-
PDF
- Split View
-
Views
-
Cite
Cite
Siobhan Chien, Paul Glen, Ian Penman, Gavin Bryce, Neil Cruickshank, Michael Miller, Andrew Crumley, Jonathan Fletcher, Perminder Phull, Ivan Gunjaca, Kevin Robertson, Jeyakumar Apollos, Grant Fullarton, the CytoSCOT group, National adoption of an esophageal cell collection device for Barrett’s esophagus surveillance: impact on delay to investigation and pathological findings, Diseases of the Esophagus, Volume 37, Issue 5, May 2024, doae002, https://doi.org/10.1093/dote/doae002
Close - Share Icon Share
Summary
High quality Barrett’s esophagus surveillance is crucial to detect early neoplastic changes. An esophageal cell collection device (OCCD) was introduced as a triage tool for Barrett’s surveillance. This study aims to evaluate whether the Scottish OCCD program (CytoSCOT) has reduced delays to Barrett’s surveillance, and whether delayed surveillance negatively impacts endoscopic pathology. All patients undergoing OCCD testing for Barrett’s surveillance across 11 Scottish health boards between 14/9/2020 and 13/9/2022 were identified. Patients were dichotomised into two groups (Year 1 vs. Year 2), with individual records interrogated to record demographics, recommended surveillance interval, time from last endoscopy to OCCD test, and OCCD result. Patients were deemed high-risk if the OCCD demonstrated atypia and/or p53 positivity. Further analysis was performed on patients who underwent endoscopy within 12 months of OCCD testing. A total of 3223 OCCD tests were included in the analysis (1478 in Year 1; 1745 in Year 2). In Year 1 versus Year 2, there was a longer median delay to surveillance (9 vs. 5 months; P < 0.001), increased proportion of patients with delayed surveillance (72.6% vs. 57.0%; P < 0.001), and more high-risk patients (12.0% vs. 5.3%; P < 0.001). 425/3223 patients (13.2%) were further investigated with upper gastrointestinal endoscopy, 57.9% of which were high-risk. As surveillance delay increased beyond 24 months, high-risk patients were significantly more likely to develop dysplasia or malignancy (P = 0.004). Delayed Barrett’s esophagus surveillance beyond 24 months is associated with increased risk of pre-cancerous pathology. The CytoSCOT program has reduced delays in surveillance, promoting earlier detection of dysplasia and reducing burden on endoscopy services.
INTRODUCTION
Esophageal adenocarcinoma (OAC) is becoming increasingly prevalent in Western countries1: OAC is associated with a poor prognosis, with 5-year survival rates of <25%.2 Barrett’s esophagus is a well-established precursor lesion to OAC, in which columnar cells demonstrating intestinal metaplasia (IM) replace the normal squamous epithelium of the esophagus.3 Barrett’s esophagus has the propensity to evolve through stepwise neoplastic changes, progressing from non-dysplastic IM to low-grade dysplasia (LGD), high-grade dysplasia (HGD) and intramucosal carcinoma (IMC), before developing into invasive OAC. Previous literature has demonstrated presence of dysplasia within the Barrett’s segment is the single best marker for risk progression to OAC.4 Thus, early detection of pre-cancerous changes via dedicated Barrett’s surveillance programs is critical to prevent progression to invasive malignancy, with improved clinical outcomes and reduced OAC-related mortality demonstrated within the Barrett’s surveillance cohort as a consequence.5
Traditionally, Barrett’s surveillance involves upper gastrointestinal (UGI) endoscopy, with this technique also allowing therapeutic intervention in cases of Barrett’s with early neoplasia. Where non-dysplastic IM is detected, the British Society of Gastroenterology (BSG) recommends regular surveillance at 3- or 5-yearly intervals, dependent on length of the Barrett’s segment.3 Clearly, the Barrett’s surveillance program in the UK requires significant resources and places substantial burden on endoscopy services. These system pressures were significantly exacerbated by the COVID-19 pandemic, during which all routine endoscopy services were temporarily halted across NHS Scotland,6 resulting in significant delays to patients’ surveillance. Therefore, alternatives to endoscopy were sought to safely enable the continuation of the Barrett’s surveillance program and minimize the risk of cancer progression.
A new minimally invasive, non-endoscopic procedure has been developed using an esophageal cell collection device (OCCD), enabling the collection of pan-esophageal mucosal cells, which then undergo cytological analysis to detect the immunohistochemical biomarker trefoil factor 3 (TFF3).7 TFF3 is a marker of columnar epithelium and therefore suggestive of the presence of IM,8 with literature demonstrating OCCD testing to have a specificity of >92% and sensitivity of >80% in the diagnosis of Barrett’s esophagus.7 Furthermore, the addition of a biomarker panel for cellular atypia (suggestive of inflammation or dysplasia) and p53 (the most prevalent biomarker for malignant transformation in Barrett’s esophagus) to OCCD testing is effective in the detection of dysplasia and neoplasia in this patient cohort.9,10 In September 2020, the CytoSCOT (SpongeCytologyOesophagealTest) program was rolled out at pace across mainland NHS Scotland with full funding as an emergency response to the COVID-19 pandemic. OCCD testing is currently used as a diagnostic triage tool to identify high-risk patients requiring urgent endoscopy,11 therefore allowing continuation of Barrett’s surveillance by reducing endoscopy demand on an already over-stretched service.
To date, the impact of delayed Barrett’s surveillance on endoscopy pathology pattern has not been reported. The COVID-19 pandemic coupled with the introduction of the CytoSCOT program provided a unique opportunity to evaluate the impact of delayed surveillance on patient outcomes. This study aims to evaluate if the CytoSCOT program has improved the delays to Barrett’s surveillance and whether delayed Barrett’s surveillance has negatively impacted endoscopic pathology patterns within this patient cohort.
MATERIALS AND METHODS
Prospectively maintained local databases were used to identify all patients who underwent Barrett’s surveillance using the OCCD (Cytosponge™) across 11 Scottish health boards (NHS Ayrshire & Arran; NHS Borders; NHS Dumfries & Galloway; NHS Fife; NHS Forth Valley; NHS Grampian; NHS Greater Glasgow & Clyde; NHS Highland; NHS Lanarkshire; NHS Lothian; NHS Tayside). All patients signed an NHS consent form to undergo OCCD testing.
All samples included in this analysis were processed centrally in a UK-based diagnostic laboratory (Cyted Ltd). As previously described, all OCCDs were placed in BD SurePath Preservative Fluid, with cells retrieved and processed to paraffin blocks (FFPE).7 Superficial and deep sections were used to prepare both Hematoxylin & Eosin (H&E) slides to assess cellular atypia (including clear-cut dysplasia and atypia of unknown significance) and FFPE slides to perform TFF3 and p53 immunohistochemistry simultaneously. p53 staining with an intensity of three was considered significant as previously published.12 In accordance with BSG guidance for reporting of dysplasia, all samples with suspected atypia or p53 positivity were reviewed by a second pathologist. Processed Cyted Ltd pathology reports were then returned to each health board, with ongoing clinical management decided locally.
Patients who underwent OCCD testing for Barrett’s surveillance within a 2-year period between 14 September 2020 and 13 September 2022 were included in this retrospective analysis, with all OCCD tests for investigation of reflux symptoms excluded. Patients were dichotomised into two groups, with those undergoing OCCD testing in Year 1 (14/9/2020 to 13/9/2021) compared to those in Year 2 (14/9/2021 to 13/9/2022).
Individual patient electronic records were interrogated to record baseline patient demographics, previous Barrett’s endoscopic morphology and pathology (including Prague classification), and OCCD test result to form a national CytoSCOT registry. Long segment Barrett’s esophagus was defined as M > 3 cm using the Prague classification. Patients were defined as high-risk if OCCD biomarkers were positive for atypia and/or p53.
The time interval from last endoscopy to OCCD test in months was recorded. In addition, the recommended Barrett’s surveillance interval in months from last endoscopy was also recorded from patients’ notes as per local guidelines: this was subtracted from the time interval from last endoscopy to OCCD test to calculate the delay to Barrett’s surveillance. This was defined as delayed if the difference between these dates was greater than 3 months, making allowances for reasonable patient-directed or administrative delays given the real-world context of this study. All repeat OCCD tests were excluded from analysis. Patients who proceeded to undergo endoscopy within 12 months of OCCD test were identified, with indication for endoscopy, endoscopic biopsy results, time to pathology result, and ongoing clinical management recorded.
Continuous parameters were presented as median and interquartile range (IQR) and categorical data as counts and percentages. The Chi-squared test was performed for comparison of categorical variables and the Mann–Whitney U test was performed for comparison of continuous variables, where appropriate. A P-value of ≤0.05 was considered statistically significant.
Ethical approval was obtained on a national level from the Caldicott guardian, as well as from local information governance teams in each health board, to undertake this analysis.
RESULTS
A total of 3223 OCCD tests were included in the analysis: 1478 tests were performed in Year 1; 1745 tests were performed in Year 2. The median follow-up time was 16 months (IQR 10-21). Table 1 demonstrates patient demographics. Of note, there was a significantly longer median delay to surveillance in Year 1 compared to Year 2 (9 months vs. 5 months; P < 0.001), and the proportion of patients with delayed surveillance was significantly higher in Year 1 compared to Year 2 (72.6% vs. 57.0%; P < 0.001).
| Demographic . | Year 1 . | Year 2 . | Significance (p) . |
|---|---|---|---|
| Number of OCCD tests for Barrett’s surveillance | 1478 | 1745 | — |
| Proportion male, n (%) | 1010 (68.3%) | 1187 (68.0%) | 0.849 |
| Median age (IQR), years | 67 (59–73) | 66 (58–73) | 0.409 |
| Median BMI (IQR), kg/m2 | 28.0 (25.2–31.3) | 27.9 (25.0–31.5) | 0.853 |
| Proportion positive smoking history, n (%) | 638 (48.3%) | 732 (46.8%) | 0.433 |
| Proportion PPI use, n (%) | 1418 (95.9%) | 1673 (95.9%) | 0.924 |
| Long segment Barrett’s (Prague M > 3 cm) | 636 (43.0%) | 586 (33.6%) | <0.001 |
| Median delay to Barrett’s surveillance (IQR), months | 9 (3-13) | 5 (1-16) | <0.001 |
| Proportion delayed Barrett’s surveillance, n (%) | 1073 (72.6%) | 995 (57.0%) | <0.001 |
| Demographic . | Year 1 . | Year 2 . | Significance (p) . |
|---|---|---|---|
| Number of OCCD tests for Barrett’s surveillance | 1478 | 1745 | — |
| Proportion male, n (%) | 1010 (68.3%) | 1187 (68.0%) | 0.849 |
| Median age (IQR), years | 67 (59–73) | 66 (58–73) | 0.409 |
| Median BMI (IQR), kg/m2 | 28.0 (25.2–31.3) | 27.9 (25.0–31.5) | 0.853 |
| Proportion positive smoking history, n (%) | 638 (48.3%) | 732 (46.8%) | 0.433 |
| Proportion PPI use, n (%) | 1418 (95.9%) | 1673 (95.9%) | 0.924 |
| Long segment Barrett’s (Prague M > 3 cm) | 636 (43.0%) | 586 (33.6%) | <0.001 |
| Median delay to Barrett’s surveillance (IQR), months | 9 (3-13) | 5 (1-16) | <0.001 |
| Proportion delayed Barrett’s surveillance, n (%) | 1073 (72.6%) | 995 (57.0%) | <0.001 |
BMI, body mass index; IQR, interquartile range; OCCD, esophageal cell collection device; PPI, proton pump inhibitor.
| Demographic . | Year 1 . | Year 2 . | Significance (p) . |
|---|---|---|---|
| Number of OCCD tests for Barrett’s surveillance | 1478 | 1745 | — |
| Proportion male, n (%) | 1010 (68.3%) | 1187 (68.0%) | 0.849 |
| Median age (IQR), years | 67 (59–73) | 66 (58–73) | 0.409 |
| Median BMI (IQR), kg/m2 | 28.0 (25.2–31.3) | 27.9 (25.0–31.5) | 0.853 |
| Proportion positive smoking history, n (%) | 638 (48.3%) | 732 (46.8%) | 0.433 |
| Proportion PPI use, n (%) | 1418 (95.9%) | 1673 (95.9%) | 0.924 |
| Long segment Barrett’s (Prague M > 3 cm) | 636 (43.0%) | 586 (33.6%) | <0.001 |
| Median delay to Barrett’s surveillance (IQR), months | 9 (3-13) | 5 (1-16) | <0.001 |
| Proportion delayed Barrett’s surveillance, n (%) | 1073 (72.6%) | 995 (57.0%) | <0.001 |
| Demographic . | Year 1 . | Year 2 . | Significance (p) . |
|---|---|---|---|
| Number of OCCD tests for Barrett’s surveillance | 1478 | 1745 | — |
| Proportion male, n (%) | 1010 (68.3%) | 1187 (68.0%) | 0.849 |
| Median age (IQR), years | 67 (59–73) | 66 (58–73) | 0.409 |
| Median BMI (IQR), kg/m2 | 28.0 (25.2–31.3) | 27.9 (25.0–31.5) | 0.853 |
| Proportion positive smoking history, n (%) | 638 (48.3%) | 732 (46.8%) | 0.433 |
| Proportion PPI use, n (%) | 1418 (95.9%) | 1673 (95.9%) | 0.924 |
| Long segment Barrett’s (Prague M > 3 cm) | 636 (43.0%) | 586 (33.6%) | <0.001 |
| Median delay to Barrett’s surveillance (IQR), months | 9 (3-13) | 5 (1-16) | <0.001 |
| Proportion delayed Barrett’s surveillance, n (%) | 1073 (72.6%) | 995 (57.0%) | <0.001 |
BMI, body mass index; IQR, interquartile range; OCCD, esophageal cell collection device; PPI, proton pump inhibitor.
Table 2 demonstrates OCCD test results. Patients were significantly more likely to have an abnormal OCCD result (atypia or p53 positive), and therefore be deemed high-risk, in Year 1 compared to Year 2 (12.0% vs. 5.3%; P < 0.001).
| OCCD test result . | All (n = 3223) . | Year 1 (n = 1478) . | Year 2 (n = 1745) . | Significance (P) . |
|---|---|---|---|---|
| TFF3 negative | 1031 (32.0%) | 398 (26.9%) | 633 (36.3%) | <0.001 |
| TFF3 positive | 1598 (49.6%) | 775 (52.5%) | 823 (47.2%) | 0.003 |
| Atypia | 151 (4.7%) | 98 (6.6%) | 53 (3.0%) | <0.001 |
| P53 | 120 (3.7%) | 80 (5.4%) | 40 (2.3%) | <0.001 |
| Insufficient | 323 (10.0%) | 127 (8.6%) | 196 (11.2%) | 0.013 |
| OCCD test result . | All (n = 3223) . | Year 1 (n = 1478) . | Year 2 (n = 1745) . | Significance (P) . |
|---|---|---|---|---|
| TFF3 negative | 1031 (32.0%) | 398 (26.9%) | 633 (36.3%) | <0.001 |
| TFF3 positive | 1598 (49.6%) | 775 (52.5%) | 823 (47.2%) | 0.003 |
| Atypia | 151 (4.7%) | 98 (6.6%) | 53 (3.0%) | <0.001 |
| P53 | 120 (3.7%) | 80 (5.4%) | 40 (2.3%) | <0.001 |
| Insufficient | 323 (10.0%) | 127 (8.6%) | 196 (11.2%) | 0.013 |
OCCD, esophageal cell collection device; TFF3, trefoil factor 3.
| OCCD test result . | All (n = 3223) . | Year 1 (n = 1478) . | Year 2 (n = 1745) . | Significance (P) . |
|---|---|---|---|---|
| TFF3 negative | 1031 (32.0%) | 398 (26.9%) | 633 (36.3%) | <0.001 |
| TFF3 positive | 1598 (49.6%) | 775 (52.5%) | 823 (47.2%) | 0.003 |
| Atypia | 151 (4.7%) | 98 (6.6%) | 53 (3.0%) | <0.001 |
| P53 | 120 (3.7%) | 80 (5.4%) | 40 (2.3%) | <0.001 |
| Insufficient | 323 (10.0%) | 127 (8.6%) | 196 (11.2%) | 0.013 |
| OCCD test result . | All (n = 3223) . | Year 1 (n = 1478) . | Year 2 (n = 1745) . | Significance (P) . |
|---|---|---|---|---|
| TFF3 negative | 1031 (32.0%) | 398 (26.9%) | 633 (36.3%) | <0.001 |
| TFF3 positive | 1598 (49.6%) | 775 (52.5%) | 823 (47.2%) | 0.003 |
| Atypia | 151 (4.7%) | 98 (6.6%) | 53 (3.0%) | <0.001 |
| P53 | 120 (3.7%) | 80 (5.4%) | 40 (2.3%) | <0.001 |
| Insufficient | 323 (10.0%) | 127 (8.6%) | 196 (11.2%) | 0.013 |
OCCD, esophageal cell collection device; TFF3, trefoil factor 3.
425/3223 patients (13.2%) were further investigated with UGI endoscopy and had available histopathology results at the time of analysis: 225/425 endoscopies (52.9%) were in the Year 1 cohort; 200/425 endoscopies (47.1%) were in the Year 2 cohort. Of note, this includes endoscopy for all indications: this is summarized in Table 3. 246/425 (57.9%) endoscopies were performed in response to an abnormal OCCD result (i.e. atypia and/or p53 positive). The median time from OCCD test to endoscopy was 2 months (IQR 1-5). The median time from endoscopy to pathology result was 16 days (IQR 8-35). Figure 1 demonstrates endoscopic pathology results categorized by delay time in Barrett’s surveillance, where p relates to the proportion of patients with confirmed dysplasia or malignancy. As delay to Barrett’s surveillance increases >24 months, the proportion of patients with dysplastic or malignant Barrett’s esophagus changes significantly increases (P < 0.001). Malignant transformation of Barrett’s esophagus to either IMC or OAC was observed in 21/3223 patients (0.7%).
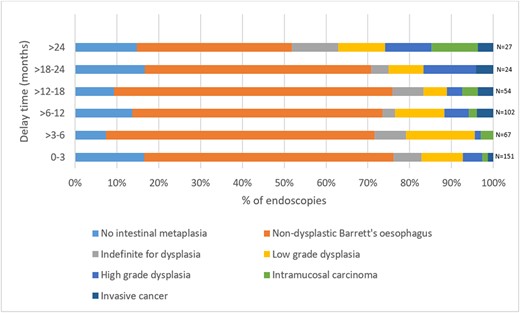
Endoscopic biopsy results categorized by delay to Barrett’s surveillance.
| Indication . | N (%) . |
|---|---|
| Abnormal OCCD result (atypia +/− p53 positive) | 246 (57.9%) |
| Insufficient OCCD result | 67 (15.8%) |
| TFF3 negative OCCD result | 47 (11.1%) |
| Investigation of red flag UGI symptoms | 40 (9.4%) |
| Assessment of ulcer healing | 23 (5.4%) |
| OCCD sponge detachment | 1 (0.2%) |
| Delayed UGI bleeda | 1 (0.2%) |
| Indication . | N (%) . |
|---|---|
| Abnormal OCCD result (atypia +/− p53 positive) | 246 (57.9%) |
| Insufficient OCCD result | 67 (15.8%) |
| TFF3 negative OCCD result | 47 (11.1%) |
| Investigation of red flag UGI symptoms | 40 (9.4%) |
| Assessment of ulcer healing | 23 (5.4%) |
| OCCD sponge detachment | 1 (0.2%) |
| Delayed UGI bleeda | 1 (0.2%) |
Patient presented with UGI bleed >6 months after OCCD test.
OCCD, esophageal cell collection device; TFF3, trefoil factor 3; UGI, upper gastrointestinal.
| Indication . | N (%) . |
|---|---|
| Abnormal OCCD result (atypia +/− p53 positive) | 246 (57.9%) |
| Insufficient OCCD result | 67 (15.8%) |
| TFF3 negative OCCD result | 47 (11.1%) |
| Investigation of red flag UGI symptoms | 40 (9.4%) |
| Assessment of ulcer healing | 23 (5.4%) |
| OCCD sponge detachment | 1 (0.2%) |
| Delayed UGI bleeda | 1 (0.2%) |
| Indication . | N (%) . |
|---|---|
| Abnormal OCCD result (atypia +/− p53 positive) | 246 (57.9%) |
| Insufficient OCCD result | 67 (15.8%) |
| TFF3 negative OCCD result | 47 (11.1%) |
| Investigation of red flag UGI symptoms | 40 (9.4%) |
| Assessment of ulcer healing | 23 (5.4%) |
| OCCD sponge detachment | 1 (0.2%) |
| Delayed UGI bleeda | 1 (0.2%) |
Patient presented with UGI bleed >6 months after OCCD test.
OCCD, esophageal cell collection device; TFF3, trefoil factor 3; UGI, upper gastrointestinal.
271/3223 patients (8.4%) were within the high-risk group (i.e. atypia and/or p53 positive). 246/271 patients (90.8%) underwent urgent UGI endoscopy within 12 months of OCCD test. The median time to endoscopy within this high-risk cohort was 2 months (IQR 1-3). 168/246 high-risk patients (68.3%) had delayed Barrett’s surveillance. Figure 2 demonstrates endoscopic biopsy results within this high-risk group categorized by delay in Barrett’s surveillance, where P relates to the proportion of patients with confirmed dysplasia or malignancy. 10/18 patients (55.5%) in this group demonstrated dysplasia when Barrett’s surveillance was delayed >24 months (P = 0.009).

Endoscopic biopsy result categorized by delay to surveillance in patients with atypia or p53 positivity on OCCD testing. (OCCD, esophageal cell collection device.)
A total of 25 high-risk patients were not included in the analysis of endoscopy results. 10/25 patients declined invitation to UGI endoscopy. 8/25 patients underwent UGI endoscopy >12 months after OCCD test: 3/8 had IM, 2/8 had no IM, 2/8 were indefinite for dysplasia and 1/8 had LGD on endoscopic biopsies. 3/25 patients underwent repeat OCCD testing in the first instance: 2/3 were TFF3 positive and returned to routine surveillance, and 1/3 was atypia positive (subsequent endoscopic biopsies showed IM). 3/25 patients died from unrelated causes before endoscopy. 1/25 patients underwent UGI endoscopy but did not have biopsies taken due to bleeding risk (follow-up remains outstanding). One high-risk patient declined invitation to UGI endoscopy, subsequently re-presented with dysphagia, and was diagnosed with esophageal adenocarcinoma 18 months after initial OCCD test.
More patients with long segment Barrett’s underwent OCCD testing in Year 1 vs. Year 2 (43.0% vs. 33.6%; P < 0.001) (Table 1). 1089/1577 patients with long segment Barrett’s had delayed surveillance, compared to 975/1646 patients with short segment Barrett’s (69.1% vs. 59.2%, P < 0.001). Table 4 demonstrates impact of delayed surveillance on endoscopy pathology in short versus long segment Barrett’s esophagus. HGD, IMC and invasive cancer were more likely to be present in patients with long segment disease with delayed surveillance. 402/1646 patients (24.4%) with short segment Barrett’s were discharged from routine surveillance following OCCD test.
Impact of delayed Barrett’s surveillance on endoscopy pathology in short versus long segment Barrett’s esophagus
| Pathology result at endoscopic biopsy . | Short segment (n = 166) . | Long segment (n = 259) . | ||
|---|---|---|---|---|
| No delay (n = 61) . | Delay > 3 months (n = 105) . | No delay (n = 90) . | Delay > 3 months (n = 169) . | |
| No intestinal metaplasia | 20 (32.8%) | 25 (23.8%) | 5 (5.6%) | 7 (4.1%) |
| Non-dysplastic Barrett’s esophagus | 33 (54.1%) | 56 (53.3%) | 57 (63.3%) | 107 (63.3%) |
| Indefinite for dysplasia | 4 (6.5%) | 8 (7.6%) | 6 (6.7%) | 8 (4.7%) |
| Low-grade dysplasia | 2 (3.3%) | 8 (7.6%) | 13 (14.4%) | 23 (13.6%) |
| High-grade dysplasia | 2 (3.3%) | 4 (3.8%) | 5 (5.6%) | 11 (6.5%) |
| Intramucosal carcinoma | 0 (0%) | 2 (1.9%) | 2 (2.2%) | 7 (4.1%) |
| Invasive cancer | 0 (0%) | 2 (1.9%) | 2 (2.2%) | 6 (3.6%) |
| Pathology result at endoscopic biopsy . | Short segment (n = 166) . | Long segment (n = 259) . | ||
|---|---|---|---|---|
| No delay (n = 61) . | Delay > 3 months (n = 105) . | No delay (n = 90) . | Delay > 3 months (n = 169) . | |
| No intestinal metaplasia | 20 (32.8%) | 25 (23.8%) | 5 (5.6%) | 7 (4.1%) |
| Non-dysplastic Barrett’s esophagus | 33 (54.1%) | 56 (53.3%) | 57 (63.3%) | 107 (63.3%) |
| Indefinite for dysplasia | 4 (6.5%) | 8 (7.6%) | 6 (6.7%) | 8 (4.7%) |
| Low-grade dysplasia | 2 (3.3%) | 8 (7.6%) | 13 (14.4%) | 23 (13.6%) |
| High-grade dysplasia | 2 (3.3%) | 4 (3.8%) | 5 (5.6%) | 11 (6.5%) |
| Intramucosal carcinoma | 0 (0%) | 2 (1.9%) | 2 (2.2%) | 7 (4.1%) |
| Invasive cancer | 0 (0%) | 2 (1.9%) | 2 (2.2%) | 6 (3.6%) |
Impact of delayed Barrett’s surveillance on endoscopy pathology in short versus long segment Barrett’s esophagus
| Pathology result at endoscopic biopsy . | Short segment (n = 166) . | Long segment (n = 259) . | ||
|---|---|---|---|---|
| No delay (n = 61) . | Delay > 3 months (n = 105) . | No delay (n = 90) . | Delay > 3 months (n = 169) . | |
| No intestinal metaplasia | 20 (32.8%) | 25 (23.8%) | 5 (5.6%) | 7 (4.1%) |
| Non-dysplastic Barrett’s esophagus | 33 (54.1%) | 56 (53.3%) | 57 (63.3%) | 107 (63.3%) |
| Indefinite for dysplasia | 4 (6.5%) | 8 (7.6%) | 6 (6.7%) | 8 (4.7%) |
| Low-grade dysplasia | 2 (3.3%) | 8 (7.6%) | 13 (14.4%) | 23 (13.6%) |
| High-grade dysplasia | 2 (3.3%) | 4 (3.8%) | 5 (5.6%) | 11 (6.5%) |
| Intramucosal carcinoma | 0 (0%) | 2 (1.9%) | 2 (2.2%) | 7 (4.1%) |
| Invasive cancer | 0 (0%) | 2 (1.9%) | 2 (2.2%) | 6 (3.6%) |
| Pathology result at endoscopic biopsy . | Short segment (n = 166) . | Long segment (n = 259) . | ||
|---|---|---|---|---|
| No delay (n = 61) . | Delay > 3 months (n = 105) . | No delay (n = 90) . | Delay > 3 months (n = 169) . | |
| No intestinal metaplasia | 20 (32.8%) | 25 (23.8%) | 5 (5.6%) | 7 (4.1%) |
| Non-dysplastic Barrett’s esophagus | 33 (54.1%) | 56 (53.3%) | 57 (63.3%) | 107 (63.3%) |
| Indefinite for dysplasia | 4 (6.5%) | 8 (7.6%) | 6 (6.7%) | 8 (4.7%) |
| Low-grade dysplasia | 2 (3.3%) | 8 (7.6%) | 13 (14.4%) | 23 (13.6%) |
| High-grade dysplasia | 2 (3.3%) | 4 (3.8%) | 5 (5.6%) | 11 (6.5%) |
| Intramucosal carcinoma | 0 (0%) | 2 (1.9%) | 2 (2.2%) | 7 (4.1%) |
| Invasive cancer | 0 (0%) | 2 (1.9%) | 2 (2.2%) | 6 (3.6%) |
In total, 43 patients were diagnosed with HGD, IMC or invasive cancer at endoscopic biopsy. 32/43 patients (74.4%) had delayed Barrett’s surveillance. 24/32 patients with delayed surveillance were suitable for endoscopic treatment with either radiofrequency ablation (RFA) or endoscopic mucosal resection (EMR), compared to 9/11 patients who underwent surveillance within the recommended time interval (75.0% vs. 81.8%). Of the remaining eight patients within the delayed group, four underwent surgical resection, two underwent chemoradiotherapy and two were palliated. In the group without delayed surveillance, only one patient had surgery and one was palliated.
DISCUSSION
With recent advancements in endoscopic therapeutic intervention, Barrett’s surveillance aims to identify dysplasia and early stage carcinoma to prevent progression to invasive OAC and reduce patient morbidity associated with esophagectomy and oncological treatments. However, with low estimated rates of malignant transformation (0.3%), the value of regular Barrett’s surveillance endoscopy has previously been questioned.13–15 Despite this, UGI endoscopy remains the gold standard for Barrett’s surveillance. The effect of delayed Barrett’s surveillance on the distribution of pathology findings has yet to be described in the literature. Our data presents the first real-world assessment of the effect of delayed Barrett’s surveillance on endoscopic pathology pattern, as a direct consequence of the impact of the COVID-19 pandemic on endoscopy services.
The pandemic negatively impacted stage migration of newly diagnosed esophageal cancers16: this effect is seen in Public Health Scotland cancer stage data from 2019 to 2021, demonstrating more esophageal cancer patients are presenting with advanced or metastatic disease.17,18 This study demonstrates that delays to Barrett’s surveillance result in significantly higher rates of dysplasia and malignancy when surveillance is delayed 24 months beyond the recommended time interval, thereby suggesting timely Barrett’s surveillance remains of critical value to improve patient outcomes and reduce cancer-related morbidity. Furthermore, within our HGD, IMC and invasive cancer patient cohort, we have demonstrated that disease is more likely to be advanced and less amenable to endoscopic treatment where Barrett’s surveillance is delayed.
Funded by NHS Scotland, the CytoSCOT program launched into clinical practice across mainland Scotland in September 2020 as an emergency response to the COVID-19 pandemic and introduced OCCD testing as the primary modality of Barrett’s surveillance when routine endoscopy services were unavailable.6 To date, nearly 6000 tests have been performed throughout Scotland, with the OCCD acting as a triage tool to identify patients requiring further investigation with UGI endoscopy. Although OCCD testing has been demonstrated to be effective within the trial setting,3 its benefits in real-world clinical practice are yet to be established. Our data is the first to demonstrate that OCCD testing can be safely used as a Barrett’s surveillance method to identify patients at risk of dysplasia and malignancy in the real-world setting.
We have successfully demonstrated that both the proportion of Barrett’s esophagus patients with delayed surveillance (72.6% in Year 1 vs. 57.0% in Year 2; P < 0.001) and median delay time to Barrett’s surveillance have significantly improved (9 months in Year 1 vs. 5 months in Year 2, P < 0.001). Undoubtedly, the CytoSCOT program plays a critical role in this phenomenon: the program was better established, with increased capacity and wider geographical availability in Year 2, with Scottish OCCD testing for Barrett’s surveillance increasing by 18.0% between the two time intervals. This expansion was reinforced by the publication of the national Endoscopy and Urology Diagnostic Recovery and Renewal Plan in November 2021, which supported implementation of new technologies (such as OCCD testing) to support endoscopy services across Scotland.19 Although the BSG published guidance to support safe resumption of routine UGI endoscopy to enable post-pandemic service recovery,20 this was slow to be implemented across NHS Scotland. Although the total number of patients on endoscopy waiting lists across Scotland improved in Year 1 compared to Year 2 (12,277 in September 2021 vs. 9635 in September 2022), this remained significantly higher than pre-pandemic numbers (7365 in September 2019). This was mirrored in the proportion of patients meeting 6-week waiting time targets in September 2019 (69.6%), September 2021 (31.7%) and September 2022 (41.4%),21 highlighting that the improvement in delayed surveillance in Year 2 was unlikely due to increased capacity of endoscopy services, further highlighting the crucial role of the CytoSCOT program in facilitating Barrett’s surveillance and supporting service recovery.
In addition to delays related to endoscopy waiting lists, the CytoSCOT program has alleviated pressures on histopathology services. The median time to urgent histopathology result from endoscopy in our cohort remained longer than the previously reported median turnaround time to OCCD result (16 vs. 8 days).9 Although many Barrett’s esophagus patients are still experiencing delays in surveillance, we would expect this to improve with time as the CytoSCOT program advances.
Increasing Barrett’s segment length is an independent risk factor for development of dysplasia.22 In our cohort, there was an increased number of patients with longer segments in Year 1 compared to Year 2 (43.0% vs. 33.6%; P < 0.001). Furthermore, our results demonstrate that this pattern of worsening pathology is amplified within the long segment Barrett’s group. Not only are these patients more likely to develop dysplasia compared to the short segment cohort, but the impact of delayed surveillance toward worsening pathology patterns becomes more pronounced in patients with long segment Barrett’s. Our results also demonstrate reduced numbers of abnormal OCCD test results (atypia and/or p53 positive) with time (12.0% in Year 1 vs. 5.3% in Year 2; P < 0.001). Clinicians may have processed higher-risk, long segment patients within Year 1 of the CytoSCOT program, thereby explaining the improvement in OCCD test pattern over time. 1031/3223 OCCD tests (32.0%) returned demonstrating TFF3 negative results in the Barrett’s cohort. We hypothesize this may be due to the inclusion of non-Barrett’s cases within our dataset, in addition to short segments <1 cm: the increased proportion of TFF3 negative results combined with the increased proportion of short segment Barrett’s in Year 2 supports this theory. Furthermore, the proportion of OCCD tests yielding ‘insufficient’ results increased in Year 2 (8.6% vs. 11.2% P = 0.013). As the CytoSCOT program expanded in Scotland between Year 1 and Year 2, more endoscopy nurses were trained to perform OCCD testing: we hypothesize that the increased number of insufficient tests may be due to the learning curve associated with undertaking this new technique.
This study is not without limitations. Firstly, although BSG guidelines provide national recommendations, Barrett’s surveillance intervals were variable between health boards based on local guidelines, introducing heterogeneity among the study group. Our cohort only included those undergoing Barrett’s surveillance with OCCD testing in the first instance: we did not include the group who underwent Barrett’s surveillance with UGI endoscopy alone as we could not access national endoscopy data, therefore it is difficult to prove that these improvements in delay time were due to the CytoSCOT program alone. Additionally, as the sensitivity of OCCD testing is 92%7 and patients with a TFF3 negative OCCD test result did not routinely undergo UGI endoscopy, we cannot prove these are true negative test results: this raises the possibility of ‘missed’ pathology within our cohort. Although UGI endoscopy is the gold standard for Barrett’s surveillance, endoscopic punch biopsies are subject to significant sampling bias, with post-endoscopy incident esophageal cancer estimated to account for 14% of the esophageal cancer burden,23 highlighting there is no perfect test for Barrett’s surveillance. Finally, the median follow-up time was only 16 months. Further work is required to determine if these improvements in delayed surveillance are sustained with the continued development of the CytoSCOT program.
In conclusion, delayed Barrett’s esophagus surveillance beyond 24 months is associated with increased rates of sinister pathology. The CytoSCOT program is a valuable resource to enable the continuation of Barrett’s surveillance at lower cost and reduce the burden on endoscopy services, focussing endoscopy on patients at higher risk and enabling earlier detection of precursor lesions.
ACKNOWLEDGMENT(S)
Many thanks to Professor Rebecca Fitzgerald for sharing her knowledge on OCCD testing. No funding was obtained for this study.
Specific author contributions: Siobhan Chien (Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Writing—original draft), Paul Glen (Conceptualization, Data curation, Supervision, Writing—original draft), Ian Penman (Data curation, Writing—original draft), Gavin Bryce (Data curation), Neil Cruickshank (Data curation), Michael Miller (Data curation), Andrew Crumley (Data curation), Jonathan Fletcher (Data curation), Perminder Phull (Data curation), Ivan Gunjaca (Data curation), Kevin Robertson (Data curation), Jeyakumar Apollos (Data curation) and Grant Fullarton (Conceptualization, Data curation, Methodology, Supervision, Writing—original draft)
Conflicts of interest: None.



