-
PDF
- Split View
-
Views
-
Cite
Cite
James Helliwell, Amanda Malone, Arjan J Bredenoord, Nam Nguyen, Hin Hin Ko, Christine Dobek, Vik Peck, Evan S Dellon, 265. INITIAL RESULTS FROM RESOLVE, A PHASE 1B DOSE-ESCALATION STUDY OF EP-104GI (EXTENDED-RELEASE FLUTICASONE PROPIONATE INTRA-ESOPHAGEAL INJECTION) FOR EOSINOPHILIC ESOPHAGITIS, Diseases of the Esophagus, Volume 37, Issue Supplement_1, September 2024, doae057.044, https://doi.org/10.1093/dote/doae057.044
Close - Share Icon Share
Abstract
Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease characterized by inflammation, influx of eosinophils and esophageal remodeling. Therapy for EoE includes swallowed/topical corticosteroids, dupilumab, and dietary elimination; but these are not always effective.
EP-104GI is an extended-release fluticasone propionate (FP) intra-esophageal injection being developed as a novel treatment for EoE. EP-104GI consists of coated FP crystals that release locally via diffusion at a pre-defined rate to reduce peak concentrations and prolong therapeutic window.
RESOLVE (NCT05608681) is a Phase 1b, multicenter, open-label, dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of EP-104GI in patients with EoE.
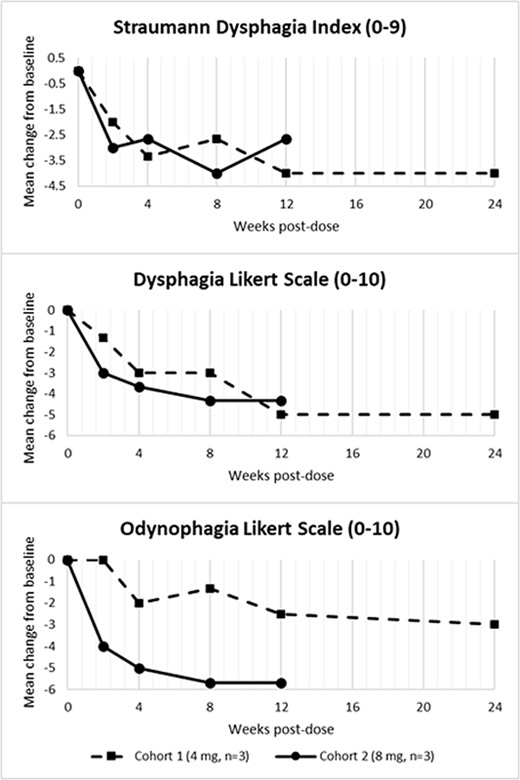
The conservative starting dose of EP-104GI consisted of 1 mg injected at each of 4 sites in 4 quadrants over two ‘rings’ in the esophagus. Dose escalations increase the dose per site and/or number of sites; the second cohort received 1 mg at each of 8 sites.
After a single administration of EP-104GI, participants are assessed for up to 24 weeks including adverse events (AEs) and laboratory tests. Efficacy assessments include histological endpoints and patient-reported symptom outcomes including the Straumann Dysphagia Index (SDI). Esophageal biopsies are collected at baseline, Week 4 and Week 12.
All subjects demonstrated decreased symptom scores compared to baseline, with generally greater responses at the higher EP-104GI dose in Cohort 2 (Fig 1). Histologically, all Cohort 2 patients showed decreases in EoEHSS scores with 2/3 having ≈50% decrease in total grade score and ≈35% decrease in total stage score at Week 4, also, eosinophil counts were reduced by ≈35% at Week 4 and ≈40% at Week 12.
Findings from Cohorts 1 and 2 of the ongoing trial include mild-moderate treatment-emergent AEs, the majority occurring on the day of treatment. No AEs were related to EP-104GI.
The initial results presented here indicate that the novel diffusion-based localized delivery of FP via EP-104GI injection into the esophagus is feasible and safe in patients with EoE and could avoid the side-effects typically associated with swallowed/topical corticosteroids. Efficacy data from the doses studied, although limited at this point, suggest a dose-response relationship with symptom outcomes and also show histologic improvements. These findings support the potential for an interval of at least 6 months between inter-esophageal injections, which may be further extended at the higher doses to be investigated in this study. Recruitment is ongoing.