-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroshi Miyata, Keijirou Sugimura, Takashi Kanemura, Tomohira Takeoka, Takahito Sugase, Masayoshi Yasui, Junichi Nishimura, Hiroshi Wada, Hiroshi Akita, Masaaki Yamamoto, Hisashi Hara, Naoki Shinno, Takeshi Omori, Masahiko Yano, Prognostic impact of nodal status and lymphovascular invasion in patients undergoing neoadjuvant chemotherapy for esophageal squamous cell carcinoma, Diseases of the Esophagus, Volume 37, Issue 9, September 2024, doae038, https://doi.org/10.1093/dote/doae038
Close - Share Icon Share
Summary
Nodal status is well known to be the most important prognostic factor for esophageal cancer patients, even if they are treated with neoadjuvant therapy. To establish an optimal postoperative adjuvant strategy for patients, we aimed to more accurately predict the prognosis of patients and systemic recurrence by using clinicopathological factors, including nodal status, in patients with esophageal cancer who received neoadjuvant chemotherapy. The clinicopathological factors associated with survival and systemic recurrence were investigated in 488 patients with esophageal squamous cell carcinoma who received neoadjuvant chemotherapy. Overall survival differed according to tumor depth, nodal status, tumor regression, and lymphovascular (LV) invasion. In the multivariate analysis, nodal status and LV invasion were identified as independent prognostic factors (P < 0.0001, P = 0.0008). Nodal status was also identified as an independent factor associated with systemic recurrence, although LV invasion was a borderline factor (P = 0.066). In each pN stage, patients with LV invasion showed significantly worse overall survival than those without LV invasion (pN0: P = 0.036, pN1: P = 0.0044, pN2: P = 0.0194, pN3: P = 0.0054). Patients with LV invasion were also more likely to have systemic, and any recurrence than those without LV invasion in each pN stage. Pathological nodal status and LV invasion were the most important predictors of survival and systemic recurrence in patients with esophageal cancer who underwent neoadjuvant chemotherapy followed by surgery. This finding could provide useful information about selecting candidates for adjuvant therapy among these patients. Our analysis showed that LV invasion was an independent prognostic factor in patients with esophageal cancer who underwent neoadjuvant chemotherapy and that combining LV invasion with pathological nodal status makes it possible to stratify the prognosis in those patients.
INTRODUCTION
Esophageal cancer is one of the most aggressive gastrointestinal cancers. Since the prognoses of patients undergoing primary surgical resection surgery are not favorable, neoadjuvant therapy to achieve better outcomes has been considered the standard treatment for advanced esophageal cancer.1–7 Neoadjuvant chemoradiotherapy (CRT) has been increasingly used as the standard treatment for advanced esophageal cancer in many countries, especially in Western countries, based on the results from the CROSS trial.3 On the other hand, in Japan, neoadjuvant chemotherapy has been mainly developed for advanced esophageal cancer. The JCOG 9907 study showed that neoadjuvant chemotherapy consisting of 5-fluorouracil (5-FU) and cisplatin (CF) was superior to postoperative adjuvant chemotherapy of the same regimen in patients with esophageal squamous cell carcinoma (ESCC).8 Moreover, recent results of JCOG 1109 showed that neoadjuvant triplet chemotherapy consisting of CF plus docetaxel improved overall survival compared with neoadjuvant doublet chemotherapy of CF.9
While neoadjuvant therapy for advanced esophageal cancer has been increasingly developed both in the eastern and western world thus far, there has not been effective postoperative adjuvant treatment in patients with esophageal cancer who underwent neoadjuvant therapy followed by surgery. However, a recent CheckMate 577 trial revealed that adjuvant therapy using nivolumab, which is a human monoclonal anti-programmed death 1 (PD-1) antibody, significantly improved disease-free survival compared with placebo in patients with esophageal cancer who underwent neoadjuvant CRT followed by surgery and had residual pathological disease.10 This result suggests that postoperative adjuvant therapy, such as nivolumab immunotherapy, can also offer a survival benefit in patients who receive neoadjuvant chemotherapy followed by surgery. To appropriately perform this adjuvant therapy strategy, it is necessary to clarify which patients most benefit from postoperative adjuvant therapy by identifying useful prognostic factors and risk factors for recurrence in patients undergoing neoadjuvant chemotherapy. Several factors, including pathological tumor regression and nodal status, have been reported as useful prognostic factors and risk factors for systemic recurrence in patients undergoing neoadjuvant CRT.11–16 We previously reported that in neoadjuvant chemotherapy, post-treatment nodal status seems to be most useful for predicting prognosis and the occurrence of systemic disease.17
In the present study, we aimed to investigate whether we can more accurately predict the prognosis of patients and systemic recurrence by combining another factor with pathological nodal status in patients with esophageal cancer who received neoadjuvant chemotherapy.
MATERIALS AND METHODS
Patients and pretreatment examination
Between January 2002 and December 2019, 513 patients with thoracic esophageal cancer underwent neoadjuvant chemotherapy followed by surgery at the Osaka International Cancer Institute. According to the principles of our hospital, neoadjuvant chemotherapy followed by surgery was performed for patients with any T (cT1–4) and lymph node involvement and no distant organ metastasis. Neoadjuvant CRT followed by surgery was basically performed for patients with thoracic esophageal cancers that were suspected to invade adjacent organs (cT4), such as the trachea and aorta, without distant organ metastasis or for those with tumors in the upper third of the thoracic esophagus that had infiltrated into the cervical esophagus. Of 513 patients who underwent neoadjuvant chemotherapy, 15 patients underwent incomplete curative resection (R1 or R2) and 10 patients had tumors that were confirmed to be histological types other than squamous cell carcinoma. Excluding these 25 patients, the remaining 488 patients with ESCC who underwent neoadjuvant chemotherapy followed by surgery were included in this study.
All 488 patients were staged by computed tomography (CT) and endoscopy, together with and 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG-PET) scan when possible. Lymph nodes were considered metastasis-positive on CT scan if they were spherical and larger than 1.0 cm in maximum transverse diameter or if focal major FDG uptake, compared with normal mediastinal activity, was detected on PET scan.
Neoadjuvant chemotherapy and surgical treatment
The neoadjuvant chemotherapy regimens used in the study period were triple therapy with either 5-FU, CF, and adriamycin (ACF) or 5-FU, CF, and docetaxel (DCF) and double therapy with 5-FU and CF. In the ACF regimen (n = 176), 5-FU was administered at 700 mg/m2 by continuous intravenous infusion on Days 1–7, with CF at 70 mg/m2 and ACF at 35 mg/m2 administered by rapid intravenous infusion on Day 1. In the DCF regimen (n = 275), 5-FU was also administered at 700 mg/m2 by continuous intravenous infusion on Days 1–5, with CF at 70 mg/m2 and DCF at 70 mg/m2 administered by rapid intravenous infusion on Day 1.7,17,18 In the CF regimen (n = 37), 5-FU was administered at 800 mg/m2 by continuous intravenous infusion on Days 1–5, with CF at 80 mg/m2 administered by rapid intravenous infusion on Day 1. Two courses of chemotherapy were used, separated by a 3- to 4-week interval.
Surgical resection was performed 3–6 weeks after the completion of chemotherapy. Our standard procedures consisted of subtotal esophagectomy with mediastinal lymphadenectomy via right thoracotomy, upper abdominal lymphadenectomy, cervical lymphadenectomy, reconstruction of the gastric tube, and anastomosis in the cervical incision. Cervical lymphadenectomy was sometimes omitted according to the pretreatment radiological diagnosis and tumor location.
The study protocol was approved by the Human Ethics Review Committee of the Osaka International Cancer Institute.
Evaluation of response to preoperative therapy
The degree of histopathological tumor regression in the surgical specimens was classified into five categories. The proportion of viable residual tumor cells within the cancerous tissue was assessed as follows: Grade 3, no viable residual tumor cells (pathological CR); Grade 2, less than 1/3 residual tumor cells; Grade 1b, 1/3–2/3 residual tumor cells; Grade 1a, more than 2/3 residual tumor cells; and Grade 0, no significant response to preoperative therapy.7,17,19,20 Patients with Grade 2 and Grade 3 findings were considered to have major responses, while those with Grade 0, Grade 1a, and Grade 1b findings were considered to have minor responses.
Lymphatic invasion and vascular invasion were microscopically assessed in hematoxylin and eosin (HE)-stained sections.
The clinical and histopathological findings were classified according to the UICC TNM classification.21
Follow-up examination and recurrence pattern
Following hospital discharge, patients were seen every 2 months for the first 2 years and every 3 months thereafter. A CT of the neck, thorax, and upper abdomen was performed every 4 months for the first 2 years and every 6 months thereafter. When recurrence was suspected by CT scan, more selective investigations, such as positron emission tomography (PET) and magnetic resonance imaging, were performed to confirm or refute recurrent disease. During follow-up periods, the first site recurrence was noted, and any additional recurrence found within 1 month was considered to have occurred simultaneously. Any first recurrence was included, regardless of the length of the postoperative period.
Local recurrence in this study was defined as recurrence within our standard surgical field, including subtotal esophagectomy with mediastinal lymphadenectomy, cervical lymphadenectomy, and upper abdominal lymphadenectomy, irrespective of the surgical procedure actually performed, and systemic recurrence was defined as recurrence beyond the surgical field with or without local recurrence.
Statistical analysis
The data are expressed as the mean ± SD. The Mann–Whitney test and Student’s t-test were used to analyze differences in clinical and pathological factors between patients with major responses and those with minor responses. Overall survival was calculated from the date of commencement of neoadjuvant therapy to the date of death or the last known date of follow-up. Recurrence-free survival was also calculated from the date of commencement of neoadjuvant therapy to the date of first recurrence after esophagectomy. Actual survival was calculated by the Kaplan–Meier method and statistically evaluated by the log-rank test. The Cox proportional hazards regression model and logistic regression analysis were used to analyze the simultaneous influences of predictive factors for survival. A P-value less than 0.05 denotes statistical significance. These analyses were performed using JMP version 9.0 software (SAS Institute, Cary, NC, USA).
RESULTS
Factors affecting the survival of patients with neoadjuvant chemotherapy
The characteristics of the 488 patients studied are listed in Table 1. The mean age of the patients was 64.7 years. Of all 488 patients, 331 patients (67.8%) were diagnosed pathologically as having lymph node metastasis (pN1–pN3), while the remaining 157 patients (32.2%) were diagnosed as having no metastasis (pN0). Seventy-three patients with M1lym had supraclavicular lymph node metastasis. Forty patients (8.2%) achieved a pathological complete response to the primary tumors (Grade 3), but 20 patients (4.1%) showed no apparent response to neoadjuvant therapy. One hundred fifty-four patients (31.1%) were classified as major responders (Grade 2–3), while 336 patients (68.9%) were minor responders (Grade 0–1). Lymphatic invasion and vascular invasion were observed in 184 patients (38.7%) and 219 patients (44.9%), respectively. As a result, lymphovascular (LV) invasion was observed in 262 patients (53.6%). LV invasion was significantly associated with pT stage, pN stage, and pathological tumor regression (Supplementary Table 1).
| . | Total n = 488 . |
|---|---|
| Age (years) | 64.67 ± 7.5 |
| Sex | |
| male | 396 (81) |
| female | 92 (19) |
| Tumor location | |
| upper third | 57 (12) |
| middle third | 245 (50) |
| lower third | 186 (38) |
| Pretherapy tumor depth | |
| cT1 | 29 (6) |
| cT2 | 108 (22) |
| cT3 | 311 (64) |
| cT4 | 40 (8) |
| cN | |
| cN0 | 68 (14) |
| cN1–3 | 420 (86) |
| Surgical approach | |
| 2-field | 152 (31) |
| 3-field | 336 (69) |
| MIE or open | |
| MIE | 108 (22) |
| open | 380 (78) |
| Number of resected lymph nodes | 72.3 ± 28.6 |
| Posttherapy tumor depth | |
| ypT0 | 40 (8) |
| ypT1 | 116 (24) |
| ypT2 | 66 (14) |
| ypT3 | 253 (51) |
| ypT4 | 13 (3) |
| ypN | |
| ypN0 | 157 (32) |
| ypN1 | 171 (35) |
| ypN2 | 84 (17) |
| ypN3 | 76 (16) |
| M1lym | |
| M0 | 415 (85) |
| M1lym | 73 (15) |
| Ly | |
| (−) | 304 (62) |
| (+) | 184 (38) |
| V | |
| (−) | 269 (55) |
| (+) | 219 (45) |
| LV | |
| (−) | 226 (46) |
| (+) | 262 (54) |
| Pathological response | |
| Grade0 | 20 (4) |
| Grade1a | 219 (45) |
| Grade1b | 97 (20) |
| Grade2 | 112 (23) |
| Grade3 | 40 (8) |
| . | Total n = 488 . |
|---|---|
| Age (years) | 64.67 ± 7.5 |
| Sex | |
| male | 396 (81) |
| female | 92 (19) |
| Tumor location | |
| upper third | 57 (12) |
| middle third | 245 (50) |
| lower third | 186 (38) |
| Pretherapy tumor depth | |
| cT1 | 29 (6) |
| cT2 | 108 (22) |
| cT3 | 311 (64) |
| cT4 | 40 (8) |
| cN | |
| cN0 | 68 (14) |
| cN1–3 | 420 (86) |
| Surgical approach | |
| 2-field | 152 (31) |
| 3-field | 336 (69) |
| MIE or open | |
| MIE | 108 (22) |
| open | 380 (78) |
| Number of resected lymph nodes | 72.3 ± 28.6 |
| Posttherapy tumor depth | |
| ypT0 | 40 (8) |
| ypT1 | 116 (24) |
| ypT2 | 66 (14) |
| ypT3 | 253 (51) |
| ypT4 | 13 (3) |
| ypN | |
| ypN0 | 157 (32) |
| ypN1 | 171 (35) |
| ypN2 | 84 (17) |
| ypN3 | 76 (16) |
| M1lym | |
| M0 | 415 (85) |
| M1lym | 73 (15) |
| Ly | |
| (−) | 304 (62) |
| (+) | 184 (38) |
| V | |
| (−) | 269 (55) |
| (+) | 219 (45) |
| LV | |
| (−) | 226 (46) |
| (+) | 262 (54) |
| Pathological response | |
| Grade0 | 20 (4) |
| Grade1a | 219 (45) |
| Grade1b | 97 (20) |
| Grade2 | 112 (23) |
| Grade3 | 40 (8) |
The data are expressed as the mean ± SD or number (percentage) of subjects.
MIE, minimally invasive esophagectomy; LV, lymphovascular invasion.
| . | Total n = 488 . |
|---|---|
| Age (years) | 64.67 ± 7.5 |
| Sex | |
| male | 396 (81) |
| female | 92 (19) |
| Tumor location | |
| upper third | 57 (12) |
| middle third | 245 (50) |
| lower third | 186 (38) |
| Pretherapy tumor depth | |
| cT1 | 29 (6) |
| cT2 | 108 (22) |
| cT3 | 311 (64) |
| cT4 | 40 (8) |
| cN | |
| cN0 | 68 (14) |
| cN1–3 | 420 (86) |
| Surgical approach | |
| 2-field | 152 (31) |
| 3-field | 336 (69) |
| MIE or open | |
| MIE | 108 (22) |
| open | 380 (78) |
| Number of resected lymph nodes | 72.3 ± 28.6 |
| Posttherapy tumor depth | |
| ypT0 | 40 (8) |
| ypT1 | 116 (24) |
| ypT2 | 66 (14) |
| ypT3 | 253 (51) |
| ypT4 | 13 (3) |
| ypN | |
| ypN0 | 157 (32) |
| ypN1 | 171 (35) |
| ypN2 | 84 (17) |
| ypN3 | 76 (16) |
| M1lym | |
| M0 | 415 (85) |
| M1lym | 73 (15) |
| Ly | |
| (−) | 304 (62) |
| (+) | 184 (38) |
| V | |
| (−) | 269 (55) |
| (+) | 219 (45) |
| LV | |
| (−) | 226 (46) |
| (+) | 262 (54) |
| Pathological response | |
| Grade0 | 20 (4) |
| Grade1a | 219 (45) |
| Grade1b | 97 (20) |
| Grade2 | 112 (23) |
| Grade3 | 40 (8) |
| . | Total n = 488 . |
|---|---|
| Age (years) | 64.67 ± 7.5 |
| Sex | |
| male | 396 (81) |
| female | 92 (19) |
| Tumor location | |
| upper third | 57 (12) |
| middle third | 245 (50) |
| lower third | 186 (38) |
| Pretherapy tumor depth | |
| cT1 | 29 (6) |
| cT2 | 108 (22) |
| cT3 | 311 (64) |
| cT4 | 40 (8) |
| cN | |
| cN0 | 68 (14) |
| cN1–3 | 420 (86) |
| Surgical approach | |
| 2-field | 152 (31) |
| 3-field | 336 (69) |
| MIE or open | |
| MIE | 108 (22) |
| open | 380 (78) |
| Number of resected lymph nodes | 72.3 ± 28.6 |
| Posttherapy tumor depth | |
| ypT0 | 40 (8) |
| ypT1 | 116 (24) |
| ypT2 | 66 (14) |
| ypT3 | 253 (51) |
| ypT4 | 13 (3) |
| ypN | |
| ypN0 | 157 (32) |
| ypN1 | 171 (35) |
| ypN2 | 84 (17) |
| ypN3 | 76 (16) |
| M1lym | |
| M0 | 415 (85) |
| M1lym | 73 (15) |
| Ly | |
| (−) | 304 (62) |
| (+) | 184 (38) |
| V | |
| (−) | 269 (55) |
| (+) | 219 (45) |
| LV | |
| (−) | 226 (46) |
| (+) | 262 (54) |
| Pathological response | |
| Grade0 | 20 (4) |
| Grade1a | 219 (45) |
| Grade1b | 97 (20) |
| Grade2 | 112 (23) |
| Grade3 | 40 (8) |
The data are expressed as the mean ± SD or number (percentage) of subjects.
MIE, minimally invasive esophagectomy; LV, lymphovascular invasion.
Overall survival differed significantly according to nodal status (5-year survival rate: pN0, 81.4%; pN1, 59.9%; pN2, 34.6%; pN3, 15.8%; Fig. 1A). Overall survival differed significantly according to pathological tumor regression (5-year survival rate: grade 3, 77.2%; grade 2, 68.9%; grade 1b, 57.2%; grade 1a, 47.4%; grade 0, 20.0%; Supplementary Fig. 1). Overall survival also differed significantly according to the LV invasion (5-year survival rate: LV(−), 74.9%; LV(+), 39.2%, P < 0.001, Fig. 1B).
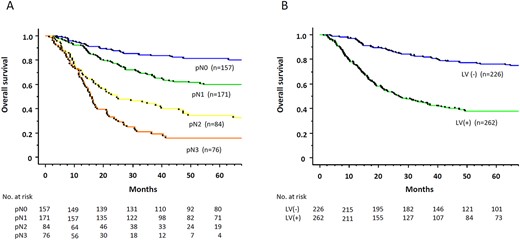
Overall survival rate of 488 patients with esophageal cancers who underwent neoadjuvant chemotherapy followed by surgery, according to pathological nodal status (A) and lymphovascular invasion (B).
Recurrence-free survival also differed significantly according to nodal status and LV invasion (Supplementary Figs. 2, 3).
In the multivariate analysis, pathological nodal status and LV invasion were identified as the most important independent prognostic factors (Table 2).
Results of univariate and multivariate analyses of the prognostic factors in patients with esophageal cancer
| . | . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . | ||
| Age | ≥70 vs. 70> | 1.01 | 0.75–1.38 | 0.939 | — | — | — |
| Sex | female vs. male | 0.84 | 0.59–1.19 | 0.323 | — | — | — |
| Tumor location | lower vs. middle/upper | 1.03 | 0.79–1.36 | 0.809 | — | — | — |
| cT | cT3–4 vs. cT1–2 | 2.06 | 1.47–2.89 | <0.0001 | 1.30 | 0.88–1.90 | 0.187 |
| cN | cN2–3 vs. cN0–1 | 2.86 | 1.66–4.90 | 0.0001 | 2.02 | 1.17–3.50 | 0.012 |
| MIE or open | MIE vs. open | 0.45 | 0.30–0.67 | <0.0001 | 0.67 | 0.44–1.02 | 0.060 |
| Number of resected node | 50> vs. ≥50 | 1.05 | 0.76–1.48 | 0.739 | — | — | — |
| Pathological response | 3–2 vs. 1b-0 | 2.14 | 1.55–2.98 | <0.0001 | 1.06 | 0.71–1.58 | 0.792 |
| Tumor depth | ypT3–4 vs. ypT0–2 | 2.47 | 1.85–3.29 | <0.0001 | 1.15 | 0.78–1.69 | 0.478 |
| Nodal status | ypN2–3 vs. ypN0–1 | 3.83 | 2.93–5.00 | <0.0001 | 2.63 | 1.96–3.52 | <0.0001 |
| LV | LV(+) vs. LV(−) | 3.16 | 2.35–4.27 | <0.0001 | 1.85 | 1.29–2.65 | 0.0008 |
| . | . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . | ||
| Age | ≥70 vs. 70> | 1.01 | 0.75–1.38 | 0.939 | — | — | — |
| Sex | female vs. male | 0.84 | 0.59–1.19 | 0.323 | — | — | — |
| Tumor location | lower vs. middle/upper | 1.03 | 0.79–1.36 | 0.809 | — | — | — |
| cT | cT3–4 vs. cT1–2 | 2.06 | 1.47–2.89 | <0.0001 | 1.30 | 0.88–1.90 | 0.187 |
| cN | cN2–3 vs. cN0–1 | 2.86 | 1.66–4.90 | 0.0001 | 2.02 | 1.17–3.50 | 0.012 |
| MIE or open | MIE vs. open | 0.45 | 0.30–0.67 | <0.0001 | 0.67 | 0.44–1.02 | 0.060 |
| Number of resected node | 50> vs. ≥50 | 1.05 | 0.76–1.48 | 0.739 | — | — | — |
| Pathological response | 3–2 vs. 1b-0 | 2.14 | 1.55–2.98 | <0.0001 | 1.06 | 0.71–1.58 | 0.792 |
| Tumor depth | ypT3–4 vs. ypT0–2 | 2.47 | 1.85–3.29 | <0.0001 | 1.15 | 0.78–1.69 | 0.478 |
| Nodal status | ypN2–3 vs. ypN0–1 | 3.83 | 2.93–5.00 | <0.0001 | 2.63 | 1.96–3.52 | <0.0001 |
| LV | LV(+) vs. LV(−) | 3.16 | 2.35–4.27 | <0.0001 | 1.85 | 1.29–2.65 | 0.0008 |
MIE, minimally invasive esophagectomy; LV, lymphovascular invasion.
Results of univariate and multivariate analyses of the prognostic factors in patients with esophageal cancer
| . | . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . | ||
| Age | ≥70 vs. 70> | 1.01 | 0.75–1.38 | 0.939 | — | — | — |
| Sex | female vs. male | 0.84 | 0.59–1.19 | 0.323 | — | — | — |
| Tumor location | lower vs. middle/upper | 1.03 | 0.79–1.36 | 0.809 | — | — | — |
| cT | cT3–4 vs. cT1–2 | 2.06 | 1.47–2.89 | <0.0001 | 1.30 | 0.88–1.90 | 0.187 |
| cN | cN2–3 vs. cN0–1 | 2.86 | 1.66–4.90 | 0.0001 | 2.02 | 1.17–3.50 | 0.012 |
| MIE or open | MIE vs. open | 0.45 | 0.30–0.67 | <0.0001 | 0.67 | 0.44–1.02 | 0.060 |
| Number of resected node | 50> vs. ≥50 | 1.05 | 0.76–1.48 | 0.739 | — | — | — |
| Pathological response | 3–2 vs. 1b-0 | 2.14 | 1.55–2.98 | <0.0001 | 1.06 | 0.71–1.58 | 0.792 |
| Tumor depth | ypT3–4 vs. ypT0–2 | 2.47 | 1.85–3.29 | <0.0001 | 1.15 | 0.78–1.69 | 0.478 |
| Nodal status | ypN2–3 vs. ypN0–1 | 3.83 | 2.93–5.00 | <0.0001 | 2.63 | 1.96–3.52 | <0.0001 |
| LV | LV(+) vs. LV(−) | 3.16 | 2.35–4.27 | <0.0001 | 1.85 | 1.29–2.65 | 0.0008 |
| . | . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . | ||
| Age | ≥70 vs. 70> | 1.01 | 0.75–1.38 | 0.939 | — | — | — |
| Sex | female vs. male | 0.84 | 0.59–1.19 | 0.323 | — | — | — |
| Tumor location | lower vs. middle/upper | 1.03 | 0.79–1.36 | 0.809 | — | — | — |
| cT | cT3–4 vs. cT1–2 | 2.06 | 1.47–2.89 | <0.0001 | 1.30 | 0.88–1.90 | 0.187 |
| cN | cN2–3 vs. cN0–1 | 2.86 | 1.66–4.90 | 0.0001 | 2.02 | 1.17–3.50 | 0.012 |
| MIE or open | MIE vs. open | 0.45 | 0.30–0.67 | <0.0001 | 0.67 | 0.44–1.02 | 0.060 |
| Number of resected node | 50> vs. ≥50 | 1.05 | 0.76–1.48 | 0.739 | — | — | — |
| Pathological response | 3–2 vs. 1b-0 | 2.14 | 1.55–2.98 | <0.0001 | 1.06 | 0.71–1.58 | 0.792 |
| Tumor depth | ypT3–4 vs. ypT0–2 | 2.47 | 1.85–3.29 | <0.0001 | 1.15 | 0.78–1.69 | 0.478 |
| Nodal status | ypN2–3 vs. ypN0–1 | 3.83 | 2.93–5.00 | <0.0001 | 2.63 | 1.96–3.52 | <0.0001 |
| LV | LV(+) vs. LV(−) | 3.16 | 2.35–4.27 | <0.0001 | 1.85 | 1.29–2.65 | 0.0008 |
MIE, minimally invasive esophagectomy; LV, lymphovascular invasion.
Prognostic impact of LV invasion in each nodal status
We performed subgroup analysis according to pathological nodal status and LV invasion. In pN0 patients, patients with LV invasion showed significantly worse overall survival (5-year survival rate: LV(+), 72.3%; LV(−), 85.4%; P = 0.0306, Fig. 2A). In pN1 patients, patients with LV invasion also showed significantly worse overall survival (5-year survival rate: LV(+), 48.7%; LV(−), 71.3%; P = 0.0044, Fig. 2B). The same tendency of worse prognosis in patients with LV invasion was similarly observed in pN2 and pN3 patients (P = 0.0194 and P = 0.0054, Fig. 2C and Fig. 2D).
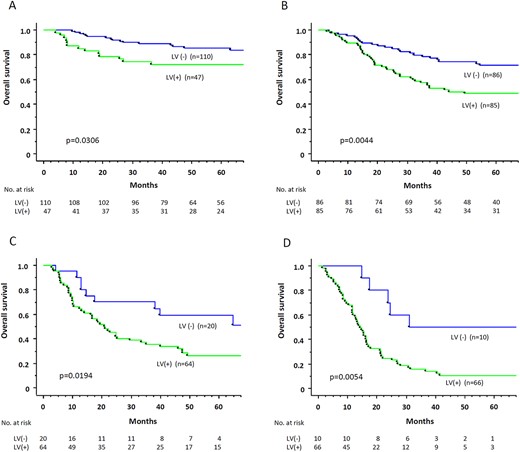
Overall survival rate, according to lymphovascular (LV) invasion, in pN0 patients (A), in pN1 patients (B), in pN2 patients (C), and in pN3 patients (D).
Regarding recurrence-free survival, patients with LV invasion tended to show worse recurrence-free survival than those without LV invasion in pN0, pN1, and pN3 patients, except for pN2 patients (pN0, P = 0.0502; pN1, P = 0.0293; pN2, P = 0.4720; pN3, P = 0.0013).
Factors affecting the occurrence of systemic disease in patients with neoadjuvant chemotherapy
Among 488 patients who underwent curative esophagectomy, any recurrence was found in 237 patients (48.6%) and systemic recurrence was found in 180 patients (36.9%). The frequency of systemic recurrence differed according to pathological nodal status (pN0, 12.7%; pN1, 33.3%; pN2, 54.8%; pN3, 75.0%; Fig. 3A). For each nodal status (pN0–pN3), patients with LV invasion were more likely to have systemic recurrence than those without LV invasion (Fig. 3A). The frequency of any recurrence also differed according to pathological nodal status (pN0, 21.0%; pN1, 46.8%; pN2, 70.2%; pN3, 85.5%; Fig. 3B). In each nodal status (pN0–pN3), there was a tendency for patients with LV invasion to be more likely to have any recurrence than those without LV invasion (Fig. 3B).
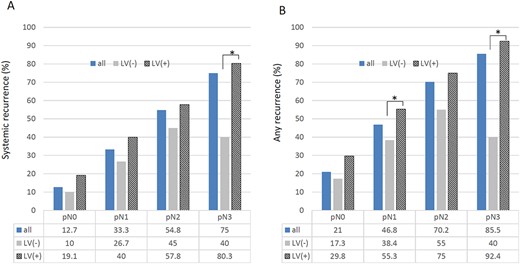
The frequency of systemic recurrence (A) and any recurrence (B) in 488 patients who underwent neoadjuvant chemotherapy followed by surgery, according to pathological nodal status and lymphovascular (LV) invasion.
Logistic regression analysis showed that pathological nodal status was an independent factor associated with systemic recurrence in patients with neoadjuvant chemotherapy, while neither pathological tumor regression nor tumor depth were. LV invasion was a borderline factor associated with systemic recurrence (Table 3).
Results of univariate and multivariate analyses of the significant factors for systemic recurrence in patients with esophageal cancer
| . | . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . | ||
| Age | ≥70 vs. 70> | 1.19 | 0.79–1.80 | 0.402 | — | — | — |
| Sex | female vs. male | 0.79 | 0.49–1.28 | 0.342 | — | — | — |
| Tumor location | lower vs. middle/upper | 1.27 | 0.87–1.85 | 0.218 | — | — | — |
| MIE or open | MIE vs. open | 0.70 | 0.44–1.10 | 0.119 | — | — | — |
| Number of resected node | 50> vs. ≥50 | 1.18 | 0.75–1.85 | 0.471 | — | — | — |
| Pathological response | 3–2 vs. 1b-0 | 2.67 | 1.72–4.13 | <0.0001 | 1.06 | 0.83–1.43 | 0.197 |
| Tumor depth | ypT3–4 vs. ypT0–2 | 2.22 | 1.51–3.25 | <0.0001 | 1.03 | 0.63–1.69 | 0.894 |
| Nodal status | ypN2–3 vs. ypN0–1 | 5.89 | 3.90–8.90 | <0.0001 | 4.56 | 2.92–7.10 | <0.0001 |
| LV | LV(+) vs. LV(−) | 3.07 | 2.08–4.54 | <0.0001 | 1.56 | 0.97–2.59 | 0.066 |
| . | . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . | ||
| Age | ≥70 vs. 70> | 1.19 | 0.79–1.80 | 0.402 | — | — | — |
| Sex | female vs. male | 0.79 | 0.49–1.28 | 0.342 | — | — | — |
| Tumor location | lower vs. middle/upper | 1.27 | 0.87–1.85 | 0.218 | — | — | — |
| MIE or open | MIE vs. open | 0.70 | 0.44–1.10 | 0.119 | — | — | — |
| Number of resected node | 50> vs. ≥50 | 1.18 | 0.75–1.85 | 0.471 | — | — | — |
| Pathological response | 3–2 vs. 1b-0 | 2.67 | 1.72–4.13 | <0.0001 | 1.06 | 0.83–1.43 | 0.197 |
| Tumor depth | ypT3–4 vs. ypT0–2 | 2.22 | 1.51–3.25 | <0.0001 | 1.03 | 0.63–1.69 | 0.894 |
| Nodal status | ypN2–3 vs. ypN0–1 | 5.89 | 3.90–8.90 | <0.0001 | 4.56 | 2.92–7.10 | <0.0001 |
| LV | LV(+) vs. LV(−) | 3.07 | 2.08–4.54 | <0.0001 | 1.56 | 0.97–2.59 | 0.066 |
MIE, minimally invasive esophagectomy; LV, lymphovascular invasion.
Results of univariate and multivariate analyses of the significant factors for systemic recurrence in patients with esophageal cancer
| . | . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . | ||
| Age | ≥70 vs. 70> | 1.19 | 0.79–1.80 | 0.402 | — | — | — |
| Sex | female vs. male | 0.79 | 0.49–1.28 | 0.342 | — | — | — |
| Tumor location | lower vs. middle/upper | 1.27 | 0.87–1.85 | 0.218 | — | — | — |
| MIE or open | MIE vs. open | 0.70 | 0.44–1.10 | 0.119 | — | — | — |
| Number of resected node | 50> vs. ≥50 | 1.18 | 0.75–1.85 | 0.471 | — | — | — |
| Pathological response | 3–2 vs. 1b-0 | 2.67 | 1.72–4.13 | <0.0001 | 1.06 | 0.83–1.43 | 0.197 |
| Tumor depth | ypT3–4 vs. ypT0–2 | 2.22 | 1.51–3.25 | <0.0001 | 1.03 | 0.63–1.69 | 0.894 |
| Nodal status | ypN2–3 vs. ypN0–1 | 5.89 | 3.90–8.90 | <0.0001 | 4.56 | 2.92–7.10 | <0.0001 |
| LV | LV(+) vs. LV(−) | 3.07 | 2.08–4.54 | <0.0001 | 1.56 | 0.97–2.59 | 0.066 |
| . | . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95%CI . | P-value . | HR . | 95%CI . | P-value . | ||
| Age | ≥70 vs. 70> | 1.19 | 0.79–1.80 | 0.402 | — | — | — |
| Sex | female vs. male | 0.79 | 0.49–1.28 | 0.342 | — | — | — |
| Tumor location | lower vs. middle/upper | 1.27 | 0.87–1.85 | 0.218 | — | — | — |
| MIE or open | MIE vs. open | 0.70 | 0.44–1.10 | 0.119 | — | — | — |
| Number of resected node | 50> vs. ≥50 | 1.18 | 0.75–1.85 | 0.471 | — | — | — |
| Pathological response | 3–2 vs. 1b-0 | 2.67 | 1.72–4.13 | <0.0001 | 1.06 | 0.83–1.43 | 0.197 |
| Tumor depth | ypT3–4 vs. ypT0–2 | 2.22 | 1.51–3.25 | <0.0001 | 1.03 | 0.63–1.69 | 0.894 |
| Nodal status | ypN2–3 vs. ypN0–1 | 5.89 | 3.90–8.90 | <0.0001 | 4.56 | 2.92–7.10 | <0.0001 |
| LV | LV(+) vs. LV(−) | 3.07 | 2.08–4.54 | <0.0001 | 1.56 | 0.97–2.59 | 0.066 |
MIE, minimally invasive esophagectomy; LV, lymphovascular invasion.
DISCUSSION
The effectiveness of postoperative adjuvant therapy in patients with esophageal cancer who undergo neoadjuvant therapy followed by surgery was first shown by a recent randomized controlled trial (CheckMate 577 trial).10 To appropriately perform this adjuvant therapy strategy, it is necessary to clarify which patients most benefit from adjuvant therapy by identifying useful prognostic factors and risk factors for recurrence in patients undergoing neoadjuvant therapy. In this study, we found that LV invasion was an independent prognostic factor along with pathological nodal status in patients with esophageal cancer who underwent neoadjuvant chemotherapy. We also found that combining LV invasion with pathological nodal status makes it possible to stratify the prognosis and risk for systemic recurrence and any recurrence in those patients.
Both pathological nodal status and pathological tumor regression have been recognized as the most important prognostic factors in patients with esophageal cancer who undergo neoadjuvant CRT or chemotherapy followed by surgery.11–17,21 In neoadjuvant chemotherapy, several studies showed that pathological nodal status was an independent prognostic factor in the multivariate analysis, although pathological tumor regression was not.17,22–24 These results suggest that nodal status may be more important as a prognostic marker than pathological tumor regression in neoadjuvant chemotherapy. In the present study, we found that LV invasion was an independent prognostic factor along with pathological nodal status, although pathological tumor regression was not identified as an independent factor.
The clinical impact of LV invasion has been mainly investigated in superficial esophageal cancer. LV invasion is known to be a prognostic factor as well as an important factor associated with LN metastasis in superficial ESCC or adenocarcinoma.25–30 In advanced esophageal cancer, several studies have focused on the prognostic impact of LV invasion in patients who underwent esophagectomy with and without neoadjuvant CRT.31–36 Chen et al. showed that LV invasion was an independent prognostic factor together with nodal status in patients who received neoadjuvant CRT.35 Hsu et al. also showed that LV invasion was an independent risk factor for distant recurrence after neoadjuvant CRT in patients with ESCC.36 However, only a few studies have addressed the clinical importance of LV invasion in patients with esophageal cancer who undergo neoadjuvant chemotherapy followed by surgery.37,38 Lagarde et al. showed that LV invasion and perineural infiltration have an important prognostic impact in patients treated with neoadjuvant chemotherapy followed by surgery.37 Oguma et al., in their study, including 388 patients with ESCC who underwent neoadjuvant chemotherapy, revealed the prognostic importance of LV invasion and advocated a novel pathological staging system that combined TNM classification with LV invasion in patients treated with neoadjuvant chemotherapy.38 In our study, we found that LV invasion was an independent prognostic factor along with pathological nodal status in patients with esophageal cancer who underwent neoadjuvant chemotherapy. This finding is consistent with those previous results. We also found that combining LV invasion with pathological nodal status enabled us to more precisely stratify prognosis in patients treated with neoadjuvant chemotherapy.
Patients who have a high risk of systemic recurrence after esophagectomy seem to be good candidates for adjuvant therapy. Peyre et al. previously reviewed the data of 1053 patients who underwent surgery alone and showed that the number of involved lymph nodes was associated with the occurrence of systemic disease.39 Our previous study showed that posttreatment nodal status rather than pathological tumor regression seems to be useful for predicting prognosis and the occurrence of systemic disease in patients with ESCC who underwent neoadjuvant chemotherapy.17 In the current study, we found that the risk of systemic recurrence was stratified by using LV invasion together with pathological nodal status. This finding can be useful for selecting candidates for adjuvant therapy after neoadjuvant therapy followed by esophagectomy.
In this study, LV invasion was present in 53.3% of patients with esophageal cancer who underwent neoadjuvant chemotherapy. Previous studies showed that LV invasion was observed in 33.8%–49.9% of patients with esophageal cancer who underwent esophagectomy without neoadjuvant treatment and that LV invasion was significantly associated with pN stage and pT stage.31–34 In the neoadjuvant CRT, several studies showed that LV invasion was present in 23.3%–39.0% of patients treated with neoadjuvant CRT.35,36,40,41 These results suggest that LV invasion may be less common in patients with neoadjuvant CRT than in patients without neoadjuvant therapy. Regarding neoadjuvant chemotherapy, Lagarde et al. showed that LV invasion was observed in 58.8% of patients treated with neoadjuvant chemotherapy.37 Oguma et al. also showed that LV invasion was present in 52.6% of patients who underwent neoadjuvant chemotherapy followed by surgery.38 These results are almost consistent with our finding of a 53.3% LV invasion rate. LV invasion seems to be common even after neoadjuvant chemotherapy, in contrast to neoadjuvant CRT. Moreover, in the present study, LV invasion was significantly associated with pN stage and pT stage. This result agrees with previous studies of patients who underwent esophagectomy without neoadjuvant therapy.31–35,37
LV invasion was an independent prognostic factor along with pathological nodal status and a borderline factor associated with systemic recurrence in patients with esophageal cancer who underwent neoadjuvant chemotherapy in this study, but we think that pathological nodal status is the most important factor associated with prognosis and recurrence in patients with esophageal cancer who undergo neoadjuvant therapy followed by surgery. In pN2–3 patients, patients with LV invasion negative had a better prognosis than those with LV invasion positive, but the 5-year survival rate is about 60%, which is not so good. pN2–3 patients may be eligible for adjuvant therapy regardless of LV invasion. On the other hand, pN0 patients had a better prognosis as a whole, but the survival rate of pN0 and LV invasion-positive patients is significantly lower than that of pN0 and LV invasion-negative. In pN1 patients, survival curves clearly differ according to LV invasion. Thus, the information about LV invasion may be useful for selecting candidates for adjuvant therapy, especially in pN0 and pN1 patients.
There are several limitations to this study. First, this study included a large number of patients with esophageal cancer who underwent neoadjuvant chemotherapy followed by surgery, but the study was retrospective in design and was conducted in only one institution. Second, we evaluated LV invasion only by using HE-stained sections. Several studies of LV invasion in patients with advanced esophageal cancer used immunohistochemical staining with antibodies, such as D2–40 and CD31, to lymph vessels and blood vessels to assess LV invasion.33,42 The diagnostic accuracy of LV invasion may increase by using additional immunohistochemical staining in this study. Further studies using the immunohistochemical method to detect LV invasion may be needed to confirm the usefulness of LV invasion as a prognostic marker and predictor for systemic recurrence in esophageal cancer patients treated with neoadjuvant therapy. Third, the study duration is relatively long, and three different chemotherapeutic regimens were used in this study. LV invasion was less frequently observed in patients who received DCF therapy than in those who received ACF or CF therapy. The analysis also showed that patients with DCF therapy tended to have fewer lymph node metastases (Supplementary Table 2). Chemotherapeutic regimens can affect the status of LV invasion and lymph node metastasis. Finally, we did not investigate biomarkers such as circulating tumor DNA (ctDNA), which might be able to detect minimal residual cancer cells in this study. Such biomarkers may be useful to select good candidates for adjuvant therapy in the near future.
In conclusion, the present study showed that pathological nodal status and LV invasion were the most important prognostic factors in patients with esophageal cancer who underwent neoadjuvant chemotherapy followed by surgery. Combining LV invasion with pathological nodal status makes it possible to stratify the prognosis and risk of systemic recurrence or any recurrence in those patients. These findings could provide useful information about selecting candidates for postoperative adjuvant therapy among patients with esophageal cancer who are treated with neoadjuvant chemotherapy followed by surgery.
Financial support
None.
Conflicts of interest
The authors declare that they have no conflict of interest.



