-
Views
-
Cite
Cite
K L Winthrop, E V Loftus Jr, D C Baumgart, W Reinisch, A J Thorpe, C I Nduaka, N Lawendy, G Chan, R D Pedersen, G S Friedman, C Su, P487 Tofacitinib for the treatment of ulcerative colitis: Analysis of infection rates from the OCTAVE clinical programme, Journal of Crohn's and Colitis, Volume 12, Issue supplement_1, February 2018, Pages S351–S352, https://doi.org/10.1093/ecco-jcc/jjx180.614
Close - Share Icon Share
Abstract
Tofacitinib is an oral, small molecule Janus kinase inhibitor that is being investigated for ulcerative colitis (UC). The safety of tofacitinib for treatment of moderate to severe UC was evaluated in 8-week Induction Phase (P) 2 (NCT00787202), 8-week Induction P3 (NCT01465763; NCT01458951) and 52-week Maintenance P3 (NCT01458574) studies,1 as well as an ongoing, open-label, long-term extension (LTE) study (OCTAVE Open, NCT01470612).2 Here, we present analysis of infections observed during the UC clinical development programme.
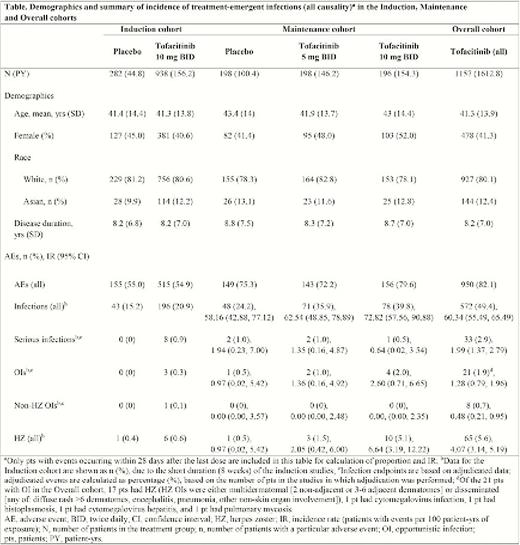
Patients who received placebo, tofacitinib 5 or 10 mg twice daily (BID) were analysed as three cohorts: Induction (P2 and P3 studies, N = 1220); maintenance (P3 study, N = 592); and overall (patients in P2, P3 and ongoing LTE studies receiving tofacitinib 5 or 10 mg BID, N = 1157). Data are shown as of 16 December 2016. Proportions and incidence rates (IRs; patients with events per 100 patient-years [PY] of exposure, 95% CI) were evaluated for infections of special interest. Opportunistic infections (OIs) were based on review by an independent adjudication committee.
In total, 1157 patients received ≥1 dose of tofacitinib 5 or 10 mg BID with 1613 PY of tofacitinib exposure (median 514 days) and ≤4.4 years of treatment. Demographics were generally similar across all treatment groups (Table). The most frequently occurring infection in all cohorts was nasopharyngitis. The serious infection events (SIEs) IR (95% CI) in the overall cohort, 1.99 (1.37, 2.79), was similar to the IRs in the maintenance cohort, 1.35 (0.16, 4.87) for 5 mg BID and 0.64 (0.02, 3.54) for 10 mg BID, suggesting that the risk of SIEs did not increase with duration of tofacitinib treatment. There was no apparent clustering of specific types of SIEs, nor apparent dose dependency in the risk of SIEs. OIs were reported in 21 patients, with an IR in the overall cohort of 1.28 (0.79, 1.96). Most OIs were herpes zoster (HZ) (17 patients, IR 1.04 [0.60, 1.66]), which was non-serious and mostly limited to skin involvement
SIEs were infrequent in the UC programme, with no apparent clustering of specific types of SIEs nor dose dependency in the risk of SIEs. OIs occurred infrequently, HZ being the most frequent, with no evidence for an increasing risk of OI with tofacitinib treatment duration. The safety profile generally appeared similar to that previously reported in rheumatoid arthritis (including increased risk of HZ)3 and that of other UC therapies including biologics.
Abstract P487
1. Sandborn WJ et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med, 2017;376(18):1723–1736.
2. Lichtenstein GR et al. Tofacitinib, an oral janus kinase inhibitor, in the treatment of ulcerative colitis: open-label, long-term extension study. Am J Gastroenterol, 2017;112(S1): Abstract 714.
3. Cohen SB et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis, 2017;76(7):1253–1262.
- osteogenesis imperfecta
- rheumatoid arthritis
- ulcerative colitis
- herpes zoster disease
- biological products
- demography
- gastroenterology
- human herpesvirus 3
- nasopharyngitis
- opportunistic infections
- safety
- skin manifestations
- infections
- immune reconstitution inflammatory syndrome
- duration of treatment
- small molecule
- janus kinase inhibitors
- tofacitinib
- internal revenue service
- oxygenation index measurement
- insulin receptor substrate proteins
- heart sound p2





