-
PDF
- Split View
-
Views
-
Cite
Cite
A Hart, T Hisamatsu, F Steinwurz, G D’Haens, W Liu, M Olurinde, P Ngqawa, Z Yang, E Merrall, N A Terry, R Panaccione, B E Sands, P0670 Efficacy of subcutaneous guselkumab induction therapy by baseline demographics and concomitant medications in participants with moderately to severely active Crohn’s disease: Results from the phase 3 GRAVITI study, Journal of Crohn's and Colitis, Volume 19, Issue Supplement_1, January 2025, Pages i1310–i1312, https://doi.org/10.1093/ecco-jcc/jjae190.0844
Close - Share Icon Share
Abstract
The GRAVITI study established the efficacy and safety of subcutaneous (SC) induction with guselkumab (GUS), a dual-acting IL-23p19 subunit inhibitor, in participants (pts) with moderately to severely active Crohn’s disease (CD) through 48 weeks of treatment.1 Here, we evaluated the efficacy of GUS SC induction in GRAVITI subgroups based on baseline demographics and concomitant CD-related medications.
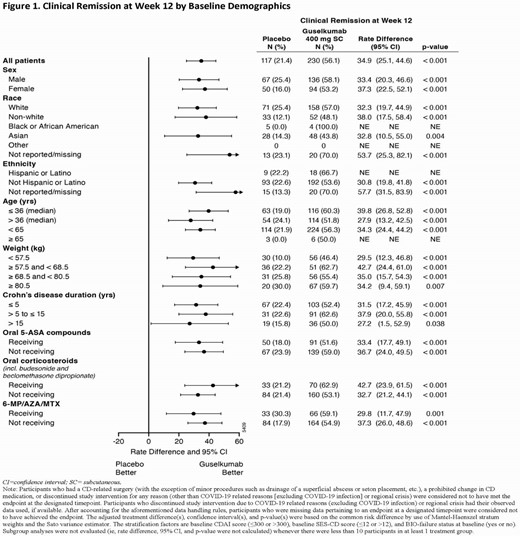
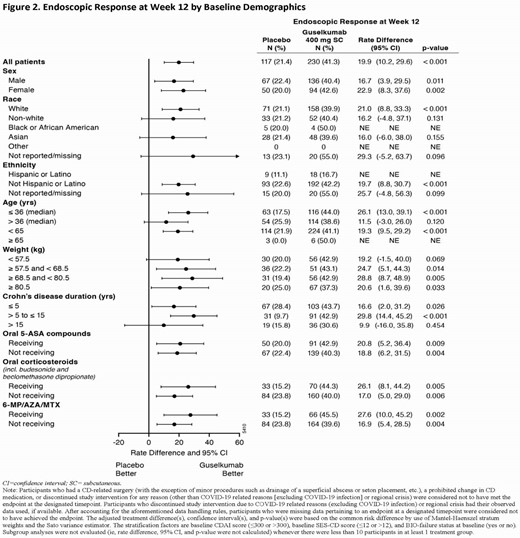
Eligible pts had a history of inadequate response or intolerance to oral corticosteroids, azathioprine (AZA), 6-mercaptopurine (6-MP), methotrexate (MTX), or biologics (ie, TNF antagonists or vedolizumab). Randomization was stratified by baseline CDAI, SES-CD, and history of inadequate response or intolerance to biologics, with 347 pts allocated 1:1:1 to GUS 400 mg SC q4w (x3) → GUS 200 mg SC q4w, GUS 400 mg SC q4w (x3) → GUS 100 mg SC q8w, or placebo (PBO). The co-primary endpoints assessed at Week 12 compared the combined GUS 400 mg SC q4w group (N=230) to PBO (N=117). Clinical remission at Week 12 (CDAI<150) and endoscopic response at Week 12 (≥50% improvement from baseline SES-CD) were evaluated for predefined subgroups based on sex (ie, male or female), race (ie, white, non-white, black or African American, Asian, other, or not reported/missing), ethnicity (ie, Hispanic or Latino, not Hispanic or Latino, or not reported/missing), age in years (ie, ≤36 [median], >36, <65, or ≥65), weight in kg by quartile (ie, <57.5, ≥57.5 to <68.5, ≥68.5 to <80.5, or ≥80.5), disease duration in years (ie, ≤5, >5 to ≤15, or >15), and concomitant CD-related medications (ie, receiving or not receiving 5-ASA compounds, oral corticosteroids, and AZA/6-MP/MTX as discrete categories).
Clinical remission at Week 12 and endoscopic response at Week 12 were achieved by greater proportions of GUS- than PBO-treated pts across all subgroups (Figures 1 and 2). Treatment differences of GUS vs PBO for clinical remission at Week 12 and endoscopic response at Week 12 were similar for each subgroup of sex, race, ethnicity, age, and disease duration. Treatment differences of GUS vs PBO for clinical remission at Week 12 and endoscopic response at Week 12 were similar across all weight quartile subgroups and for each subgroup of concomitant CD-related medications.
In GRAVITI, GUS SC induction was effective in inducing clinical remission at Week 12 and endoscopic response at Week 12 across predefined subgroups of sex, race, age, weight quartile, and concomitant CD-related medications among pts with moderately to severely active CD.
1)Panaccione R, Hart A, Steinwurz F, et al. Efficacy and safety of subcutaneous guselkumab induction therapy in patients with moderately to severely active Crohn’s disease: Results through week 48 from the phase 3 GRAVITI study. Presented at American College of Gastroenterology 2024.
- azathioprine
- adrenal corticosteroids
- crohn's disease
- endoscopy
- glucocorticoids
- 6-mercaptopurine
- biological products
- demography
- ethnic group
- gastroenterology
- hispanics or latinos
- mesalamine
- methotrexate
- neoadjuvant therapy
- safety
- mineralocorticoids
- african american
- asian
- surrogate endpoints
- tumor necrosis factor inhibitors
- illness length
- crohn's disease activity index
- disease remission
- vedolizumab
- guselkumab



