-
PDF
- Split View
-
Views
-
Cite
Cite
M H B C Stenstra, F van Workum, F J H van den Wildenberg, F Polat, C Rosman, Evolution of the surgical technique of minimally invasive Ivor-Lewis esophagectomy: description according to the IDEAL framework, Diseases of the Esophagus, Volume 32, Issue 3, March 2019, doy079, https://doi.org/10.1093/dote/doy079
Close - Share Icon Share
Summary
Totally minimally invasive Ivor–Lewis esophagectomy (Ivor Lewis TMIE) is a technically challenging procedure and is associated with a learning curve. Refinement of surgical technique is an important part of this learning curve. However, detailed descriptions of these refinements according to the idea, development, exploration, assessment, and long-term follow-up (IDEAL) framework are lacking and this study was undertaken to fill this knowledge gap. From 2010 until 2016, all consecutive patients (n = 164) were included from the first patient undergoing Ivor Lewis TMIE. Surgical reports were analyzed and surgeons were interviewed to determine surgical refinements. These data were used to describe the transition of the surgical technique from IDEAL stage IIB to stage III. The main findings were that four refinements were made to the surgical procedure in IDEAL stage IIB: (1) At case 9, the use of the 25 mm OrVil was abandoned, exchanged for a 28 mm EEA stapler and a large omental wrap around the anastomosis was introduced; (2) at case 27, the omental wrap was reduced in volume; (3) at case 60, the omental wrap was refined to cover the full 360° of the anastomosis and (4) at case 77, the fixation of the anvil with the Endostitch was replaced by fixation with two Endoloops®. During the transition from IDEAL stage IIB to stage III, the incidence of anastomotic leakage decreased from 26.0% to 4.6% (P < 0.001) and the incidence of textbook outcome increased from 31.2% to 47.1% (P = 0.039). In conclusion, this study describes the surgical refinements that were made during the progression of Ivor Lewis TMIE from IDEAL stage IIB to IDEAL stage III. During IDEAL stage IIB, postoperative outcome improved as surgical proficiency was gained and the technique was refined.
INTRODUCTION
Totally minimally invasive esophagectomy (TMIE) is associated with reduced postoperative morbidity [1] and has been shown to be oncologically [2] safe. Worldwide, TMIE is increasingly being used and the totally minimally invasive Ivor–Lewis esophagectomy (Ivor Lewis TMIE) is gaining popularity [3]. However, Ivor Lewis TMIE is a technically challenging technique. The basic steps of the Ivor–Lewis esophagectomy have been described for open surgery [4], but there is no detailed, generally accepted standardized technique for Ivor Lewis TMIE. Therefore, individual surgeons starting implementing Ivor Lewis TMIE in regular practice refined their technique during implementation [5,6]. This leads to heterogeneous surgical techniques for Ivor Lewis TMIE (e.g. different anastomotic techniques) [7]. This process is ultimately undesirable because refinement processes may be associated with decreased patient safety and the simultaneous development of heterogeneous surgical techniques may complicate the conduction of surgical research.
The IDEAL (idea, development, exploration, assessment, and long-term follow-up) framework can be used to describe the development process of an innovative surgical procedure (Table 1) [8]. These descriptions can enhance the development toward a fully developed standard surgical technique and might shorten a surgeon's search for the most optimal surgical technique. Detailed reports describing surgical refinements during the transition from IDEAL stage IIB to IDEAL stage III for Ivor Lewis TMIE are currently lacking. Therefore, the aim of this study is to describe the refinements in the surgical technique of Ivor Lewis TMIE that were made during the transition from IDEAL stage IIB to IDEAL stage III.
| Stage . | . | Description [7] . |
|---|---|---|
| I | Idea | Proof of concept, evolving intervention, single digit patients |
| IIa | Development | Developing procedure, few patients |
| IIb | Exploration | Procedure refinement, many patients |
| III | Assessment | Stable intervention, randomized controlled trial |
| IV | Long-term study | Stable intervention, long-term outcomes |
| Stage . | . | Description [7] . |
|---|---|---|
| I | Idea | Proof of concept, evolving intervention, single digit patients |
| IIa | Development | Developing procedure, few patients |
| IIb | Exploration | Procedure refinement, many patients |
| III | Assessment | Stable intervention, randomized controlled trial |
| IV | Long-term study | Stable intervention, long-term outcomes |
| Stage . | . | Description [7] . |
|---|---|---|
| I | Idea | Proof of concept, evolving intervention, single digit patients |
| IIa | Development | Developing procedure, few patients |
| IIb | Exploration | Procedure refinement, many patients |
| III | Assessment | Stable intervention, randomized controlled trial |
| IV | Long-term study | Stable intervention, long-term outcomes |
| Stage . | . | Description [7] . |
|---|---|---|
| I | Idea | Proof of concept, evolving intervention, single digit patients |
| IIa | Development | Developing procedure, few patients |
| IIb | Exploration | Procedure refinement, many patients |
| III | Assessment | Stable intervention, randomized controlled trial |
| IV | Long-term study | Stable intervention, long-term outcomes |
PATIENTS AND METHODS
Study type
Single center descriptive cohort study: The research was ethically assessed and approved by the ethical committee of the Radboudumc and the need to obtain informed consent for the use of patient data was waived.
Study setting
This study was conducted at a regional expert center for esophageal cancer surgery in which at least 40 Ivor Lewis TMIE procedures are performed annually. Three surgeons that all had previous experience with other minimally invasive surgical procedures and open esophagectomies implemented the new Ivor Lewis TMIE. Together, the surgeons had previously performed 11 totally minimally invasive transhiatal esophagectomy procedures [9] and 27 totally minimally invasive transthoracic esophageal resections with cervical anastomosis [10]. At the time of implementation of Ivor Lewis TMIE, totally minimally invasive McKeown esophagectomy was standard treatment. The standardized enhanced recovery program, which was already used before the introduction of Ivor Lewis TMIE, remained unchanged during the study period. Each operation was performed by two surgeons.
Patients
All consecutive patients with esophageal carcinoma undergoing elective, curative resection (cT1b-4a N0-3 M0) for esophageal carcinoma were included during the period of December 2010 to April 2016. Patients were scheduled to receive neoadjuvant chemoradiotherapy according to the CROSS scheme [11] as standard treatment according to national guidelines.
Surgical technique
The initial technique was laparothoracoscopic esophagectomy with intrathoracic esophagogastric anastomosis and placement of a chest tube, jejunostomy feeding tube, and a mediastinal drainage tube.
The abdominal phase was performed with the patient in supine position. Initially, a 10 mm balloon trocar (30° camera port) was placed via a cut-down technique just above the umbilicus. Carbon dioxide was insufflated in order to create a pneumoperitoneum of 12 mmHg. Two 10–12 mm ports were placed: 5–8 cm to the left and right of the 10 mm balloon trocar respectively, and one 5 mm port subcostally on the right midclavicular line (tissue grasper ports). Finally, a Nathansen liver retractor (Cook Medical, USA) was introduced approximately 3 cm distally to the xypoid process and attached to the retractor arm after lifting the left hepatic lobe. Lymphadenectomy was performed of the suprapancreatic lymph nodes, nodes along the celiac trunk and along the left gastric artery. The stomach was mobilized and the dissection of the lower esophagus is performed through the gastric hiatus. A gastric tube with a diameter of 3–5 cm was created using multiple cartridges of a 60 mm linear endostapler (Endo-GIA, Covidien) introduced through the 10–12 mm grasper port at the right side. The gastric tube was sutured to the gastric cardia in order to make it possible to pull up the gastric tube into the thorax during the second phase. No pyloroplasty was performed and at the time of implementation, no omentum was spared for a later omentoplasty. A feeding jejunostomy tube was created in the left upper quadrant. Abdominal wounds were closed.
For the thoracoscopic phase, the patient was turned to prone position. Initially, a 10 mm balloon trocar (30° camera port) was placed via a cut-down technique just distally to the tip of the right scapula. Carbon dioxide was insufflated until a pressure of 8 mm Hg was reached. One 10–12 mm port was introduced 5–8 cm distally from the balloon trocar and one proximally at the medial edge of the scapula. Finally a 5 mm port was introduced at the medial and upper side of the clavicle. A formal lymphadenectomy was performed up to the level of the carina, including paraesophageal lymph nodes, lymph nodes along the aortic arch, along the descending aorta and along the left and right main bronchus. The azygos vein was dissected with a vascular stapler. After mobilization of the thoracic esophagus, it was divided at the level of the carina with a linear endostapler. The intra-abdominal gastric tube was pulled up into the thoracic cavity and the resection specimen was extracted through an OctoPort (DalimSurgNet, Seoul, Korea) placed at the level of the diaphragm after removing the distal 10–12 mm port. The esophagus was opened and the anvil of the 25 mm OrVil (Covidien) device was placed in the distal part of the remaining esophagus. The proximal end of the gastric tube was resected and the OrVil stapler was introduced in the gastric tube outside the patient. The anvil was connected to the central rod of the stapler after the rod had perforated the gastric tube on the opposite side of the linear staples that were used to create the gastric tube. The device was fired and an end-to-side anastomosis was created. The opening in the gastric tube was closed with a linear endostapler. A chest tube and a mediastinal drainage tube near the anastomosis were placed and thoracic wounds were closed.
IDEAL framework
The IDEAL framework can be used to systematically describe the development process of an innovative surgical procedure (Table 1). Stage I corresponds to the phase when surgeons perform a surgical technique for the first time. In stage IIA, the development stage, surgeons further develop the technical aspect of the procedure. If a procedure is implemented when the basic steps of a procedure are defined, but refinements to the final surgical technique are still being made, this corresponds to IDEAL stage IIB (exploration stage). In IDEAL stage III, the surgical technique has been fully developed and standardized. Descriptions of refinements in surgical technique during the transition from IDEAL stage IIB to IDEAL stage III (assessment stage, with a fully developed surgical technique) can inform surgeons about experiences from other groups. The last stage of the IDEAL framework, stage IV (long-term study), is when long term outcomes are studied [8].
Assessment of evolution of surgical technique
Surgical reports were reviewed and surgeons were interviewed to detect refinements in surgical technique. All refinements in surgical technique were listed. The timing of the refinement, the part of the operation that was refined and the reasons for this refinement were noted. The transition to IDEAL stage III was considered to be complete when no more changes to surgical technique were made.
Casemix parameters
Casemix parameters were extracted from our esophagectomy database in order to present what types of cases were included in this study. The parameters that were extracted were age, sex, BMI, ASA classification score, tumor location, tumor histology, clinical tumor stage, and whether patients had received neoadjuvant treatment.
Outcome parameters
Outcome parameters were anastomotic leakage and textbook outcome. Anastomotic leakage was defined according to the esophagectomy complications consensus group (ECCG) as: ‘Full thickness GI defect involving esophagus, anastomosis, staple line, or conduit irrespective of presentation or method of identification’ [12]. Textbook outcome is a combined outcome parameter that can be seen as a ‘success rate’. Textbook outcome was scored when a patient has a radical (R0) resection, no peroperative complications, 15 or more extracted lymph nodes, no reinterventions or reoperations, no complications of Clavien-Dindo grade ≥3 [13], no intensive care unit (ICU) readmission, no hospital length of stay of >21 days, no hospital readmission <30 days, and no mortality <30 days or mortality during the admission [14]. Other outcome parameters were operative time in minutes and reoperation rate during admission or within 30 days after surgery. Pulmonary complications were defined as pneumonia for which treatment was started, pleural empyema, pneumothorax, pleural effusion, and mediastinitis. Cardiac complications were defined as clinical diagnosed arrhythmias, cardiac ischemic events, pericarditis, and asystolie. Reintervention rate was categorized in bedside chest tube placement, radiologic reinterventions, and endoscopic reinterventions. Length of hospital and ICU stay in days, hospital- and ICU readmission rate within the first 30 days after discharge. Mortality was defined as 30- and 90-days mortality after surgery including in-hospital mortality.
Statistical analysis
Trends in anastomotic leakage and textbook outcome were presented in incidence graphs which were created using weighted centered moving average analysis [15]. Calculations were performed in R and the graphs were plotted using IBM SPSS for Windows (version 22.0, Armonk NY, USA). The chi-square test was used to evaluate whether differences in binomial variables were statistically significant.
RESULTS
Patients
This study included 164 patients. The average age was 64 years, 79.9% were men, and 57% had ASA II classification. The predominant tumor type was adenocarcinoma. Tumors were most often located at the distal esophagus. Clinical stage III was highly represented with 64%. Of all included patients, 96.9% received neoadjuvant treatment (Table 2).
| Parameter . | All patients . |
|---|---|
| Age (years)—mean (SD) | 64 (10) |
| BMI—mean (SD) | 26.1 (4.3) |
| Male sex—N (%) | 131 (79.9%) |
| ASA score—N (%) | |
| ASA I | 21 (12.9%) |
| ASA II | 93 (57.1%) |
| ASA III | 47 (28.8%) |
| ASA IV | 2 (1.2%) |
| Tumor type—N (%) | |
| Adenocarcinoma | 127 (77.9%) |
| Squamous cell carcinoma | 32 (19.6%) |
| Other | 3 (1.8%) |
| Tumor location—N (%) | |
| Mid esophagus | 15 (9.3%) |
| Distal esophagus | 127 (78.4%) |
| Gastroesophageal junction | 20 (12.3%) |
| Clinical stage—N (%) | |
| Stage I | 12 (8.6%) |
| Stage II | 38 (27.3%) |
| Stage III | 89 (64.0%) |
| Neoadjuvant treatment—N (%) | 158 (96.9%) |
| Parameter . | All patients . |
|---|---|
| Age (years)—mean (SD) | 64 (10) |
| BMI—mean (SD) | 26.1 (4.3) |
| Male sex—N (%) | 131 (79.9%) |
| ASA score—N (%) | |
| ASA I | 21 (12.9%) |
| ASA II | 93 (57.1%) |
| ASA III | 47 (28.8%) |
| ASA IV | 2 (1.2%) |
| Tumor type—N (%) | |
| Adenocarcinoma | 127 (77.9%) |
| Squamous cell carcinoma | 32 (19.6%) |
| Other | 3 (1.8%) |
| Tumor location—N (%) | |
| Mid esophagus | 15 (9.3%) |
| Distal esophagus | 127 (78.4%) |
| Gastroesophageal junction | 20 (12.3%) |
| Clinical stage—N (%) | |
| Stage I | 12 (8.6%) |
| Stage II | 38 (27.3%) |
| Stage III | 89 (64.0%) |
| Neoadjuvant treatment—N (%) | 158 (96.9%) |
ASA, American Society of Anesthesiologists classification; SD, standard deviation.
| Parameter . | All patients . |
|---|---|
| Age (years)—mean (SD) | 64 (10) |
| BMI—mean (SD) | 26.1 (4.3) |
| Male sex—N (%) | 131 (79.9%) |
| ASA score—N (%) | |
| ASA I | 21 (12.9%) |
| ASA II | 93 (57.1%) |
| ASA III | 47 (28.8%) |
| ASA IV | 2 (1.2%) |
| Tumor type—N (%) | |
| Adenocarcinoma | 127 (77.9%) |
| Squamous cell carcinoma | 32 (19.6%) |
| Other | 3 (1.8%) |
| Tumor location—N (%) | |
| Mid esophagus | 15 (9.3%) |
| Distal esophagus | 127 (78.4%) |
| Gastroesophageal junction | 20 (12.3%) |
| Clinical stage—N (%) | |
| Stage I | 12 (8.6%) |
| Stage II | 38 (27.3%) |
| Stage III | 89 (64.0%) |
| Neoadjuvant treatment—N (%) | 158 (96.9%) |
| Parameter . | All patients . |
|---|---|
| Age (years)—mean (SD) | 64 (10) |
| BMI—mean (SD) | 26.1 (4.3) |
| Male sex—N (%) | 131 (79.9%) |
| ASA score—N (%) | |
| ASA I | 21 (12.9%) |
| ASA II | 93 (57.1%) |
| ASA III | 47 (28.8%) |
| ASA IV | 2 (1.2%) |
| Tumor type—N (%) | |
| Adenocarcinoma | 127 (77.9%) |
| Squamous cell carcinoma | 32 (19.6%) |
| Other | 3 (1.8%) |
| Tumor location—N (%) | |
| Mid esophagus | 15 (9.3%) |
| Distal esophagus | 127 (78.4%) |
| Gastroesophageal junction | 20 (12.3%) |
| Clinical stage—N (%) | |
| Stage I | 12 (8.6%) |
| Stage II | 38 (27.3%) |
| Stage III | 89 (64.0%) |
| Neoadjuvant treatment—N (%) | 158 (96.9%) |
ASA, American Society of Anesthesiologists classification; SD, standard deviation.
Evolution of surgical technique
A summary of the refinements that were made to the surgical technique is shown in Table 3, along with the date of the refinement and the consecutive case number after which the refinement was made.
| After case number . | Date . | Refinement . |
|---|---|---|
| 9 | May 2011 | Addition of omental wrap and changing of stapling device from 25mm OrVil to 28 mm circular EEA stapler |
| 27 | April 2012 | Reduction of omental wrap volume |
| 60 | March 2013 | Modification to 360° omental wrap |
| 77 | September 2013 | Changing from endostitch to endoloop anvil fixation |
| After case number . | Date . | Refinement . |
|---|---|---|
| 9 | May 2011 | Addition of omental wrap and changing of stapling device from 25mm OrVil to 28 mm circular EEA stapler |
| 27 | April 2012 | Reduction of omental wrap volume |
| 60 | March 2013 | Modification to 360° omental wrap |
| 77 | September 2013 | Changing from endostitch to endoloop anvil fixation |
| After case number . | Date . | Refinement . |
|---|---|---|
| 9 | May 2011 | Addition of omental wrap and changing of stapling device from 25mm OrVil to 28 mm circular EEA stapler |
| 27 | April 2012 | Reduction of omental wrap volume |
| 60 | March 2013 | Modification to 360° omental wrap |
| 77 | September 2013 | Changing from endostitch to endoloop anvil fixation |
| After case number . | Date . | Refinement . |
|---|---|---|
| 9 | May 2011 | Addition of omental wrap and changing of stapling device from 25mm OrVil to 28 mm circular EEA stapler |
| 27 | April 2012 | Reduction of omental wrap volume |
| 60 | March 2013 | Modification to 360° omental wrap |
| 77 | September 2013 | Changing from endostitch to endoloop anvil fixation |
First refinement
Before the first refinement, the operation was performed in the way that is described in the methods section. After the first nine patients, the anastomotic technique was critically reviewed because a high percentage of anastomotic leakage was experienced. After internal discussions within the team of three dedicated esophageal surgeons and the rest of the treatment team, the anastomotic technique was changed after the 9th patient (May 2011).
The use of the 25 mm OrVil was abandoned and exchanged for a 28 mm EEA stapler (Covidien) (Figs 1–3), because we hypothesized that tissue necrosis occurred lateral from the double staggered stapled rings of the OrVil. In addition, the surgical procedure was modified to include a large omental wrap because research had shown promising results on the effect of an omental wrap in reducing clinically relevant anastomotic leakage [16]. For the omental wrap, all available omental tissue that could be spared was positioned in the thorax and was used to cover the dorsal 180° circumference of the anastomotic site. The omental wrap was fixated to the pleura.
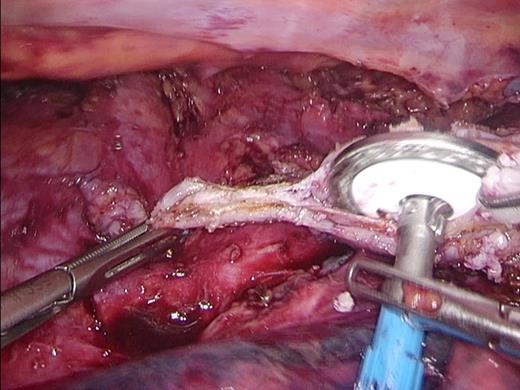
Introduction of the Anvil. The 28 mm Anvil is introduced into the esophagus after removing the staple line.

28 mm stapler. The 28 mm circular extralarge EEA stapler is introduced into the opened tip of the gastric tube.
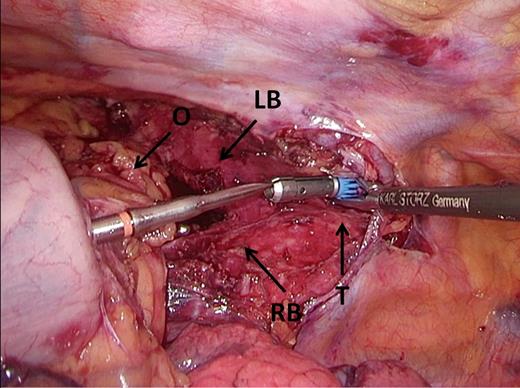
Docking. Omental tissue is positioned ventrally from the gastric tube, covering the bronchus and trachea, and the trocar is perforated just dorsal to the omental tissue. LB, left main stem bronchus; O, omental tissue, RB, right main stem bronchus, T, trachea.
Second refinement
After case 27 (April 2012), the postoperative results were critically reviewed by the treatment team. Although the incidence of anastomotic leakage had decreased, pulmonary complications occurred in 44% of all patients. We hypothesized that the large intrathoracic omental volume could impair postoperative pulmonary function. This might have led to a higher incidence of pulmonary complications. The volume of the omental wrap was reduced and after this refinement omental tissue from 5 cm of the left gastroepiploic artery was spared. In addition, the treatment team decided to administer 1 mg/kg dexamethasone intravenously before anesthesia because there was some evidence that this could reduce pulmonary complications [17].
Third refinement
After case 60 (March 2013), the surgical team noticed that two patients developed a fistula from the gastric tube to the bronchus (managed by reoperation) and that one patient died after developing a fistula from the gastric tube to the aorta. After Ivor Lewis TMIE, the gastric tube and the anastomosis lie in close proximity to both the bronchi and the aorta. Possibly, inflammation of this area (for example after anastomotic leakage) can predispose to fistula formation. The surgical team concluded that the omental wrap should cover the whole 360° of the anastomosis instead of only the dorsal 180° (Fig. 4). After this refinement, the full 360° of the anastomosis was draped with omental tissue. The omentum was sutured to the pleura so that it could not displace.
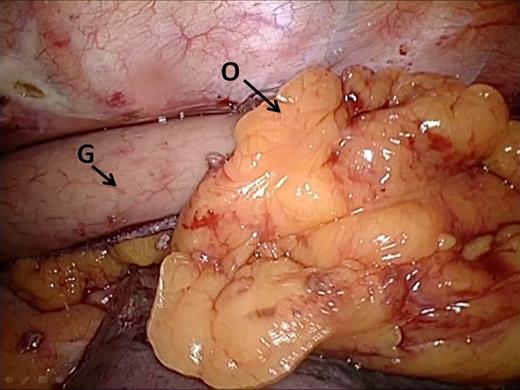
Omental wrap. Omental tissue is draped around the dorsal side of the anastomosis and fixated onto the pleura in order to create a 360° omentoplasty. G, gastric tube; O, omental tissue.
Fourth refinement
The final refinement was made after case 77 (September 2013). By gaining surgical experience, the surgical team had become convinced that good fixation of the anvil of the stapler was paramount to obtaining two ‘complete donuts’ after firing the circular stapler. In turn, obtaining two ‘complete donuts’ seemed to be related to an anastomosis of good quality. Because fixation of the anvil in the esophagus with the Endostitch device was considered to be technically challenging, it was replaced by fixation of the anvil with two Endoloops® (Ethicon), without performing a purse string. This enabled an easy to use and reproducible and secure fixation of the anvil in the distal esophagus (Figs 5,6).
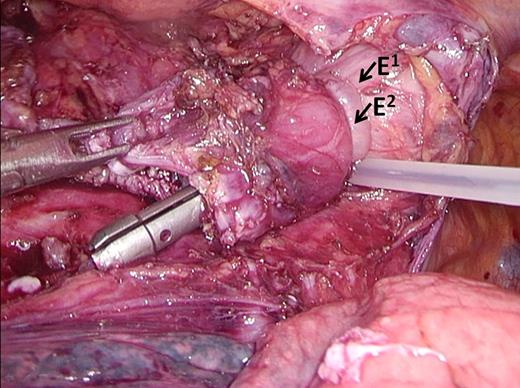
Endoloops. The anvil is secured in the esophagus by two endoloops. E1 = Endoloop 1, E2 = Endoloop 2.
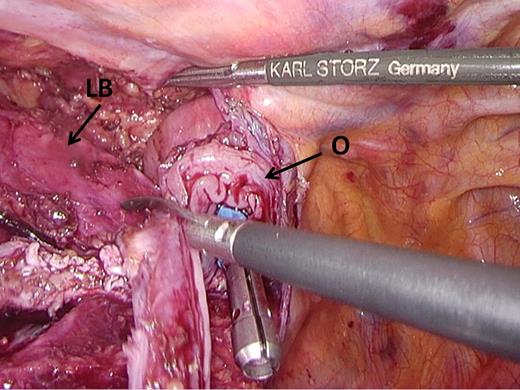
Excision of redundant tissue. Redundant esophageal tissue is excised to prevent interposition into the staple line. LB, left main stem bronchus; O, esophagus.
After the fourth refinement, the surgical technique had entered IDEAL stage III since no more modifications were made to the surgical technique.
Outcome parameters
Outcome parameters are shown in Table 4. The overall anastomotic leakage rate was 14.6% and the overall textbook outcome was 40.2%. Hospital length of stay was 11 days and ICU length of stay was 3 days. The incidence of cardiac complications was 24% and pulmonary complications were almost 52%. Thirty-five of 164 patients needed reoperation and the total reintervention rate was 28%. Thirty- and 90-day mortality was 3.6% and 6.7%, respectively.
| Outcome parameter . | N . | % . |
|---|---|---|
| Operative time in minutes—median/IQR | 270 | 90.5 |
| Anastomotic leakage (AL)—N (%) | ||
| AL grade 1 | 2 | 1.22 |
| AL grade 2 | 5 | 3.05 |
| AL grade 3 | 17 | 10.37 |
| Total | 24 | 14.63 |
| Pulmonary complications—N (%) | 85 | 51.83 |
| Cardiac complications—N (%) | 40 | 24.39 |
| Reintervention rate—N (%) | ||
| Chest tube (bedside) | 10 | 6.10 |
| Radiologic | 28 | 17.07 |
| Endoscopic | 17 | 10.37 |
| Total | 46 | 28.0 |
| Reoperation rate | 35 | 21.34 |
| Length of stay in days—median/IQR | ||
| ICU | 3 | 5 |
| Hospital | 11 | 10 |
| Readmission rate—N (%) | ||
| ICU | 25 | 15.24 |
| Hospital | 26 | 15.85 |
| Mortality—N (%) | ||
| 30-day | 6 | 3.66 |
| 90 day | 11 | 6.71 |
| Textbook outcome—N (%) | 66 | 40.24 |
| Outcome parameter . | N . | % . |
|---|---|---|
| Operative time in minutes—median/IQR | 270 | 90.5 |
| Anastomotic leakage (AL)—N (%) | ||
| AL grade 1 | 2 | 1.22 |
| AL grade 2 | 5 | 3.05 |
| AL grade 3 | 17 | 10.37 |
| Total | 24 | 14.63 |
| Pulmonary complications—N (%) | 85 | 51.83 |
| Cardiac complications—N (%) | 40 | 24.39 |
| Reintervention rate—N (%) | ||
| Chest tube (bedside) | 10 | 6.10 |
| Radiologic | 28 | 17.07 |
| Endoscopic | 17 | 10.37 |
| Total | 46 | 28.0 |
| Reoperation rate | 35 | 21.34 |
| Length of stay in days—median/IQR | ||
| ICU | 3 | 5 |
| Hospital | 11 | 10 |
| Readmission rate—N (%) | ||
| ICU | 25 | 15.24 |
| Hospital | 26 | 15.85 |
| Mortality—N (%) | ||
| 30-day | 6 | 3.66 |
| 90 day | 11 | 6.71 |
| Textbook outcome—N (%) | 66 | 40.24 |
| Outcome parameter . | N . | % . |
|---|---|---|
| Operative time in minutes—median/IQR | 270 | 90.5 |
| Anastomotic leakage (AL)—N (%) | ||
| AL grade 1 | 2 | 1.22 |
| AL grade 2 | 5 | 3.05 |
| AL grade 3 | 17 | 10.37 |
| Total | 24 | 14.63 |
| Pulmonary complications—N (%) | 85 | 51.83 |
| Cardiac complications—N (%) | 40 | 24.39 |
| Reintervention rate—N (%) | ||
| Chest tube (bedside) | 10 | 6.10 |
| Radiologic | 28 | 17.07 |
| Endoscopic | 17 | 10.37 |
| Total | 46 | 28.0 |
| Reoperation rate | 35 | 21.34 |
| Length of stay in days—median/IQR | ||
| ICU | 3 | 5 |
| Hospital | 11 | 10 |
| Readmission rate—N (%) | ||
| ICU | 25 | 15.24 |
| Hospital | 26 | 15.85 |
| Mortality—N (%) | ||
| 30-day | 6 | 3.66 |
| 90 day | 11 | 6.71 |
| Textbook outcome—N (%) | 66 | 40.24 |
| Outcome parameter . | N . | % . |
|---|---|---|
| Operative time in minutes—median/IQR | 270 | 90.5 |
| Anastomotic leakage (AL)—N (%) | ||
| AL grade 1 | 2 | 1.22 |
| AL grade 2 | 5 | 3.05 |
| AL grade 3 | 17 | 10.37 |
| Total | 24 | 14.63 |
| Pulmonary complications—N (%) | 85 | 51.83 |
| Cardiac complications—N (%) | 40 | 24.39 |
| Reintervention rate—N (%) | ||
| Chest tube (bedside) | 10 | 6.10 |
| Radiologic | 28 | 17.07 |
| Endoscopic | 17 | 10.37 |
| Total | 46 | 28.0 |
| Reoperation rate | 35 | 21.34 |
| Length of stay in days—median/IQR | ||
| ICU | 3 | 5 |
| Hospital | 11 | 10 |
| Readmission rate—N (%) | ||
| ICU | 25 | 15.24 |
| Hospital | 26 | 15.85 |
| Mortality—N (%) | ||
| 30-day | 6 | 3.66 |
| 90 day | 11 | 6.71 |
| Textbook outcome—N (%) | 66 | 40.24 |
Trend analysis
A graphical display of anastomotic leakage and textbook outcome is shown in Figure 7. The four surgical refinements listed above are also displayed in this figure. The incidence of anastomotic leakage was 26.0% during IDEAL stage IIB and 4.6% during the IDEAL stage III (P < 0.001). A graphical display of the incidence of textbook outcome, with the timing of surgical refinements annotated, is shown in Figure 8. The incidence of textbook outcome was 31.2% during IDEAL stage IIB and 47.1% during the IDEAL stage III (P = 0.039).
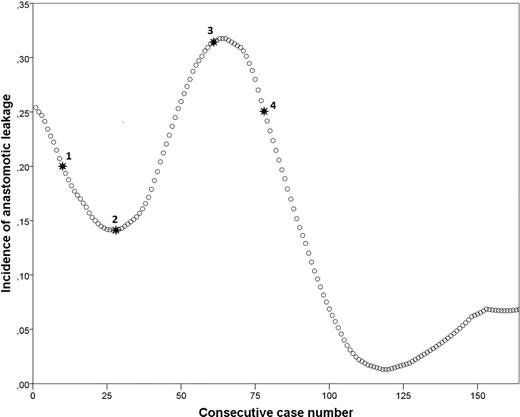
Incidence graph of anastomotic leakage with surgical refinements annotated. 1: Addition of omental wrap and changing of stapling device from 25 mm OrVil to 28 mm circular EEA stapler, 2: Reduction of omental wrap volume, 3: Modification to 360° omental wrap, 4: Changing from Endostitch to Endoloop® anvil fixation.
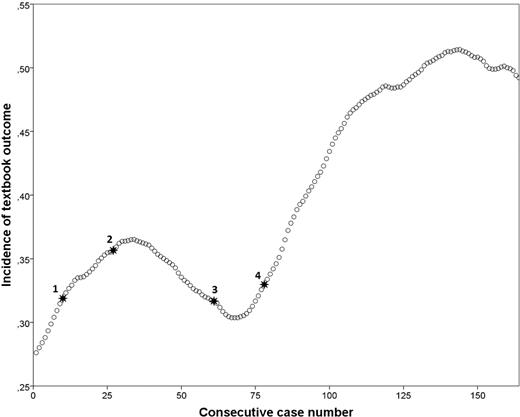
Incidence graph of textbook outcome with surgical refinements annotated. 1: Addition of omental wrap and changing of stapling device from 25 mm OrVil to 28 mm circular EEA stapler, 2: Reduction of omental wrap volume, 3: Modification to 360° omental wrap, 4: Changing from Endostitch to Endoloop® anvil fixation.
DISCUSSION
The evolution of the surgical technique progressed from IDEAL stage IIB to IDEAL stage III after 77 cases. During 33 months of IDEAL stage IIB, four significant refinements were made to the surgical technique of Ivor Lewis TMIE. All refinements were preceded by an internal discussion of the previous results by the treatment team in an attempt to improve results. These discussions led to a critical evaluation of the surgical technique and ultimately led to a stable treatment program with favorable results. This underlines the importance of prospective outcome registration and evaluation after introduction of a complex surgical technique in IDEAL stage IIB, as has been reported by other groups [18,19].
Postoperative outcome improved significantly as the surgical technique progressed to IDEAL stage III. A trend reversal in the incidence of anastomotic leakage and textbook outcome was observed after the omental wrap procedure was improved to cover 360° of the anastomosis (refinement 3) and the anvil fixation method was improved (refinement 4). However, it is well known that outcomes improve as surgeons gain proficiency in esophagectomy [20–23], regardless of refinements in operative technique. Therefore, we were unable to determine to what extent improvements in outcome were related to the refinements in surgical technique and to what extent improvements were related to the proficiency gain of surgeons.
Implementation of a complex surgical technique in IDEAL stage IIB can also have important disadvantages compared to implementation in IDEAL stage III. We hypothesize that a surgical technique in IDEAL stage IIB is learned at a slower rate than a surgical technique in IDEAL stage III, because in IDEAL stage IIB, surgeons still have to find their optimal surgical technique in addition to gaining the strict surgical skills. If this hypothesis is correct, this might have important implications for patients, surgeons, and policy makers because patient safety can be compromised during surgical proficiency gain [20,21]. Longer surgical proficiency gain curves are associated with more morbidity. Implementation of a surgical technique in IDEAL stage III could reduce this morbidity and more research is needed to investigate this hypothesis.
In addition, implementation in IDEAL stage IIB in multiple centers during the same period of time can result in multiple variations of a surgical technique. For example, Ivor Lewis TMIE is performed with stapled end-to-side or semistapled side-to-side anastomosis in the Netherlands [24]. Recently, two other Dutch centers refined their anastomotic technique and now perform robot-assisted hand-sewn end-to-end anastomosis for Ivor Lewis TMIE. Diverging surgical techniques can complicate the establishment of a generally accepted surgical technique, that can in turn be implemented by other centers in IDEAL stage III. This is important because it has been shown that high quality teaching of surgical procedures can maximize the benefits from training [25]. An example of how a structured training program can be established is the case of the national training program for laparoscopic colorectal surgery in the United Kingdom [26].
These considerations should be taken into account by surgeons before implementing an innovative surgical technique in IDEAL stage IIB.
Strengths and limitations
Strength of this study is that recommendations on how to describe implementation of surgical techniques according to the IDEAL framework were followed [27]. By using the IDEAL framework, surgeons can obtain a detailed and structured description of the evolution of new surgical techniques, which may aid in implementation processes and may lead to a more standardized surgical technique.
In addition, this study provides a detailed description of IDEAL stage IIB for Ivor Lewis TMIE. The description of our experience can be used by surgeons that consider implementing Ivor Lewis TMIE in their practice. Another strength is that a sufficient number of patients were included in IDEAL stage III and this makes it plausible that the surgical technique is now stable. A limitation is that we were unable to test whether surgical refinements were directly related to improving trends in outcome parameters. Finally, the listings of refinements to surgical technique were performed retrospectively. However, substantial efforts were made to include all refinements by analyzing surgical reports and interviewing surgeons.
CONCLUSION
This study describes the surgical refinements that were made during the progression of Ivor Lewis TMIE from IDEAL stage IIB to IDEAL stage III. During IDEAL stage IIB, postoperative outcome improved as surgical proficiency was gained and the technique was refined.
Notes
Specific author contributions: Marianne Stenstra, Frans van Workum, and Camiel Rosman contributed to the design of the study. All authors were involved in acquisition of the data. Analysis was performed by Marianne Stenstra and Frans van Workum. All authors were involved in interpretation of the work. Marianne Stenstra, Frans van Workum, and Camiel Rosman drafted the first manuscript. All authors were involved in critically revising the manuscript for intellectual content. All authors approve of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
No funds were received in support of this study.
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.



