-
PDF
- Split View
-
Views
-
Cite
Cite
Walter Reinisch, Silvio Danese, Laurent Peyrin-Biroulet, Edward V Loftus, on behalf of the International Organization for the Study of Inflammatory Bowel Diseases [IOIBD], Clinical Trials for Inflammatory Bowel Disease: Global Guidance During the COVID-19 Pandemic, Journal of Crohn's and Colitis, Volume 14, Issue Supplement_3, October 2020, Pages S815–S819, https://doi.org/10.1093/ecco-jcc/jjaa119
Close - Share Icon Share
Abstract
The severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]-causing coronavirus disease [COVID]-19 pandemic poses major challenges for patients with inflammatory bowel disease [IBD] to be recruited and maintained in clinical trials. However, clinical trials offer patients who have failed multiple drugs access to study medications with alternative modes of action and the potential for relief from inflammation-mediated symptoms. Therefore, the continuation of clinical trials in IBD during the COVID-19 pandemic is important both for participants and for the community of IBD patients, due to the dire need for an expanded therapeutic armamentarium. As the safety of patients in clinical trials is the leading principle, we are providing ten specific rules to guide patients and principal investigators safely through the challenging time.
1. Introduction
In recent years, the inflammatory bowel diseases [IBD], ulcerative colitis [UC] and Crohn’s disease [CD], have attracted major interest by pharmaceutical companies as indications for new drug development. A survey from 2019 (i.e. the year before the outbreak of the severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]-related coronavirus disease [COVID]-19 pandemic) revealed 47 companies recruiting up to 50 000 patients into phase II and III clinical trials on IBD for a total of 68 new investigational drugs.1 Impressively, despite rising numbers of approved biologics and small molecules, these figures also represent the unmet demand for novel medical therapies in IBD. As such, clinical trials provide physicians and IBD patients with access to new drugs with alternative modes of action, increasingly for patients who have previously failed multiple medical interventions.
The COVID-19 crisis has numerous implications for the care of patients with IBD, resulting in shifting priorities for treating sites and patients as outlined in detail elsewhere.2 From the availability of general protective measures to IBD-specific opinion-based management recommendations, guidance documents have been published to help safely steer physicians and patients through this time of exceptionally infectious circumstances.3 However, there are also several challenges eroding the initiation and continuation of clinical trials in IBD. The integrity of a clinical trial may be jeopardized by travel limitations, quarantine separations, and site/region/country-specific public health measures with resulting consequences including dropping of non-urgent procedures such as endoscopy for endpoint assessment, interruption to the supply chain and patients’ reluctance over concerns about a potentially increased infection risk from the study medication.
The Food and Drug Administration [FDA], the European Medicines Agency [EMA] and other regulatory authorities have issued regularly updated general guidance documents for industry, investigators, and institutional review boards [IRBs] to assist in ensuring the safety of trial participants, maintaining compliance with good clinical practice [GCP] and minimizing risks to trial integrity during the COVID-19 pandemic.4,5 In contrast, the literature addressing the specific conduct of clinical trials in IBD during COVID-19 is sparse and mostly limited to considerations generated by the COVID-19 IBD working group of the British Society of Gastroenterology [BSG].6 The International Organization for the Study of Inflammatory Bowel Disease [IOIBD], an international, global organization to promote the health of people with IBD, has established numerous task forces to address the various aspects of management of IBD during the COVID-19 pandemic. Here, we report guidance for investigators of clinical trials on patients with IBD.
2. General Considerations Regarding Clinical Trials in IBD During the COVID-19 Pandemic
The feasibility of screening and recruiting new trial participants in an ongoing study and continuing enrolled participants should be carefully assessed, individually, at each study site, in collaboration with the sponsor and IRB. Additional considerations include country or regional regulatory measures, maintenance of supply chains for study drug and samples, time, and each site’s clinical resources and safety. As an overarching principle, protecting the safety of trial participants and study staff is of paramount importance. The specific guidance from the other IOIBD COVID-19 task forces on risk mitigation measures in terms of acute care, infusion centres, endoscopy and telehealth also funnel into the safe conduct of clinical trials in IBD. Without repetition, we encourage detailed study of those publications elsewhere in this issue of the Journal of Crohn’s and Colitis.
Whereas recruitment hold for new participants has been issued by sponsors and new site activation has been suspended until further notice for most studies in IBD, some studies still permit enrolment of new patients if conditions allow.
Patients lacking therapeutic alternatives [e.g. patients who are steroid-dependent despite multiple biologics and small molecules, patients with a history of serious adverse events such as severe infections or paradoxical inflammation in association with approved mode of actions, or patients who lack access to approved drugs] might still be candidates for enrolment into clinical trials. However, the decision to recruit a new patient in a trial mandates balancing the potential benefits of receiving a trial medication against the risk of trial participation including travel to the study site, face-to-face visits, and the unknown effects of the investigational medicinal product [IMP] on the course of COVID-19, as also suggested by the BSG.6 Preventing increased disease activity, continuous/repetitive use of corticosteroids and unanticipated [emergent] surgeries are leading principles in the treatment of IBD patients that continues to apply during the coronavirus pandemic. The increased risk of serious and opportunistic infections in patients with CD with higher disease activity and with concomitant use of corticosteroids is noteworthy in that context and could be determining factors for the recruitment decision in patients lacking approved therapeutic alternatives.7 It is important to note that high doses of corticosteroids have been associated with delayed viral clearance during previous coronavirus outbreaks in Middle East respiratory syndrome [MERS] and SARS and interim guidance from the World Health Organization [WHO] advised against the use of steroids for SARS-CoV2-induced lung injury.8,9 Furthermore, endocrinologists have issued warnings that individuals taking steroid hormones are at a higher risk of acquiring SARS-CoV2 infection and, if infected, might be prone to a more severe course.10 Early results from the SECURE-IBD registry, recruiting IBD patients with proven COVID-19, support an increased risk of severe COVID-19, defined as a composite of intensive care unit admission, ventilator use and/or death, in association with concomitant use of glucocorticoids.11 In an exploratory analysis, combination therapy of a tumour necrosis factor [TNF] antagonist with thiopurines or methotrexate compared to TNF antagonist monotherapy was positively associated with COVID-19-related hospitalization or death, suggesting against double immunosuppression treatment strategies during the time of pandemic if avoidable.
Most single-target mode of actions, including blockade of interleukin [IL]-23 and anti-adhesion strategies, currently subjected to clinical phase III trials in IBD, appear to be involved in viral clearance of SARS-CoV-2.9,12 The jury is still out on Janus kinase [JAK] inhibitors, which target JAK1 and JAK3, as those affect multiple cytokines such as type I interferons, IL-2, IL-15, IL-21 and interferon-γ involved in antiviral responses. Sphingosine-1-phosphate receptor 1 modulators have been reported to protect from death in animal models caused by severe influenza infection. Whether those results could be extrapolated to patients with SARS-CoV-2 remains to be determined.13
For recruited participants in ongoing IBD trials, COVID-19 illness and/or COVID-19 mitigation measures can negatively affect compliance with protocol-specified study procedures and schedules, including IMP administration, adherence to protocol-specified visits and endpoint evaluations such as endoscopy for outcome assessment. Therefore, protocol modifications and deviations are expected and recognized by regulatory agencies and IRBs. In addition to ensuring the integrity of patient data, sponsors need to evaluate the potential impact on the safety not of only study participants but also study staff. Partnering and communications between investigators [and their IRBs], clinical research organizations [CROs] and industry sponsors should determine how to best approach challenges and develop solutions. Due to the acuteness of the situation, alternative study processes intended to immediately mitigate the risk from the COVID-19 pandemic may be implemented prior to approval by IRB/regulatory authorities, which may not meet regularly due to the crisis and must be notified afterwards. The FDA and EMA require that these changes must be clearly and reproducibly documented in the clinical study report [e.g. home administration of the IMP, local laboratory utilization, missing and remote visits, alternative treatment failure assessment when endoscopy is not available, transient or permanent discontinuation of treatment], by also providing details on the duration of these changes and which trial subjects have been impacted to exactly what extent.
In general, the continuation of clinical trials for patients with moderate or severe IBD may be critical, in particular for those showing a clear clinical benefit regarding patient-reported outcomes [PROs] that are corroborated by improvement of objective signs of inflammation, such as endoscopic disease activity and/or inflammatory biomarkers (faecal calprotectin [FCP] and serum C-reactive protein [CRP]). Discontinuing patients with objective evidence of improvement, even in the absence of completed endpoint assessments, can potentially compromise their overall well-being by exposure to recurrent inflammation and the associated risk of a flare necessitating ‘rescue’ corticosteroids. As outlined above, both enhanced disease activity and corticosteroids should be avoided whenever possible. Furthermore, in several countries, patients have no other access to advanced targeted therapies, including biologics, leaving them insufficient treatment options after trial discontinuation.
Maintaining the scientific integrity of a clinical trial, in particular of phase III studies, is critical as it protects the contributions that have been made by the patients to date, but also as provision to achieve the pre-defined statistical objectives. The COVID-19 situation may introduce a number of significant events that may have potential impact on the evaluation and power of study objectives from missing or alternative assessments (e.g. by replacing endoscopic endpoints with PROs supported by biomarkers [CRP, FC]). To minimize that risk, efforts by study staff based on benefit–risk considerations are warranted to adhere to protocol-mandated study procedures scheduled for endpoint visits, while taking into account local guidance. Delay in endoscopy assessments especially when it is the primary endpoint can be negotiated to complement secondary endpoint criteria that may be available.
Regulatory agencies encourage conversion of physical visits into phone or video visits, utilization of alternative locations for assessments including local laboratory and imagining centres, home delivery and administration of the IMP, and even postponement or complete cancellation of visits if the situation is too challenging and does not ensure safety.4,5 Each visit should be assessed case by case with tailored solutions. The COVID-19 restrictions are providing prime opportunities for the evolution to telemedicine modalities and the FDA is providing specific guidance on remote monitoring during the crisis.14 In exceptional situations, the transfer of participants to other investigational sites, either already participating or new ones, performing under safer conditions, could occur, particularly if the trial participant urgently needs to stay in the trial.
3. Ten Specific Rules Regarding Clinical Trials in IBD During the COVID-19 Pandemic
Figure 1 summarizes specific rules for the various stages of a clinical trial in patients with IBD.
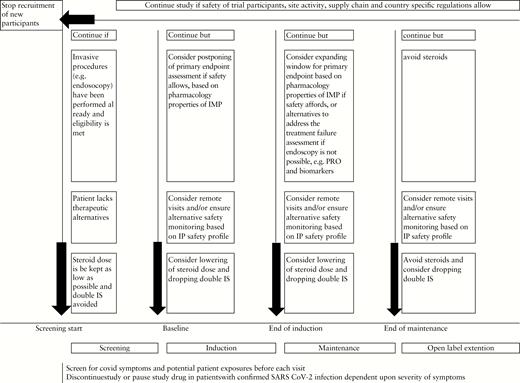
Guidance for conduct of clinical trials in inflammatory bowel disease during the COVID-19 pandemic.
Consider continuing recruited participants in ongoing trials if subject safety, site activity, supply chain, travel limitations and national/regional/local COVID-19-related restrictions allow. Particular attention should be paid to patients who lack therapeutic alternatives to approved drugs and who objectively benefit from the study.
Consider completing recruitment of screened patients who lack therapeutic alternatives and who have already undergone an invasive procedure and meet eligibility.
Patients who are suspected of having COVID-19 or have tested positive for SARS-CoV-2 should not be enrolled or initiated on protocol treatment until resolution of symptoms or negative re-testing dependent upon the provision of sponsors. For ongoing patients who are exhibiting symptoms consistent with COVID-19, consider holding dosing of the IMP and consult the medical monitor. In the event that a patient tests positive for COVID-19 consistent with the institutional standard of care, or if no testing is available, immediately report to the sponsor and consider holding dosing until such time as symptoms resolve, before resuming to dose. A COVID-19 test may be mandatory before endoscopy at some sites. The outcome of potential COVID-19 cases should be tracked during the trial.
Try to keep the exposure to corticosteroids as low as possible with careful balancing of the advantages and disadvantages.
If possible, try to avoid or minimize the duration of double immunosuppression during the conduct of the trial.
Consider unblinding of participants only if unavoidable such that disclosed information would determine further management [e.g. severe COVID-19 disease].
Consider mitigation strategies [telemonitoring of PROs or adverse events, local laboratory for critical safety labs, home-based drug delivery, endoscopy at another site, etc.] to keep participants in the trial if on-site visits are exposing patients to an increase risk of coronavirus infections. Mitigations should also be considered if patients are not allowed to travel because of lockdown. Again, national/regional/local research guidance may help to determine what is considered safe.
Consider a more expanded interpretation of the timing of study visit windows, including endoscopy, dependent upon the pharmacology properties of the IMP [e.g. −2 to +4 weeks] in consultation with industry sponsors and CROs. These should be documented before dropping visits or mandated procedures, in particular for endpoint assessment. However, criteria should be developed for patients to continue in the trial even without specified endoscopic examinations [e.g. by switching responders to open-label extensions if unable to attend endpoint procedures].
Ensure that all COVID-19 emergency-related changes and/or modifications from the clinical study protocol [e.g. missed or late study visits, missed doses of study medication] are documented in the study file and notify the IRB/ethics committee. Ensure a continuous bidirectional communication between sponsors, CROs and sites.
If a sponsor holds or stops a clinical trial, investigators should resume standard therapy dependent upon disease severity in line with national and international scientific guidance.
4. Summary
We recognize that conducting a clinical trial in patients with IBD during the rapidly evolving situation elicited by COVID-19 with different geographical considerations poses numerous challenges regarding trial recruitment, retention and evaluations. Here we provide guidance in an attempt to manoeuvre patients safely through the crisis by maintaining the study’s scientific power and integrity. IBD affects patients not only due to debilitating and embarrassing signs and symptoms, but also as a result of social functioning and disability. As such, in addition to the development of novel therapies crucial to improve outcomes for these chronic inflammatory conditions, clinical trials also enable access to alternative modes of action in patients, with a progressive disease course, who lack therapeutic alternatives. However, the safety of patients, investigators and staff involved in clinical trials is of paramount importance.
Conflict of Interest
W.R. has served as a speaker for Abbott Laboratories, Abbvie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor and Yakult; as a consultant for Abbott Laboratories, Abbvie, Aesca, Algernon, Amgen, AM Pharma, AMT, AOP Orphan, Arena Pharmaceuticals, Astellas, Astra Zeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, DSM, Elan, Eli Lilly, Ernest & Young, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Intrinsic Imaging, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestle, Nippon Kayaku, Novartis, Ocera, OMass, Otsuka, Parexel, PDL, Periconsulting, Pharmacosmos, Philip Morris Institute, Pfizer, Procter & Gamble, Prometheus, Protagonist, Provention, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Sigmoid, Sublimity, Takeda, Therakos, Theravance, Tigenix, UCB, Vifor, Zealand, Zyngenia and 4SC; as an advisory board member for Abbott Laboratories, Abbvie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, DSM, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Sandoz, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zealand, Zyngenia and 4SC. S.D. reports consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ely Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc. and Vifor. L.P.B. has received personal fees from AbbVie, Janssen, Genentech, Ferring, Tillots, Pharmacosmos, Celltrion, Takeda, Boerhinger Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestle, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen, BMS, Vifor, Norgine; Mylan, Lilly, Fresenius Kabi, Oppilan Pharma, Sublimity Therapeutics, Applied Molecular Transport, OSE Immunotherapeutics, Enthera and Theravance; grants from Abbvie, MSD and Takeda. E.V.L. has consulted for AbbVie, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion Healthcare, Genentech, Gilead, Eli Lilly, Janssen, Pfizer, Takeda and UCB Biopharma; and has received research support from AbbVie, Bristol-Myers Squibb, Celgene, Genentech, Gilead, Janssen, Pfizer, Robarts Clinical Trials, Takeda and UCB Biopharma.
Author Contributions
W.R. generated the idea of the manuscript and wrote the first draft of the manuscript. S.D., L.P.B. and E.V.L. critically reviewed and modified the draft in an iterative fashion.
Funding
This paper was published as part of a supplement financially supported by ECCO and IOIBD.
Acknowledgments
All authors are members of the International Organization for the Study of Inflammatory Bowel Diseases [IOIBD].
References
U.S. Food & Drug Administration. FDA Guidance on conduct of clinical trials of medical products during COVID-19 public health emergency. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/ fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency. Accessed June 30, 2020.
European Medicines Agency. Guidance on the management of clinical trials during the COVID-19 (coronavirus) pandemic. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guidanceclinicaltrials_covid19_en.pdf. Accessed June 30, 2020.
U.S. Food & Drug Administration. Enforcement policy for non-invasive remote monitoring devices used to support patient monitoring during the coronavirus disease 2019 (COVID-19) public health emergency (revised). https://www.fda.gov/regulatory-information/search-fda-guidance- documents/enforcement-policy-non-invasive-remote-monitoring-devices-used-support-patient-monitoring-during. Accessed June 30, 2020.



