-
Views
-
Cite
Cite
W J Sandborn, B E Sands, R Panaccione, C D O’Brien, H Zhang, J Johanns, L Peyrin-Biroulet, G Van Assche, S Danese, S R Targan, M T Abreu, T Hisamatsu, P Szapary, C Marano, OP37 Efficacy and safety of ustekinumab as maintenance therapy in ulcerative colitis: Week 44 results from UNIFI, Journal of Crohn's and Colitis, Volume 13, Issue Supplement_1, March 2019, Pages S025–S026, https://doi.org/10.1093/ecco-jcc/jjy222.034
Close - Share Icon Share
Abstract
The study objective was to evaluate the safety and efficacy of SC ustekinumab (UST) as maintenance therapy in UC patients who were in clinical response to a single IV induction dose of UST.
This was a Ph3, double-blind, randomised withdrawal study in patients with moderate–severe active UC who failed conventional or biologic therapy (including anti-TNF and/or vedolizumab) and were in clinical response 8 weeks after receiving a single UST IV induction dose. The primary study population included 523 patients randomised 1:1:1 to placebo (PBO) SC, UST 90 mg SC q8w or q12w at Week 0. Primary endpoint was clinical remission at Week 44 (52 weeks after IV induction); key secondary endpoints were maintenance of clinical response, endoscopic healing, corticosteroid-free clinical remission, and maintenance of clinical remission among patients who achieved clinical remission at baseline.
Baseline (induction Week 0) demographics, UC disease characteristics, concomitant UC medications, and medication history were generally similar among treatment groups. Significantly greater proportions of UST q8w and q12w patients were in clinical remission at Week 44 (43.8% and 38.4%, respectively) vs. PBO patients (24.0%; p < 0.001 and p = 0.002, respectively). Significantly greater proportions of UST q8w and q12w patients maintained clinical response through Week 44 and achieved endoscopic healing and corticosteroid-free clinical remission vs. PBO patients. Clinical remission through Week 44 was maintained for a significantly greater proportion of q12w patients and a numerically greater proportion of q8w vs. PBO patients.
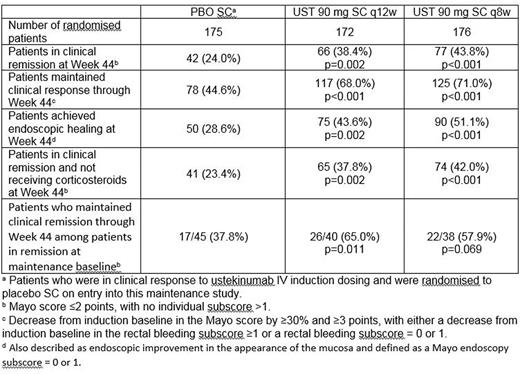
Abstract OP037 – Table 1. Primary and key secondary endpoints.
The proportions of patients with AEs, serious AEs, infections, and serious infections in the UST groups were generally comparable to PBO group. The proportions of patients who discontinued study agent were lower with UST q8w and q12w vs. PBO. Among the primary population in the maintenance study: no deaths, 2 malignancies other than NMSC (1 colon cancer,q8w; 1 papillary renal cell carcinoma, q12w) were reported. One patient-reported NMSC (2 SCC events, q12w).
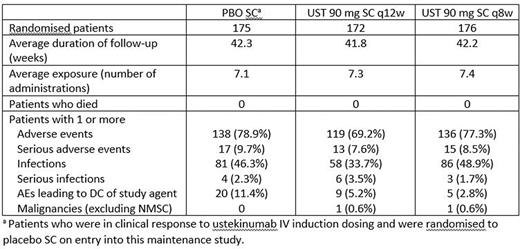
Abstract OP037 – Table 2. Summary of key safety findings through Week 44.
Both UST 90 mg q8w and q12w SC achieved clinical remission and maintained clinical response and were effective in achieving endoscopic healing and corticosteroid-free remission among patients with moderate–severe UC induced into clinical response with single IV dose of UST. The safety for UST in UC patients was consistent with the known safety profile of UST in CD.





