-
PDF
- Split View
-
Views
-
Cite
Cite
Russell Greenberg, Brian J. Olsen, Matthew A. Etterson, Patterns of Seasonal Abundance and Social Segregation in Inland and Coastal Plain Swamp Sparrows in a Delaware Tidal Marsh, The Condor: Ornithological Applications, Volume 112, Issue 1, 1 February 2010, Pages 159–167, https://doi.org/10.1525/cond.2010.080060
Close - Share Icon Share
Abstract.
The Coastal Plain Swamp Sparrow (Melospiza georgiana nigrescens) breeds in coastal marshes of the mid-Atlantic United States. During the nonbreeding season, these marshes are occupied by this subspecies and the two interior-breeding subspecies M. g. georgiana and M. g. ericrypta (collectively, interior Swamp Sparrows). From September to May, 2005–2006 and 2007–2008, we surveyed marshes in which nigrescens breeds; >90% of these birds had been color-banded by the end of the previous breeding season. This allowed us to determine the nonbreeding occupancy of the breeding site by individuals that bred locally, Coastal Plain Swamp Sparrows that did not breed there, and interior Swamp Sparrows. Locally breeding birds disappeared from the breeding site by mid-October, although nonlocal nigrescens occupied it from October to late November. Locally breeding birds reappeared in April without any pulse of migration of nigrescens. Interior-breeding sparrows were abundant in the autumn and appeared in smaller numbers later in the nonbreeding season. The seasonal pattern of nigrescens was similar in both years, and its departure and arrival dates appear to coincide with changes in the frequency of freezing temperatures. The temporal pattern of interior Swamp Sparrows in the two years was more varied. Finally, during the autumn peak of Swamp Sparrow migration, the subspecies tended strongly to segregate in subspecies-specific flocks, suggesting that Swamp Sparrows preferentially interact with morphologically similar birds, a behavior which may have implications for divergence in species recognition mechanisms and speciation.
Resumen.
Melospiza georgiana nigrescens se reproduce en marismas de los estados localizados en la costa central del Atlántico de Norteamérica. Durante el periodo no reproductivo, estas marismas son ocupadas por esta subespecie y por otras dos subespecies del interior, M. g. georgiana y M. g. ericrypta. Desde septiembre a mayo de 2005–2006 y 2007–2008, realizamos conteos en marismas en que se reproduce la subespecie nigrescens. Hasta el fin del periodo reproductivo anterior, más del 90% de estas aves habían sido marcadas con anillos de colores. Esto nos permitió determinar la ocupación durante el periodo no reproductivo del sitio de cría por parte de individuos que se reproducen localmente, de individuos de M. g. nigrescens que no se reproducen en esa localidad y por parte de las otras dos subespecies del interior. Los individuos que se reproducen localmente desaparecieron del sitio de cría a mediados de octubre, a pesar de que individuos no locales de la subespecie nigrescens ocuparon el sitio desde octubre hasta fines de noviembre. Los individuos que se reproducen en la localidad reaparecieron en abril, sin un pulso migratorio de la subespecie nigrescens. Las subespecies que se reproducen en el interior fueron abundantes durante el otoño y aparecieron en números menores durante las etapas más tardías de la época de cría. El patrón estacional de la subespecie nigrescens fue similar en ambos años, y sus fechas de partida y llegada parecieron coincidir con cambios en la frecuencia con que se registran temperaturas bajo cero. El patrón temporal de las subespecies del interior durante los dos años fue más variado. Finalmente, durante el periodo de mayor actividad migratoria del otoño, las subespecies tendieron a agruparse en bandadas específicas para cada subespecie, lo que sugiere que estas especies de pinzones prefieren interactuar con individuos morfológicamente más similares entre sí que con individuos de otras subespecies, un comportamiento que puede tener implicaciones importantes para la divergencia y mecanismos de reconocimiento entre especies y para los procesos de especiación.
Introduction
Most accounts of the residency of species on their breeding grounds during the nonbreeding season are based on species-level surveys. To obtain a complete understanding of the life history of local populations, however, it is necessary to determine the degree of nonbreeding residency of specific members of the local population rather than of the species as a whole. Where it can be reliably accomplished in the field, the identification of subspecies (especially those with a limited distribution) can provide information on the regional residency of populations. The resighting of locally banded individuals can then provide information on the site fidelity of individuals. Using subspecies identification and individual marking together can provide a picture of residency on both the regional and local scales. We employed this two-pronged approach in determining the nonbreeding status of Coastal Plain Swamp Sparrows (Melospiza georgiana nigrescens) at breeding sites in coastal Delaware, U.S. The ability to identify birds to subspecies (or subspecies groups) also allowed us to study the social interactions between birds of regional versus more distant origins. In particular, we were interested in whether birds from morphologically distinct populations showed any tendency to segregate into separate flocks, implying population-specific recognition. As assortative mating is a necessary step for speciation under the biological species concept (Coyne and Orr 2004), the development of such population recognition during the nonbreeding period may have implications for future speciation, even among populations that are currently allopatric during the breeding season.
The Swamp Sparrow breeds across a broad band of the northern U.S. and Canada (Mowbray 1997). Most Swamp Sparrows breed in freshwater wetlands, and these populations have been separated into two subspecies (M. g. georgiana or M. g. ericrypta, collectively, interior Swamp Sparrows) that occupy the southern and northern portion of the continental range (Mowbray 1997). The third subspecies, M. g. nigrescens, is restricted to brackish coastal marshes from northern Virginia to northern New Jersey (Beadell et al. 2003, Watts et al. 2008) and is distinguished from interior Swamp Sparrows by the combination of a larger bill, blacker markings in the head and dorsal plumage, and grayer overall body coloration (Greenberg et al. 2008), although means of distinguishing it by mitochondrial DNA (Greenberg et al. 1998) or microsatellite loci (R. Fleischer, unpubl. data) have not been identified. This lack of difference in neutral genetic markers (which has been found in a number of other salt-marsh subspecies of birds, Chan et al. 2006) suggests that nigrescens has either diverged very recently or is subject to continued gene flow from the interior subspecies.
In the breeding season, nigrescens is allopatric with the interior subspecies except for a narrow zone of intergradation with georgiana in northern New Jersey (Greenberg and Droege 1990). The degree of overlap in distribution during the nonbreeding season, however, remains poorly understood. Swamp Sparrows winter commonly throughout the southeastern United States with the highest concentrations in the Lower Mississippi Alluvial Valley and along the Gulf of Mexico (Root 1988). Within this broad range, the nonbreeding distribution of Coastal Plain Swamp Sparrow requires further definition. In the original description of the subspecies Bond and Stewart (1951) considered it to be largely resident on the breeding grounds, although they listed a few extralimital records. Subsequently, Greenberg and Droege (1990) identified the nonbreeding specimens reported by Bond and Stewart as interior Swamp Sparrows.
Greenberg et al. (2007) reported Coastal Plain Swamp Sparrows wintering in coastal North Carolina and Virginia with interior Swamp Sparrows. An analysis of isotopes in winter-molted crown feathers collected from individuals on the breeding grounds had predicted that at least a portion of the population migrates to these southern marshes in the winter (Greenberg et al. 2007). Because of a steep climatic gradient between Delaware and North Carolina, Greenberg et al. (2007) hypothesized that the short migration (250–500 km) of the Coastal Plain Swamp Sparrow to North Carolina allows the subspecies to avoid freezing conditions. They suggested further that the subspecies' propensity to forage for invertebrates in tidal mud makes it particularly sensitive to freezing substrates. For example, Bear, Delaware (a weather station 22 km from our study location), experiences an average of 99 freezing days (66%) between 1 November and 1 April (ACON 2008), whereas Cedar Island, North Carolina (a wintering site for the Coastal Plain Swamp Sparrow), reports only 25 freezing days (17%) in the same period, on the basis of 1996-2006 averages (NCDC 2009).
Despite our observations away from the breeding range, not all Coastal Plain Swamp Sparrow may migrate; some individuals may remain at the breeding sites year round. Swamp Sparrows (of unknown subspecies) are observed between October and March in the marshes where Coastal Plain Swamp Sparrows breed, sometimes in high abundance. Prior to this study the relative nonbreeding abundance and seasonal patterns of interior and Coastal Plain Swamp Sparrows within the latter's breeding range were unknown. In this paper, we report on systematic surveys of the abundance of Coastal Plain and interior Swamp Sparrows during the nonbreeding seasons of 2005–2006 and 2007–2008 in marshes in which the Coastal Plain Swamp Sparrow breeds. We use these data to address the following: (1) the seasonal pattern of occurrence of each category of Swamp Sparrow; (2) the seasonal pattern of occurrence of locally versus nonlocally breeding Coastal Plain Swamp Sparrows; (3) the response of the Coastal Plain Swamp Sparrow's abundance to the onset of freezing temperatures and the arrival of interior Swamp Sparrows; and (4) population-specific spatial or social segregation.
Study Site and Methods
Study Site
Surveys were conducted along an approximately 1-km transect through portions of two existing 10-ha study plots established for a study of breeding of the Coastal Plain Swamp Sparrow at the Woodland Beach Wildlife Management Area, Kent County, Delaware (for a detailed description see Olsen 2007). The study areas were established in 2002 and 2003. At the end of the 2005 breeding season they contained 139 sparrows color-banded as adults and 92 color-banded as nestlings; at the end of the 2007 season they contained 125 color-banded adults and 57 color-banded nestlings. The dominant habitat along the transect was high tidal marsh with various species of Spartina, high-tide bush (Iva frutescens), eastern baccharis (Baccharis halimifolia), common reed (Phragmites australis), and bulrush (Schoenoplectus americanus).
Survey Methods
We completed 19 surveys between mid-September and early May, 2005–2006 and 2007–2008. We surveyed between 07:00 and 10:00 by walking slowly and, at approximately 25-m intervals, playing a 30-sec tape recording of an interior Swamp Sparrow's distress call (sensu Stefanski and Falls 1972) recorded during the winter in North Carolina. In previous field work, the recording we used in this study has proven effective at exciting and attracting both interior and Coastal Plain Swamp Sparrows, and experiments by Stefanski and Falls (1972) demonstrated that the distress calls of Song (Melospiza melodia) and Swamp Sparrows evoked similar responses across species. All Swamp Sparrows detected within 25 m of the transect were included in the survey results. We could not distinguish between the two inland subspecies in the field, so they are pooled. Whenever possible, Swamp Sparrows were identified to subspecies or subspecies group by plumage coloration and bill size (Greenberg et al. 2008). Of the total 1035 Swamp Sparrow sightings, 90% were identified as of either interior or Coastal Plain Swamp Sparrows. Because not all Swamp Sparrows were identified to one of these categories, the ratio of interior to Coastal Plain Swamp Sparrows among unidentified individuals was assumed to match that of identified birds for that day. We plotted the estimated total number of interior and Coastal Plain Swamp Sparrows (total birds seen multiplied by the proportion of identified birds of each form) so that we could depict the migration peaks more accurately. During days with a large number of Swamp Sparrows, more birds were left unidentified so that the survey could be completed before winds gained velocity in the late morning. Because of observations made in the first season that suggested flocks segregating by subspecies, in 2007 we kept more detailed notes on the sparrows' spatial distribution, and all birds observed within 25 m of each other were considered a flock. Furthermore, we analyzed flocks' composition to determine if they were dominated by a single subspecies or subspecies group when both categories occurred on the same plot (October–November).
Statistical Analysis
Annual concordance in phenology of occurrence. We divided the nonbreeding season into 47 five-day periods starting with 19 September, then calculated the mean number of each category of Swamp Sparrow recorded per survey during each period and year. We calculated the between-year correlation (Pearson r) separately for each subspecies on the basis of these means for all 5-day periods in which surveys were conducted in both years (n = 14). The strength of this correlation between the two categories of sparrows was compared by a test for the homogeneity of the two correlation coefficients using the Fisher r-to-z transformation (Lowry 2009). In addition, we used correlation analysis within each year between the 10-day average abundances of each category of sparrow to examine the degree of direct temporal displacement between them.
The relationship between freezing temperatures and the seasonal abundance of interior and Coastal Plain Swamp Sparrows. With separate linear regressions, we examined the relationship between the total number of each subspecies group detected in a 10-day period (data pooled for both years) and the decadal average number of days with temperatures below freezing for the same 10-day period. We transformed the proportion of freezing days per 10-day period with an arcsine transformation prior to calculating the r2 values for these regressions. The individual regression residuals met assumptions of normality and homogeneity of variance (StatSoft 2003). The decadal averages are based on 1996–2006 data from the Bear, Delaware, station (39.6° N, 75.6° W) of the Maryland/Delaware/D.C. chapter of the Atlantic Coast Observer Network (ACON 2008), which is approximately 20 km from our study site. We tested for homeogeneity between the interior and Coastal Plain Swamp Sparrows in the strength of the correlation between the number of freezing days and abundance with a Fisher r-to-z transformation (Lowry 2009) of the arcsine-transformed data.
Spatial segregation of interior and Coastal Plain Swamp Sparrows by habitat. The survey plots comprised two major habitats: roadside edge (with sufficient elevation to support a high density of common reed, high-tide bush, and eastern baccharis) and the marsh core (dominated by Spartina grasses and bulrush). Over the two October–November periods we summed the total number of each category of Swamp Sparrow within 25 m of a road versus within the interior of the marsh (pooled for both years and all survey dates) and tested for differences in the spatial distribution of the two types of Swamp Sparrow by using a χ2 contingency test.
Segregation of Swamp Sparrows by subspecies. To test the hypothesis that Swamp Sparrow flocks were not segregated by subspecies (or subspecies group), we performed a series of four logistic regression analyses with goodness-of-fit tests. In these analyses, our response variable, y, was the number of Coastal Plain Swamp Sparrows in a given flock conditional on n and p, where n is the number of individuals identified to subspecies (n >y) and p is the probability (estimated in the logistic regression) that a given individual within a flock is a Coastal Plain Swamp Sparrow. The primary goal of this analysis was to make an inference about the variance of y by using model residuals. If the variance in y is too large, then we can reject the hypothesis that flocks are not segregated by subspecies. In other words, we can conclude that flocks are not assembled according to the relative abundance of each subspecies in the environment but that individuals preferentially form flocks with other individuals of the same form.
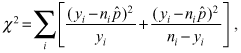
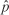 is the estimated probability of a Coastal Plain Swamp Sparrow from the logistic regression, should follow a χ2 distribution with the appropriate degrees of freedom (number of flocks minus the number of estimated parameters). We performed this goodness-of-fit test on all four models described below. When cell sizes are small (flock sizes in this case), however, observed values of χ2 may deviate substantially from their expectation under a χ2 distribution. Therefore we also performed Monte Carlo tests on χ2 as follows. We assumed a binomial distribution (as above) and repeatedly generated sets of data with exactly the same number of flocks and flock sizes as our observed data, conditional on n. and
is the estimated probability of a Coastal Plain Swamp Sparrow from the logistic regression, should follow a χ2 distribution with the appropriate degrees of freedom (number of flocks minus the number of estimated parameters). We performed this goodness-of-fit test on all four models described below. When cell sizes are small (flock sizes in this case), however, observed values of χ2 may deviate substantially from their expectation under a χ2 distribution. Therefore we also performed Monte Carlo tests on χ2 as follows. We assumed a binomial distribution (as above) and repeatedly generated sets of data with exactly the same number of flocks and flock sizes as our observed data, conditional on n. and 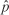 (the latter coming, as before, from the logistic regression). With each simulated data set we estimated new values of χ2 and recorded whether the simulated value of χ2 was larger than the value derived from the empirical analysis. Unlike the comparison to a χ2 distribution, above, this test is not sensitive to small sample size. For each of the four models below, we repeated the Monte Carlo analysis 106 times and report the proportion of simulations that produce a value of χ2 greater than that observed in our empirical analysis.
(the latter coming, as before, from the logistic regression). With each simulated data set we estimated new values of χ2 and recorded whether the simulated value of χ2 was larger than the value derived from the empirical analysis. Unlike the comparison to a χ2 distribution, above, this test is not sensitive to small sample size. For each of the four models below, we repeated the Monte Carlo analysis 106 times and report the proportion of simulations that produce a value of χ2 greater than that observed in our empirical analysis.We performed four logistic regression analyses on flock composition. Our simplest model assumed a single probability of the Coastal Plain Swamp Sparrow across all dates (i.e., a single value of p) and considered all flocks separately. In this model large residuals could occur because of subspecific segregation or because of the dependence of p on some other factor not included in the model. In particular, we suspected that flock composition varied with date (our data were derived from four surveys on 10 October, 23 October, 8 November, and 13 November). Our second simplified model again assumed a single value of p, but it combined all flocks on a given date into a single large date-specific flock, resulting in four “flocks.” In this case large model residuals could come only from dependence of p on date. For our third model, we resumed our analysis of individual flocks but specified date as a categorical covariate to p in the logistic regression analysis. Therefore, we estimated four separate values of p corresponding to the four dates. Finally, given the unequal spacing among surveys, we analyzed flocks separately but specified date as a continuous covariate to p, resulting in two estimated parameters (an intercept and slope describing the relationship between p and date). In the latter two cases, large model residuals would indicate that, even controlling for a Swamp Sparrow community changing temporally, observed flocks still tend to be dominated by one or the other subspecies. Finally, to ensure that our conclusions about flock segregation were not confounded by a tendency for one or the other subspecies to join larger flocks, we estimated the mean and variance of flock size for each subspecies separately. All analyses of flock size and composition were done in MATLAB (Mathworks 2009). For all other analyses we used Statistica Version 6 (Statsoft 2003).
Results
Seasonal Patterns of Swamp Sparrow Abundance
Swamp Sparrows occurred on the plots throughout the nonbreeding season, with peak abundance late October–November and late April–early May (Fig. 1). The Coastal Plain Swamp Sparrow's abundance varied seasonally with sightings decreasing from the initial survey to near zero from December to early April. Interior Swamp Sparrows showed a more complex temporal pattern that consisted of several peaks (late October, December, and mid-March).
During the early autumn surveys (19 September—10 October), 11% of Coastal Plain Swamp Sparrows sighted in 2005 (n = 77) and 14% of those in 2007 (n = 68) had been color-banded (Fig. 2). On the plot, Coastal Plain Swamp Sparrows were observed singing and producing flight songs (Nowicki et al. 1991) until 10 October. No banded individuals were seen from mid-October to the end of March in either year, and all color-banded birds observed in September and October were adults. During the pre-breeding-season peak (31 March—2 May), 49% and 44% of observed Coastal Plain Swamp Sparrows (2006 and 2007, respectively) had been color-banded in previous years. The Coastal Plain Swamp Sparrow's seasonal pattern of abundance in the two years showed an extremely high level of concordance (r = 0.94, n = 14) (Figs. 1, 3a), while the inland Swamp Sparrows' abundance was more variable (r = 0.74, n = 14). The correlation coefficients for the concordance of abundance between years of the two categories differed significantly (Z = 2.03, P = 0.04). The seasonal change in abundance of the Coastal Plain Swamp Sparrow closely tracks the average seasonal distribution of the frequency of freezing temperatures (Fig. 4), with the population declining during the period between late November and mid April when freezing occurred on 50% or more of the days in a 10-day period (on the basis of 1996–2006 averages). The number of freezing days per 10-day period during the two study years was highly correlated (r2 = 0.81 and 0.93 for 2005–2006 and 2007–2008, respectively), so using a single year's data does not change the pattern presented in Fig. 4. The average frequency of freezing days per 10-day period accounts for a large portion of the variance in the average number of Coastal Plain Swamp Sparrows observed per 10-day period (means summed for the two years; r2 = 0.66, n = 23, P < 0.001), whereas this is not the case for interior Swamp Sparrows in 2005–2006 (r2 = 0.01), 2007–2008 (r2 = 0.07), or both years pooled (r2 = 0.02). The corresponding r values from the tests with years pooled (Coastal Plain = 0.81, interior = 0.14) differ significantly (Z = 2.97, P = 0.002), indicating a tighter relationship between abundance and freezing temperatures on the Coastal Plain than on the interior Swamp Sparrows. Finally, the correlation between the 10-day mean abundances of the two categories resulted in a very low r value for both years (2005–2006 = 0.14, 2007–2008 = 0.20), indicating that other than the small numbers of interior Swamp Sparrows that were detected in mid-winter when the Coastal Plain subspecies was absent, there was no overall significant temporal displacement between the two forms.
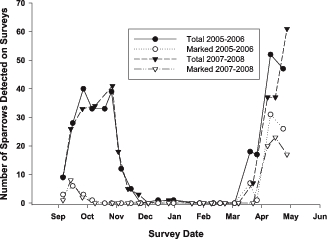
The numbers of Coastal Plain (black symbols) and interior (white symbols) Swamp Sparrows observed on surveys at Woodland Beach Wildlife Management Area in the nonbreeding seasons of 2005–2006 (circles) and 2007–2008 (triangles).

The number of total (black symbols) and color-banded (white symbols) Coastal Plain Swamp Sparrows observed on surveys at Woodland Beach Wildlife Management Area in the nonbreeding seasons of 2005–2006 (circles) and 2007–2008 (triangles).
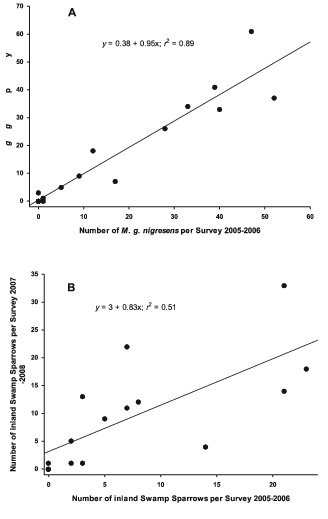
The regression of numbers of Coastal Plain Swamp Sparrows seen within matched 5-day periods during the two winters of the study (r2 = 0.89). Note that only 14 of these periods had surveys in both years.
Microhabitat Segregation of the Coastal Plain and Interior Swamp Sparrows
Coastal Plain Swamp Sparrows had a small but significant tendency (χ21 = 6.42, P = 0.01) to occupy habitat separate from that occupied by the interior subspecies on our study site: Coastal Plain Swamp Sparrows constituted 63% (n = 235) of the roadside birds but only 50% (n = 159) of the birds in the marsh's interior.
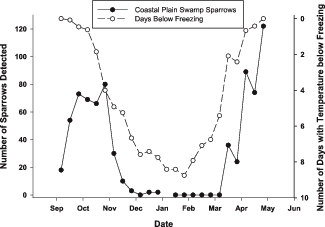
The average number of Coastal Plain Swamp Sparrows observed per survey within 10-day periods (black circles) plotted against the average number of days with minimum temperatures below 0 °C in the same period for a weather station 20 km from the study area (white circles). Data for each period are summed for the two winters.
Social Segregation of the Coastal Plain and Interior Swamp Sparrows During Migration
Goodness-of-fit tests on the logistic regression models confirmed that Swamp Sparrow subspecies tend to segregate in flocks dominated by one or the other (Table 1, Fig. 5). Model 1, which assumed a single proportion of the Coastal Plain Swamp Sparrow in flocks, regardless of date, showed extreme overdispersion (Table 1). Comparisons with the second model, in which we pooled all flocks by date (removing any trace of subspecific flock segregation), still showed significant overdispersion resulting from the proportion of the Coastal Plain Swamp Sparrow declining as the season progressed (Table 1, row 2). Models 3 and 4, in which date was included as a categorical and then continuous covariate, respectively, continued to show highly significant overdispersion despite controlling for the variation due to date (Table 1), thus indicating that flock membership was not random in regard to subspecies.
Logistic regression analyses of Swamp Sparrow flock composition. The first two models assume a single proportion of the Coastal Plain Swamp Sparrow across all four dates (10, 23 October, 7, 12 November). Model 1 treats flocks separately. Model 2 pools flocks by date. Models 3 and 4 use separate proportions for each date estimated by specifying p as a function of date in the logistic regression. Model 3 treats date as a categorical covariate. Model 4 treats date as a continuous covariate.
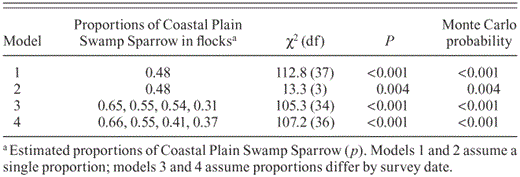
Logistic regression analyses of Swamp Sparrow flock composition. The first two models assume a single proportion of the Coastal Plain Swamp Sparrow across all four dates (10, 23 October, 7, 12 November). Model 1 treats flocks separately. Model 2 pools flocks by date. Models 3 and 4 use separate proportions for each date estimated by specifying p as a function of date in the logistic regression. Model 3 treats date as a categorical covariate. Model 4 treats date as a continuous covariate.

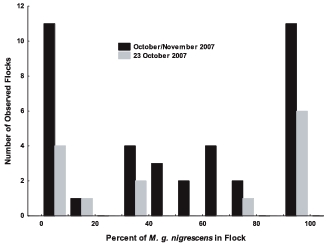
The proportion of the Coastal Plain subspecies in Swamp Sparrow flocks (3–11 birds per flock) during the fall migration of 2007 and for the single date 23 October when the overall abundance ratio of the two subspecies was 50:50.
The above goodness-of-fit tests demonstrate that there is significantly greater variance in the number of the Coastal Plain subspecies in Swamp Sparrow flocks, even after the effects of its seasonal decline in abundance is controlled for, than would be expected if flocks were assembled at random according to their proportions in the environment. In all cases, the Monte Carlo results were very similar to the results of the χ2 goodness-of-fit tests (Table 1). Analysis of flock size further shows that this tendency toward segregation is not due to the subspecies preferring flocks of different sizes. The mean (SD) size of flocks containing at least one Coastal Plain Swamp Sparrow was 5.6 (3.74) birds, whereas the mean size of flocks containing at least one interior Swamp Sparrow was 5.9 (3.69) birds. Thus, flocks of a certain size (within the range of sizes we observed) do not appear to be preferred by either subspecies group.
Discussion
Seasonal Patterns of Abundance
We found no Coastal Plain Swamp Sparrows in the Delaware marsh we surveyed from December through the end of February, supporting the hypothesis that this subspecies migrates from its breeding grounds (Greenberg et al. 2007). Furthermore, the lack of color-banded birds observed during most of the autumn suggests that even when Coastal Plain Swamp Sparrows occur in the nonbreeding season at sites where they breed, the population does not consist of locally breeding individuals. Rather, after the last territorial birds disappear in early October, the post-breeding period appears to be one of movement and flux. Because the breeding range of the Coastal Plain Swamp Sparrow is so limited (sizable breeding populations are found no farther than 80 km from our study site to the northeast and east, Beadell et al. 2003), this fall influx of unmarked birds must represent local or regional movement rather than long-distance migration.
The low number of Coastal Plain Swamp Sparrows observed in the first surveys (19 and 20 September) deserves comment. Swamp Sparrows were unusually unresponsive on this date; three individuals observed at close range were color-banded (local breeders), in visibly heavy molt. It is possible that at this time molting Swamp Sparrows are shyer and more difficult to detect than later in the autumn, as has been reported for other passerines (Haukioja 1971, Swaddle and Witter 1997). Coastal Plain Swamp Sparrows were distinctly more conspicuous at the end of September and beginning of October after the completion of molt, and birds were frequently observed singing at this time. During this period (until approximately 10 October), a number of color-banded adults were observed (10–15% of the total Coastal Plain Swamp Sparrows observed), but the last marked bird was seen alone on 20 October 2007.
The arrival of Coastal Plain Swamp Sparrows in the spring appears to be more synchronous than fall departure and is composed from the beginning primarily of returning color-banded birds. The 49% value for the percentage of sightings involving marked individuals (all color-banded as adults in previous years) from late March to May is very similar to the average return rate of marked adults in the population (50%: Olsen et al. 2008), which suggests that we detected few, if any, passage migrants.
Although the seasonal patterns of both the Coastal Plain and interior Swamp Sparrows in the two years of the study were similar, the former showed a much greater temporal correlation between years. In fact, the similarity between the two years is astonishingly high (r2 = 0.89). All of the observations of the interior subspecies and most of those of the Coastal Plain subspecies were of birds moving in from outside the study area. The Coastal Plain Swamp Sparrows, however, were drawn from a small regional population, whereas the interior Swamp Sparrows (particularly if both subspecies were involved) could have originated from breeding localities across the continent. The predictable timing of numbers the Coastal Plain subspecies on the study site is probably a result of the birds' ability to time their movements more finely to the local climate calendar (mean conditions). It is clear from Fig. 4 that the Coastal Plain Swamp Sparrow's movements are consistently repeated each year and that the timing of departure and arrival coincides closely with the beginning and end of a daily probability of freezing >50%.
Competitive interactions can result in temporal and geographic displacement of various populations or subspecies during migration and winter. The most commonly cited pattern is that of leapfrog migration, in which later-arriving northern-breeding birds migrate to a wintering area south of that of more southern-breeding birds (e.g., Swarth 1920). In the case of Swamp Sparrows in Delaware, the opposite is the case: the more northerly interior-breeding subspecies arrive later, and at least some individuals remain throughout the winter, whereas the locally breeding subspecies migrates south. The phenological pattern of migration, however, does not show any clear pattern of displacement (Fig. 1), as the peak abundance of both subspecies occurs at approximately the same time. Furthermore, the patterns of abundance over time of the two forms are not correlated.
Relative Specialization on Coastal Marshes by Interior and Coastal Plain Swamp Sparrows During the Nonbreeding Season
From this study and surveys in brackish marshes in coastal Virginia and the Carolinas (Greenberg et al. 2007), it appears that interior Swamp Sparrows may be more abundant in coastal marshes during the winter than are Coastal Plain Swamp Sparrows. This observation could call into question the significance of adaptations for tidal-marsh living proposed the latter (Greenberg and Droege 1990, Olsen 2007). Considering the overall global abundance of the interior subspecies, which vastly outnumber the Coastal Plain Swamp Sparrow (Beadell et al. 2003), we can surmise that only a small proportion of the former spend time during the winter in coastal marshes, whereas to date, all wintering Coastal Plain Swamp Sparrows have been found along the coast. Therefore, as a subspecies, the Coastal Plain Swamp Sparrow appears to be more specialized on coastal marshes in the nonbreeding season. Furthermore, our survey data suggest that interior Swamp Sparrows' use of Delaware's coastal marshes fluctuates considerably throughout the winter. There are a number of scenarios that could explain these patterns of use. First, these habitats may be marginal for all interior Swamp Sparrows, and the observed occupation may represent birds that are moving through the habitat continuously. Alternatively, it could be that grass seeds (produced largely after the cessation of breeding) allow Swamp Sparrows of all subspecies to concentrate along the marsh edge during the late fall and early winter, where selection for a tidal-marsh phenotype is reduced. Third, the largest challenges for living in coastal marshes may be the difficulties of breeding successfully, such as significant nest predation, nest flooding, high temperatures, and osmoregulatory challenges to nestlings' growth (Olsen 2007, Olsen et al. 2008). Survival during the nonbreeding period, on the other hand, may require less adaptation to tidal marshes, especially since adults can avoid locally extreme conditions (e.g., salinity and tides) better than nestlings, constrained by nest placement). For example, adults may avoid some of the nestlings' osmoregulatory challenges (Olsen 2007) by drinking fresh water along the marsh edge and in upland habitats. Further studies of the fitness consequences of the Coastal Plain and interior Swamp Sparrows' use of tidal marshes, especially during the nonbreeding periods, could help evaluate these possibilities.
Social Segregation and Subspecies Recognition of the Coastal Plain and Interior Swamp Sparrows
The most surprising result of this study is the strong tendency for the sparrows to form subspecies-specific flocks. This tendency toward social segregation is apparently not simply due to locally breeding birds associating with each other because almost all of the observations involved unmarked birds after the locally breeding birds had departed. The social segregation is only marginally attributable to the small degree of habitat segregation detected in a small area (the observations come from portions of two 10-ha study plots). The result is all the more surprising since Swamp Sparrows commonly associate in loose flocks with other bird species, particularly the Song Sparrow, in the nonbreeding season. Nonbreeding social segregation between such similar and closely related taxa is poorly documented but may indicate behavioral or morphological differences between the taxa that Swamp Sparrows recognize, even in the nonbreeding season.
The morphological divergence of the Coastal Plain Swamp Sparrow is likely the result of selection on traits that are adaptive for life in tidal marshes (Greenberg and Droege 1990), selection that has occurred even, as neutral genetic markers suggest, in the face of recent or continuing genetic exchange with inland populations (Greenberg et al. 1998). Partial or complete isolation from conspecifics in a geographically distinct range or habitat may lead to such divergence but not necessarily to species formation. Not all subspecies become species and, at least under the biological species concept, the evolution of pre-zygotic isolating mechanisms is a necessary step in the process of speciation (Coyne and Orr 2004). Assortative mating is a hallmark of the speciation process, but it generally requires that the animal be able to distinguish (and then prefer not to mate with) individuals from different populations. Such recognition systems may be aided by some degree of sympatry, where reinforcement (defined generally as selection for assortative behavior) is possible if there are fitness consequences for individuals that do not recognize differences among populations (e.g., Sætre et al. 1999, Svedin et al. 2008, reviewed in Randler 2008). Migration often brings members of allopatric breeding populations together during the autumn and winter. While mate choice may not occur during these times of mixing, in species that flock or are otherwise social species-recognition processes may be invoked, and individual fitness may depend on specific flock associations (in terms of nonbreeding survival or the maintenance of condition). Thus, these periods of sympatry outside of the breeding season represent unique opportunities for the reinforcement of species recognition and population preference, processes that could have important consequences for present genetic isolation and future divergence.
Acknowledgments
We thank the Department of Natural Resources of Delaware for allowing access to the study areas. Jessica Hardesty and Greg Shriver assisted in several surveys. Two anonymous reviewers provided insightful comments on earlier versions of the manuscript.
Literature Cited



