomatic differences, which must involve distinct neurobiology. Even though melancholia probably evolved more recently, it may not be unique to humans since the inescapable shock model of depression is symptomatically similar (Table 3.1).
The unique symptoms of melancholia suggest that the modification is attributable to natural selection for a cognitive function. That is, the neurological mechanisms that produce melancholic symptoms may be an adaptation for enhanced cognition that evolved as an extension of the energy reallocation machinery of sickness behavior. First, ruminative thinking involves an analytical processing style in which complex problems are broken into smaller, more manageable components, which are then studied sequentially (Andrews & Thomson, 2009). Analysis is a highly useful approach to solving complex problems, such as in science, mathematics, and many areas of modern life. Second, rumination is resistant to distraction (Andrews & Thomson, 2009). To keep track of the components, analysis requires working memory (WM), a memory system in which information is kept active because it is useful for ongoing processing. As WM load increases, tasks become more vulnerable to interruption because it is easier for task-irrelevant stimuli to displace relevant information (Kane & Engle, 2002). The distraction-resistant nature of rumination may promote analysis by reducing the vulnerability to interruption (Andrews & Thomson, 2009). Third, the increase in REM sleep also points to a cognitive function, since REM sleep helps consolidate hippocampal memory representations that encode complex information (Rasch & Born, 2013).
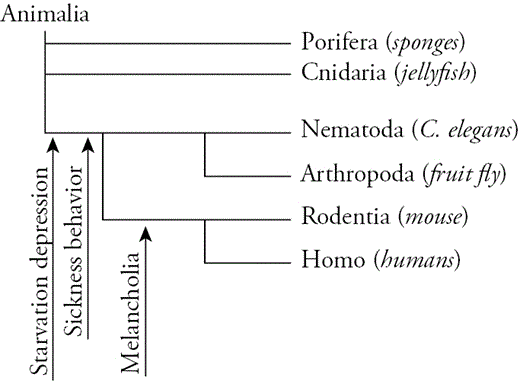
Tentative phylogeny of starvation depression, sickness behavior, and melancholia.
Each symptom, by itself, probably reflects nontrivial neurological modification. The confluence of them together suggests that melancholia exhibits highly nonrandom biological organization for the sustained processing of complex information. The fact that evolution by natural selection is the only known source of highly nonrandom biological organization suggests that there is an adaptation for producing melancholic symptoms in response to complex stressors, assisting in the processing and resolution of those stressors. To be clear, the claim is that the adaptation is in the neurological machinery for producing melancholic symptoms, not in the symptoms itself, as those could also be produced by a malfunction in that machinery.
Prior research has proposed that depression evolved as a response to complex problems in which the symptoms help promote uninterrupted analysis (Andrews & Thomson, 2009). This analytical rumination hypothesis (ARH) was explicitly framed in resource allocation logic. Specifically, the ARH is based on the fact that WM resources are limited, which makes cognitive processes, including analysis, increasingly vulnerable to interruption as WM load increases. Under the ARH, the problem that triggered the depressive episode is complex and important to resolve, so it should get prioritized access to WM resources. To do this, other processes that could draw WM resources away from the triggering problem must be inhibited, and depressive symptoms help coordinate this trade-off. For instance, anhedonia reduces interest in normally pleasurable activities that would otherwise draw attention away from the triggering problem. Thus, the ARH can be framed in terms of the generalized resource allocation hypothesis wherein the limited resource is WM. As described below, rumination is likely to be energetically expensive as well. We now turn to several testable implications of the ARH.
Analytical Processing is Promoted Throughout the Continuum of Melancholic Symptoms
The analytical processing style could exist throughout the continuum of symptom severity for melancholia (Andrews & Thomson, 2009). There is, in fact, substantial evidence for this prediction. An analytical rumination style called “reflective pondering” has been found in both subclinical and clinical samples (Joormann, Dkane, & Gotlib, 2006; Treynor, Gonzalez, & Nolen-Hoeksema, 2003). In our own work, we have developed a new instrument specifically for assessing analytical rumination, and it shows positive correlations with depressive symptoms in cross-sectional studies of both clinical and subclinical samples (Barbic, Durisko, & Andrews, 2014; Durisko et al., unpublished).
If depression focuses attention on analyzing the triggering problem, then fewer cognitive resources should be available for other things. Moreover, this effect should be present across the range of depressive symptomology. Consistent with this, depressed patients often perform worse than nondepressed controls on abstract cognitive tasks in the laboratory (Austin, Mitchell, & Goodwin, 2001). That the performance decrements are due to an altered focus of attention, rather than impaired cognition, is well supported. In clinical and subclinical samples, the performance decrements can be eliminated by simple attentional interventions such as thinking about a black umbrella for a few minutes (reviewed in Andrews & Thomson, 2009). In short, focused analysis of the triggering problem appears to explain performance decrements on abstract analytical tasks.
People with depression are more likely to show performance enhancements on laboratory tasks similar to the problems in their lives (Andrews & Thomson, 2009). As we discuss below, depressed people often face problems in their lives that involve cost–benefit trade-offs. Consequently, they seem to reliably outperform nondepressed controls on laboratory tasks that involve making a decision based on an optimization or a cost–benefit analysis (Table 3.2). This enhanced performance has been found across the range of depressive symptomology, including people with severe symptoms (Keller, Lipkus, & Rimer, 2002; Overall & Hammond, 2013) and patients hospitalized for depression (von Helversen, Wilke, Johnson, Schmid, & Klapp, 2011).
Depression Is Triggered by Complex Problems
The ARH predicts that depression is triggered by complex problems for which sustained, uninterrupted analysis is an adaptive response. We discuss two complex problems with evolutionary relevance. First, a social dilemma is a conflict with a close partner (mate, family member, friend) with whom it is important to maintain cooperative relations (Andrews & Thomson, 2009). Social dilemmas are complex problems because they are difficult to resolve in one’s favor without risking the erosion of the cooperative bond. Social dilemmas are a stronger predictor of depressive symptoms than simple interpersonal conflict (Gautam, Saito, Houde, & Kai, 2011; Pagel, Erdly, & Becker, 1987). Second, resource management dilemmas, in which people have too little time, money, or other resources to meet demands, are complex because a cost–benefit analysis must be performed to determine how scarce resources should be allocated. Resource management dilemmas are positively associated with depressive symptoms (Roxburgh, 2004).
| Effect . | Level of Depression . | Reference . |
|---|---|---|
Sensitivity to costs and benefits of social options | Experimentally induced | |
Subclinical (BDI > 10) | ||
Subclinical (BDI-SF > 5) | ||
Accurate risk assessment | Major depression (CES-D ≥ 16) | |
Major depression (HAM-D > 13) | ||
Optimal choices | Experimentally induced | |
Major depression (hospitalized) | ||
Salary after graduating college | Subclinical (Likert scale) | |
Awareness of impairment in brain injury and schizophrenia | Subclinical (various) | |
Assessment of romantic partner’s commitment | Subclinical (CES-D) |
| Effect . | Level of Depression . | Reference . |
|---|---|---|
Sensitivity to costs and benefits of social options | Experimentally induced | |
Subclinical (BDI > 10) | ||
Subclinical (BDI-SF > 5) | ||
Accurate risk assessment | Major depression (CES-D ≥ 16) | |
Major depression (HAM-D > 13) | ||
Optimal choices | Experimentally induced | |
Major depression (hospitalized) | ||
Salary after graduating college | Subclinical (Likert scale) | |
Awareness of impairment in brain injury and schizophrenia | Subclinical (various) | |
Assessment of romantic partner’s commitment | Subclinical (CES-D) |
BDI, Beck Depression Inventory; BDI-SF, Beck Depression Inventory-Short Form; CES-D, Center for Epidemiologic Studies-Depression; HAM-D, Hamilton Rating Depression Scale.
Bereavement is a specific type of stressor that might seem paradoxical under the ARH because analysis cannot bring back the dead. However, analysis might prevent other deaths in the future. Due to the fitness consequences, parents may be particularly likely to analyze the circumstances of a child’s death to assess if they could have done anything differently. They may grieve a lost child for years, particularly if they had negative interactions with the child immediately before the child’s death (Thieleman & Cacciatore, 2014). Bereavement may also be depressogenic if it causes resource management dilemmas, such as financial stress or household management difficulties (Liu & Chen, 2006; Umberson, Wortman, & Kessler, 1992).
Analytical Rumination Helps People Solve, Manage, or Cope with the Triggering Problem
It is widely thought that depressive cognition is maladaptive, but the evidence for this is not strong (reviewed in Andrews & Thomson, 2009). Depression is associated with increased pessimism, but this may be an honest assessment that one faces complex problems that are difficult to solve. Moreover, such an assessment may be an important psychological state that triggers sadness and analytical rumination. People with depression also show increased attention to negative information, but such information could be useful when trying to understand and solve such problems. Finally, it is commonly argued that depressed people have poor social problem-solving skills. Of course, the most important metric for assessing this is the ability to achieve social goals that are relevant to the depressed person’s episode. However, research in this area tends to focus on proxy variables that researchers assume are associated with effective social problem solving (empathy, voice tone, cooperativeness).
One area of research that might be taken as evidence against effective problem solving proposes that depression alters the brain, increases the depressogenic impact of minor stressors, and eventually disassociates episodes from stressors. This kindling hypothesis is widely thought to have substantial empirical support (e.g., Monroe & Harkness, 2005), and it suggests that depression cannot solve problems when they occur in the absence of problems. For instance, among people currently experiencing a bout of depression, people with greater histories of depression report fewer precipitating stressors. Although consistent with the kindling hypothesis, it is also consistent with the alternative that people with greater histories of depression have a stable genetic sensitivity to stressors. One study attempted to rule out this alternative using a longitudinal within-person design (Kendler, Thornton, & Gardner, 2000). Using Cox proportional hazards analysis, the authors reported a significant interaction between the number of prior episodes of depression and exposure to a stressor on the risk of a new episode of depression. But its value was less than 1, which could suggest that a history of depression, rather than sensitizing the brain, protects against new episodes of depression. Finally, kindling studies rarely ensure that participants are unmedicated, which is problematic because antidepressant medications (ADMs) can produce a pattern similar to kindling (Andrews, Kornstein, Halberstadt, Gardner, & Neale, 2011). ADMs disturb monoamine neurotransmitter levels, and the brain’s homeostatic mechanisms attempt to restore equilibrium. When ADMs are discontinued, these homeostatic forces can cause relapses that are unassociated with stressors.
The most crucial issue for the ARH is whether depressive rumination helps people solve or cope with the problem that triggered the episode (Andrews & Thomson, 2009). The current evidence does not allow conclusive determination because most studies evaluate depressive cognition using laboratory tasks rather than the triggering problem. One relevant experiment explored the effects of mood in a simulated market (Au, Chan, Wang, & Vertinsky, 2003). Participants were finance or economics students with knowledge of, or experience with, simulated or real financial trading. On each round participants were provided with historically accurate charts about the daily closing prices of currencies in the past 3 years and with news items that described influential market factors, the market movement, and comments from leading practitioners and economists from prominent investment banks. Careful analysis of this information would allow participants to make good predictions about the relative movement of Deutsche marks and Swiss francs. Performance was assessed by whether participants decided to buy or sell the correct currency on that round (accuracy) and by how much money the participants gained or lost (profit), which in turn depended on accuracy and the amount invested. Mood was manipulated by providing participants with false feedback on the first round. In the positive mood induction, participants received a high profit for their decision, regardless of what they actually did. In the sad mood induction, participants took a substantial loss. In the neutral mood induction, participants broke even. Participants who had sad mood induced by false negative feedback on the first round made more accurate decisions on subsequent rounds than those in the positive and neutral conditions. This interesting study suggests that sadness may help promote analysis of the triggering problem.
Factor analyses of rumination scales show two different subtypes: one focused on the past (“brooding”) and another associated with increased analysis (“reflective pondering”) (Joormann et al., 2006; Treynor et al., 2003). In longitudinal studies, brooding is associated with higher depressive symptoms over time, and it is commonly thought to be maladaptive. However, reflective pondering is associated with lower symptoms over time and may reflect a positive effect of analysis on problem solving (Joormann et al., 2006; Treynor et al., 2003). Similarly, interventions that naturally encourage rumination (e.g., journal writing about your strongest thoughts and feelings related to the episode) tend to shorten the duration of clinical episodes (Krpan et al., 2013). Therapies that foster analysis of problems also reduce symptoms. Rumination-Focused Cognitive–Behavioral Therapy helps promote the reflective (analytical) style of rumination through the use of functional analytic techniques (Watkins et al., 2007). Similarly, Concreteness Training involves the depressed person imagining personally relevant emotional events, focusing on the unique details of the event, and attempting to understand why the event occurred (Watkins, Baeyens, & Read, 2009).
Analytical Rumination Is Energetically Expensive
Rodents exposed to inescapable shock show an increase in glycolytic pathways to generate adenosine triphosphate (ATP), the primary molecule used to fuel biological processes (Mallei et al., 2011; Uehara, Sumiyoshi, Itoh, & Kurachi, 2007). Glycolysis generates less ATP per glucose molecule than oxidative phosphorylation, but it produces ATP at a faster rate (Pfeiffer, Schuster, & Bonhoeffer, 2001), so glycolysis is an indicator of metabolic activity (Shulman, Hyder, & Rothman, 2001). Moreover, glycolysis does not use oxygen, and in the brain it occurs in astrocytes that rely on stored glucose rather than blood-borne glucose. Consequently, neuronal activity and blood flow can be decoupled in regions relying heavily on glycolysis (Shulman et al., 2001), such as the prefrontal cortex (Vaishnavi et al., 2010). In fact, the correlation between regional activity and blood flow, normally positive in nondepressed people, becomes negative in many brain regions of patients with unmedicated depression (Dunn et al., 2005). This evidence suggests that depressive cognition is so energetically expensive it can be supported only by glycolysis.
Other Energetically Expensive Processes Are Downregulated
Further evidence that depression is energetically expensive comes from the fact that other energetically expensive activities are downregulated, such as growth and reproduction. For instance, hippocampal neurogenesis is downregulated, which may contribute to a decline in hippocampal volume during depression (Arnone et al., 2012). Although many researchers consider this pathological, many organisms show plasticity in neurogenesis in response to environmental demands (Gross, 2000). For instance, the attenuation of synapse strength in the hippocampus (a phenomenon called long-term depression) is important in WM tasks (Laroche, Davis, & Jay, 2000). Long-term depression is associated with a loss of dendritic spines in hippocampal neurons (Zhou, Homma, & Poo, 2004), which may contribute to the shrinkage in this region. Moreover, the shrinkage appears to be reversible, since depressed patients who remit without medication have normal hippocampal volumes (Arnone et al., 2012). Thus, neurogenesis may be temporarily downregulated because it is expensive and interferes with WM processes.
Other Depressive Adaptations?
Again, our analysis suggests that any hypothesis for depression based in resource allocation logic warrants closer scrutiny. For instance, depression may have evolved to inhibit effort that is likely to be wasted because circumstances are unpropitious (Nesse, 2000).
Another hypothesis proposes that depression can have negative effects on close social partners who otherwise benefit from the relationship (e.g., mates, kin, allies). The withdrawal of those benefits during depression could motivate social partners to help depressed individuals resolve their problems. Thus, depression could have evolved to divert energy, time, effort, or other valuable resources from close social partners to motivate them to provide help (Hagen, 2003; Watson & Andrews, 2002). This hypothesis provides a possible explanation for the link between depression and suicidal behavior and deliberate self-harm. Although suicidal behavior is commonly considered to be evidence of mental disorder, there are clear examples of adaptation for suicide in nature (Andrews & Thomson, 2010). One type of suicidal behavior has the social goal of seeking or leveraging help from others (Stengel & Cook, 1958). Such suicide attempts are sometimes thought to be “bluffs,” but the risk of death must be great enough to influence social partners (Andrews, 2006). Thus, some suicide attempts could be a desperate gamble in which a nontrivial risk of death is incurred to leverage help from social partners. Overall, the gamble can be favored by natural selection if the social rewards are great enough, but some people may die from the attempt. Depression and suicidal behavior may therefore be part of the same tactical dimension for leveraging help from others. Natural selection can also favor extremely deadly suicide attempts that the attempter has little chance of surviving. The circumstances favoring the evolution of such attempts require the individual to have little chance of future reproduction, and close genetic relatives must have reduced fitness by caring for the individual (e.g., the individual has chronic health problems). Some empirical support for this burdensomeness-to-kin hypothesis has been found in humans (deCatanzaro, 1995). Both types of suicidal behavior may require analysis of the individual’s situation (e.g., the likely risks and benefits of making a suicide attempt, and the effects of the attempt on kin), which may also contribute to the association with depression (Andrews & Thomson, 2010). Indeed, adolescents appear to engage in a cost–benefit analysis before making a suicide attempt during conflict with their parents (Andrews, 2006).
Conclusions
Sickness behavior, starvation depression, and melancholia share partially overlapping symptoms, neurobiology, and genes. The common neurobiology probably originated to coordinate a response to either infection or starvation, whereas melancholia involved the subsequent cooption and modification of this machinery. These three syndromes share a functional similarity: coordinating trade-offs in limited resources in response to persistent threats (starvation, infection, complex problems such as social or resource management dilemmas).
The energy reallocation that occurs in melancholia may often be the output of a properly functioning adaptation. First, melancholia is often triggered by complex problems that (like starvation and infection) are so persistent they likely require a sustained reallocation of energy. Second, by directing energy toward promoting analysis of those problems, the cognitive effects of melancholia show an ecological correspondence to the situational causes. The correspondence suggests that the analytical processing style might help depressed people solve the problems that triggered their episodes. Some evidence supports this prediction, though it should be researched in greater detail. Third, by upregulating cognition and downregulating growth and reproduction, melancholia appears to coordinate various biological systems in the body in a nonrandom fashion to promote uninterrupted analysis. Given the evidentiary demands of demonstrating adaptation, this prediction should also be tested more rigorously. The distraction-resistant nature of rumination is particularly worthy of further study because it is thought to promote unproductive, repetitive, circular thinking. However, distraction resistance may have evolved to promote problem solving by adaptively reducing the vulnerability of analysis to interruption (Andrews & Thomson, 2009). The ARH predicts that many of the behavioral and cognitive symptoms of depression—anhedonia, social withdrawal, lethargy, sleeping and eating less, difficulty concentrating—act in a highly nonrandom, coordinated fashion to facilitate analytical rumination by promoting distraction resistance (Andrews & Thomson, 2009).
Evolutionary theory offers hope for a better understanding of the etiology of depressive phenotypes, including some currently classified as clinical disorders. This, in turn, could lead to more effective treatments. Future research will need to test whether this framework can improve clinical practice in mental health.
References
Akiskal, H. S. (
American Psychiatric Association (APA). (
Andrews, P. W. (
Andrews, P. W., Gangestad, S. W., & Matthews, D. (
Andrews, P. W., Kornstein, S. G., Halberstadt, L. J., Gardner, C. O., & Neale, M. C. (
Andrews, P. W., & Thomson, J. A., Jr. (
Andrews, P. W., & Thomson, J. A., Jr. (
Angst, J., Gamma, A., Benazzi, F., Ajdacic, V., & Rössler, W. (
Arnone, D., McKie, S., Elliott, R., Juhasz, G., Thomas, E. J., Downey, D., … Anderson, I. M. (
Au, K., Chan, F., Wang, D., & Vertinsky, I. (
Austin, M. P., Mitchell, P., & Goodwin, G. M. (
Barbic, S. P., Durisko, Z., & Andrews, P. W. (
Chambers, M. C., & Schneider, D. S. (
Christensen, L., & Brooks, A. (
Dantzer, R. (
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W., & Kelly, K. W. (
Deak, T., Meriwether, J. L., Fleshner, M., Spencer, R. L., Abouhamze, A., Moldawer, L. L., … Maier, S. F. (
de Catanzaro, D. (
Dess, N. K. (
Dunn, R. T., Willis, M. W., Benson, B. E., Repella, J. D., Kimbrell, T. A., Ketter, T. A., … Post, R. M. (
Durisko, Z., Mulsant, B. H., & Andrews, P. W. (
Engel, G. L., & Schmale, A. H. (
Exner, C., Hebebrand, J., Remschmidt, H., Wewetzer, C., Ziegler, A., Herpertz, S., … Klingenspor, M. (
Fleminger, S., Oliver, D. L., Williams, W. H., & Evans, J. (
Fuhrman, J. A. (
Gautam, R., Saito, T., Houde, S. C., & Kai, I. (
Greenberg, G. (
Gross, C. G. (
Hagen, E. H. (
Hagen, E. H. (
Hart, B. L. (
Hertel, G., Neuhof, J., Theuer, T., & Kerr, N. L. (
Hokanson, J. E., Sacco, W. P., Blumberg, S. R., & Landrum, G. C. (
Iyengar, S. S., Wells, R. E., & Schwartz, B. (
Jackson, R. L., Maier, S. F., & Rapaport, P. M. (
Joormann, J., Dkane, M., & Gotlib, I. H. (
Kane, M. J., & Engle, R. W. (
Kaplan, R. F., Trevino, R. P., Johnson, G. M., & Levy, L. (
Keller, P. A., Lipkus, I. A., & Rimer, B. K. (
Kendler, K. S., Thornton, L. M., & Gardner, C. O. (
Keys, A., Brozek, J., & Henschel, A. (
Kramer, L., Bauer, E., Funk, G., Hofer, H., Jessner, W., Steindl-Munda, P., … Ferenci, P. (
Krpan, K. M., Kross, E., Berman, M. G., Deldin, P. J., Askren, M. K., & Jonides, J. (
Laroche, S., Davis, S., & Jay, T. M. (
Larson, S. J., & Dunn, A. J. (
Lee, R. K. K., & Maier, S. F. (
Leff, M. J., Roatch, J. F., & Bunney, W. E. (
Liu, R. X., & Chen, Z.-y. (
Lochmiller, R. L., & Deerenberg, C. (
MacFadyen, U., Oswald, I., & Lewis, S. (
Mallei, A., Giambelli, R., Gass, P., Racagni, G., Mathé, A. A., Vollmayr, B., & Popoli, M. (
McCue, M. D. (Ed.). (
Minor, T. R., Jackson, R. L., & Maier, S. F. (
Monroe, S. M., & Harkness, K. L. (
Mundt, C., Reck, C., Backenstrass, M., Kronmüller, K., & Fiedler, P. (
Nelson, J. C., & Mazure, C. (
Nesse, R. M. (
Neumann, I. D., Wegener, G., Homberg, J. R., Cohen, H., Slattery, D. A., Zohar, J., … Mathe, A. A. (
O’Malley, M. W., Fishman, R. L., Ciraulo, D. A., & Datta, S. (
Overall, N. C., & Hammond, M. D. (
Overmann, S. R. (
Pagel, M. D., Erdly, W. W., & Becker, J. (
Pfeiffer, T., Schuster, S., & Bonhoeffer, S. (
Pietromonaco, P. R., & Rook, K. S. (
Prentice, A. M. (
Prentice, A. M., & Keneba, M. (
Raison, C. L., & Miller, A. H. (
Rasch, B., & Born, J. (
Rausch, J. L., Johnson, M. E., Corley, K. M., Hobby, H. M., Shendarkar, N., Fei, Y., … Leibach, F. H. (
Rising, R., Keys, A., Ravussin, E., & Bogardus, C. (
Roxburgh, S. (
Ruiz-Núñez, B., Pruimboom, L., Dijck-Brouwer, D. A. J., & Muskiet, F. A. J. (
Schwartz, M. W., & Seeley, R. J. (
Shulman, R. G., Hyder, F., & Rothman, D. L. (
Smoski, M. J., Lynch, T. R., Rosenthal, M. Z., Cheavens, J. S., Chapman, A. L., & Krishnan, R. R. (
Stengel, E., & Cook, N. G. (
Taylor, M. A., & Fink, M. (
Thieleman, K., & Cacciatore, J. (
Tooby, J., & Cosmides, L. (
Tooby, J., & Cosmides, L. (
Treynor, W., Gonzalez, R., & Nolen-Hoeksema, S. (
Uehara, T., Sumiyoshi, T., Itoh, H., & Kurachi, M. (
Umberson, D., Wortman, C. B., & Kessler, R. C. (
Vaishnavi, S. N., Vlassenko, A. G., Rundle, M. M., Snyder, A. Z., Mintun, M. A., & Raichle, M. E. (
Vollmayr, B., & Henn, F. A. (
von Helversen, B., Wilke, A., Johnson, T., Schmid, G., & Klapp, B. (
Watkins, E., Scott, J., Wingrove, J., Rimes, K., Bathurst, N., Steiner, H., … Malliaris, Y. (
Watkins, E. R., Baeyens, C. B., & Read, R. (
Watson, P. J., & Andrews, P. W. (
Wolowczuk, I., Verwaerde, C., Viltart, O., Delanoye, A., Delacre, M., Pot, B., & Grangette, G. (
Xiang, Y.-T., Wang, G., Hu, C., Guo, T., Ungvari, G. S., Kilbourne, A. M., … Chiu, H. F. K. (
Zhou, Q., Homma, K. J., & Poo, M.-m. (
| Month: | Total Views: |
|---|---|
| October 2022 | 5 |
| November 2022 | 5 |
| December 2022 | 7 |
| January 2023 | 20 |
| February 2023 | 13 |
| March 2023 | 8 |
| April 2023 | 3 |
| May 2023 | 9 |
| June 2023 | 6 |
| July 2023 | 2 |
| August 2023 | 5 |
| September 2023 | 9 |
| October 2023 | 9 |
| November 2023 | 5 |
| December 2023 | 11 |
| January 2024 | 5 |
| February 2024 | 5 |
| March 2024 | 7 |
| April 2024 | 7 |
| May 2024 | 3 |
| June 2024 | 4 |
| July 2024 | 3 |
| August 2024 | 2 |
| September 2024 | 1 |
| October 2024 | 2 |
| November 2024 | 6 |
| December 2024 | 5 |
| January 2025 | 11 |
| February 2025 | 3 |
| March 2025 | 7 |
| May 2025 | 2 |
