-
PDF
- Split View
-
Views
-
Cite
Cite
L Peyrin-Biroulet, Y Bouhnik, X Hebuterne, M Fumery, C Taxonera, A Gutierrez-Casbas, S Howaldt, J Stein, J Lindsay, J Monnet, E Simoes, F Dolfi, P0830 Use of FK-adalimumab biosimilar (MSB 11022) in context of biologic switch in Inflammatory Bowel Diseases : 6 months data of the real-world IDEA study, Journal of Crohn's and Colitis, Volume 19, Issue Supplement_1, January 2025, Pages i1582–i1583, https://doi.org/10.1093/ecco-jcc/jjae190.1004
Close - Share Icon Share
Abstract
Biosimilars offer similar efficacy and safety when compared to their originators, thereby enhancing patient access to more affordable treatments. FK-adalimumab (FK-ada) is an adalimumab biosimilar approved for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC). FK-ada development included 1,234 subjects treated up to 52 weeks (total 420 patients-year). Since first approval, there have been no unexpected safety signals despite more than 115,000 patient-years of exposure. IDEA is an ongoing real-world study aimed to assess persistence with FK-ada and develop a predictive model of persistence at 12 months. In this study, adult patients with IBD who switched from adalimumab originator or another adalimumab biosimilar to FK-ada are followed up to one year.
Data collected include patient characteristics, reasons for treatment switch, clinical status at each follow-up visit including disease activity scores (Harvey-Bradshaw Index (HBI) and Montreal classification), and faecal calprotectin results. Adult patients with either CD or UC were included, those receiving treatment outside of routine clinical care were excluded. Data are presented using descriptive statistics and a logistic regression model will be employed to identify potential predictive factors at 12 months. We present here the baseline characteristics of IBD patients, and the 6 months intermediate results.
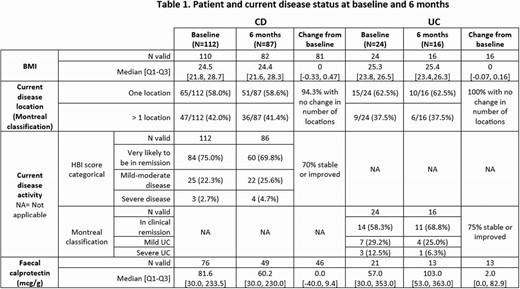
A total of 136 IBD patients (CD: 112, UC: 24) were recruited across France, Germany and Spain, 57% being males, median age of 43 years and median BMI of 25.2 kg/m2. At the time of switch 97.3% of patients with CD and 87.5% with UC were in clinical remission or had mild to moderate disease activity. 54.4% of patients switched to FK-ada from originator, and 45.6% from another biosimilar, with most switches occurring for administrative reasons. A total of 103 IBD patients (CD: 87, UC: 16) completed the 6 months visit. Patients and disease status are summarized in Table 1. The persistence rate of FK-ada at 6 months was 76.4% (CD: 79%, UC: 65.2%). Most permanent treatment discontinuations occurred around 3 months after switch (median 3.6 months), primarily due to adverse events. Since the start of the study 46 patients (34%) experienced at least one adverse event (AE); 16 (12%) had at least one moderate to severe AE and 30 (22%) had AE(s) attributable to FK-ada.
6 months after the switch, FK-ada persistence rate was 76,4%. The majority of patients were still in remission or experiencing mild to moderate disease activity. Treatment and tolerance data from the study completion (52-week) will be critical for identifying predictive factors influencing drug persistence after the switch.
- body mass index procedure
- crohn's disease
- inflammatory bowel disease
- ulcerative colitis
- adult
- feces
- germany
- safety
- spain
- individuals with disabilities education act
- adalimumab
- predictor variable
- follow-up visit
- montreal
- descriptive statistics
- adverse event
- leukocyte l1 antigen complex
- biosimilar pharmaceuticals
- disease remission



