-
PDF
- Split View
-
Views
-
Cite
Cite
N Ünal Gülsen, O K Bakkaloglu, T Kav, H T Kani, A Akpinar, P Akincioglu, T Eskazan, M Balamir, G Dagci, I Sendur, I Buyuktorun, S Barutcu, G Bengi, B Cavus, N Oruc, Y Alabdah, O Atug, D Dincer, I Hatemi, Y Z Erzin, A Tezel, M Toruner, F Akyuz, A F Celik, P1015 Upadacitinib as a Rescue Therapy in Patients with Steroid- and Infliximab-Refractory Acute Severe Ulcerative Colitis: Real-Life Multicentre Results, Journal of Crohn's and Colitis, Volume 19, Issue Supplement_1, January 2025, Pages i1877–i1878, https://doi.org/10.1093/ecco-jcc/jjae190.1189
Close - Share Icon Share
Abstract
Ulcerative colitis (UC) is a chronic inflammatory bowel disease with remissions and flares. Approximately 20% of UC Patients develop acute severe ulcerative colitis (ASUC), a serious condition that is refractory to standard therapies and leads to morbidity and mortality. Rescue therapy (salvage therapy) with steroids, cyclosporine, and/or infliximab is recommended by guidelines.1 The colectomy rate in ASUC remains at around 36% even in the biological millennium.2 Upadacitinib (UPA) is a JAK1 selective inhibitor used effectively to treat UC. The role of UPA as a rescue therapy in ASUC is not clear. This study aims to evaluate the outcomes of UPA as a rescue therapy in ASUC patients.
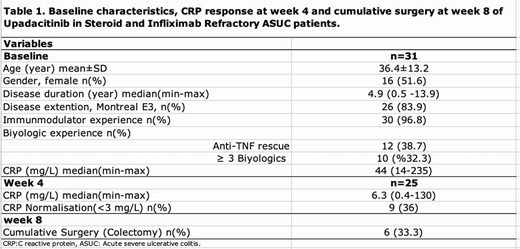
A total of 31 steroid- and infliximab-refractory ASUC patients from 10 IBD centers from all over Türkiye were included between April 2023 and October 2024 in our study. Demographics of the patients and disease characteristics were recorded. Stool frequency (per day), rectal bleeding scores, and C reactive protein (CRP) were assessed from the electronic database at baseline (week 0), week 1, 4, 8, and 16 retrospectively. Complete clinical response was defined as stool frequency ≤3 per day and rectal bleeding score 0-1.
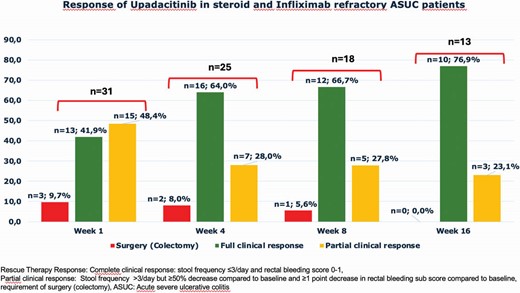
Mean age was 36±13years; 16(51.6%) were female, mean disease duration was 6.9±5.4 years, 30(96.8%) had immunomodulator experience (Table1). A total of 10 patients had ≥ 3 biologics failures. Complete clinical response at weeks, 4, 8, and 16 were in 13 (42%), 16 (64%), 12 (67%), and 10 (77%) patients respectively.
Rescue therapy responses were not statistically significant between ≥3 and <3 biologics failure groups (p=0.580) at week1. CRP normalization (<3 mg/L) at week 4 was found in 9(36%) patients. By the end of the week, 6 (33%) patients had undergone colectomy. Clinical responses and surgery rates of UPA at week1,4,8, and 16 were detailed in Figure 1.
UPA provided a complete clinical response as a rescue therapy within the first week in 42% of cases in anti-TNF and steroid non-responders even with multiple biologics experience. Over half of the patients showed clinical remission by weeks 4 and 8.
1.Raine T, Bonovas S, Burisch J, et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohns Colitis. 2022;16(1):2-17. doi:10.1093/ecco-jcc/jjab1782.
2.Moore AC, Bressler B. Acute Severe Ulcerative Colitis: The Oxford Criteria No Longer Predict In-Hospital Colectomy Rates. Dig Dis Sci. Feb 2020;65(2):576-80.
- tumor necrosis factors
- colectomy
- crohn's disease
- inflammatory bowel disease
- ulcerative colitis
- immunologic adjuvants
- biological products
- colitis
- demography
- feces
- salvage therapy
- spinal cord injuries
- steroids
- surgical procedures, operative
- c-reactive protein
- cyclosporine
- guidelines
- morbidity
- mortality
- surgery specialty
- rectal bleeding
- infliximab
- illness length
- medical management
- disease remission
- upadacitinib
- skin cancer index



