-
PDF
- Split View
-
Views
-
Cite
Cite
Erik I. Johnson, Jennifer K. DiMiceli, Philip C. Stouffer, Timing of Migration and Patterns of Winter Settlement by Henslow's Sparrows, The Condor: Ornithological Applications, Volume 111, Issue 4, 1 November 2009, Pages 730–739, https://doi.org/10.1525/cond.2009.080025
Close - Share Icon Share
Abstract.
Fall arrival is an important period in the life history of migratory birds because processes during this period determine where birds spend the winter, which is linked to subsequent survival and condition at the time of spring migration. Henslow's Sparrows (Ammodramus henslowii) winter in savannas of longleaf pine (Pinus palustris), but their secretive behavior limits our knowledge of their winter ecology, including behavioral processes that lead to their documented preference of an ephemeral habitat—recently burned savannas. We expected that upon arrival Henslow's Sparrows actively seek out recently burned savannas and that their over-winter location is dependent on intraspecific interactions during the arrival period. We conducted a 2-year mark—recapture study in southeastern Louisiana longleaf pine savannas to examine these predictions. Bird densities were highest in savannas burned during the previous growing season. There was a large turnover of transient individuals in October and early November, but the proportion of dispersing birds was not related to the number of years since fire or to the bird's age or sex. Early-season movements did not result in skewed age or sex distributions associated with years since fire, suggesting the lack of a class-mediated competitive hierarchy. Birds were then site faithful from late November through spring departure, which began in March and lasted through mid April, with males departing first.
Resumen.
El periodo de llegada en el otoño es importante en la historia de vida de las aves migratorias porque los procesos que ocurren durante este periodo determinan donde las aves van a estar en el invierno, hecho que esta ligado a la supervivencia y condición en la siguiente migración de primavera. Ammodramus henslowi inverna en las sabanas de Pinus palustris, pero su comportamiento sigiloso limita el conocimiento que tenemos sobre su ecología durante el invierno, incluyendo los procesos comportamentales que llevan a su preferencia por un hábitat efímero: las sabanas recién quemadas. Se esperaba que Ammodramus henslowi buscara activamente a su llegada las sabanas recién quemadas y que la ubicación de las areas de invernada dependiera de las interacciones intraespecíficas durante este periodo. Realizamos un estudio de marca-recaptura en las sabanas de Pinus palustris del sudeste de Louisiana para examinar estas predicciones. La densidad de aves fue mayor en las sabanas quemadas durante la temporada de crecimiento previa. Además, hubo un recambio grande de individuos transeúntes en octubre y en el comienzo de noviembre, pero la proporción de aves que se dispersaron no estuvo relacionada con el numéro de años desde el último incendio o con la edad y sexo del individuo. Los movimientos durante la primera parte de la temporada no produjeron distribuciones sesgadas de edad o sexo, hecho que sugiere la falta de jerarquía competitiva entre clases. Finalmente, las aves fueron fieles a sus sitios de invernada desde finales de noviembre hasta su partida en la primavera, la cual comenzó en marzo, duro hasta mediados de abril y en la cual los machos partieron primero.
Introduction
Migratory birds have remarkably complex life histories, and seasonal changes in their ecology and distribution have fascinated ornithologists for many years. For the breeding period, there has been great interest in understanding patterns of arrival, including the timing of arrival by sex and age classes, the establishment of territories, and subsequent consequences (Nyström 1997, Kokko 1999, Morbey and Ydenberg 2001, Tottrup and Thorup 2008). Interest in wintering ecology has been recently fueled by observations that variation in wintering habitat quality can correlate with future breeding success (Marra et al. 1998). There is accumulating evidence that age and sex classes of migratory species segregate either regionally (differential migration; Cristol et al. 1999, Jenkins and Cristol 2002, Stouffer and Dwyer 2003) or locally by habitat; it is often assumed that local age and sex distributions are mediated by competitive interactions (Greenberg 1986, Stutchbury 1994, Catry et al. 2004, but see Brown et al. 2002). As in spring migration, fall migration can be sequential (Mills and Sealy 2005), but generalized patterns and processes of the fall arrival period, a period crucial for understanding the ecology of migratory birds on their wintering grounds, have not been well developed, probably because of a lack of quantitative data.
Although relatively well monitored on its breeding grounds, Henslow's Sparrow (Ammodramus henslowii) is extremely secretive on its wintering grounds, limiting our understanding of its winter ecology. We do know that this species of high conservation concern (Burhans 2002, Rich et al. 2004) is associated with fire management; bird density is highest in pine savannas burned during the previous growing season and declines to near zero 3 years after a fire (Carrie et al. 2002, Tucker and Robinson 2003, Bechtoldt and Stouffer 2005), but no age and sex data are available to relate demographic structuring to habitat quality. Within a winter, the site fidelity of Henslow's Sparrow is high, the birds occupying small (<1 ha) home ranges that overlap slightly with those of neighbors, suggesting that these patches are highly sought after and are at least partially defended (Plentovich et al. 1998, Thatcher 2003, Bechtoldt and Stouffer 2005). Our objective in this study was to use individually marked birds to link fall arrival, which is undescribed for Henslow's Sparrow, with the better-understood pattern of winter habitat selection. In addition, we examine the maintenance of site fidelity through spring departure.
We hypothesized that two factors, habitat selection and social structure, contribute to the over-wintering location of Henslow's Sparrows. First, we expected that upon arrival most Henslow's Sparrows actively seek out recently burned savanna before establishing a small winter home range. Second, we predicted that if a social dominance hierarchy were present, the proportion of the dominant age or sex class (e.g., adult males) would vary by habitat through time and the subordinate class would move more (Marra 2000). Alternatively, if a social dominance hierarchy were not present, we predicted that upon arrival all age and sex classes would move at similar rates and that age and sex ratios would not differ by habitat or through time. We tested these hypotheses by examining movements of birds of known age and sex from fall arrival through spring departure in savannas of longleaf pine (Pinus palustris) in southeastern Louisiana.
Methods
Study Area
We conducted this study at four conservation areas for longleaf pine savanna in St. Tammany and Tangipahoa parishes in southeastern Louisiana. These include Camp Whispering Pines (Girl Scouts of America; 30° 40′ N, 90° 28′ W), Sandy Hollow Wildlife Management Area (WMA; Louisiana Department of Wildlife and Fisheries [LDWF]; 30° 50′ N, 90° 25′ W), Abita Creek Flat woods Preserve (The Nature Conservancy [TNC]; 30° 31′ N, 89° 58′ W), and Lake Ramsay WMA (TNC and LDWF; 30° 32′ N, 90° 10′ W). Sites had a sparse overstory of longleaf pine, with scattered loblolly pines (Pinus taeda) intermixed. A species-rich community of grasses (Poaceae) and sedges (Cyperaceae) with scattered forbs constituted the ground cover. Detailed analyses of habitat structure and plant species composition for these sites are available in Bechtoldt and Stouffer (2005) and Johnson (2006).
The sites were managed on a 2-year fire rotation, with portions of each conservation area burned between 12 March and 1 June during our study. Following Bechtoldt and Stouffer (2005), we defined study plots as year 0 (burned <1 year earlier) and year 1 (burned >1 year earlier). At the onset of our study all study plots had at least 10 years of continuous fire management. At our study sites fire-management regimes are broadly similar to the within- and between-year patterns of historic fire regimes in the region (Platt 1999, Huffman 2006) and were applied to restore and maintain biodiversity and natural processes of this ecosystem.
Sparrow Sampling
We sampled Henslow's Sparrows at ten 2.25-ha study plots during two consecutive winters from 2003 to 2005. One plot was at Camp Whispering Pines, two at Sandy Hollow WMA, two at Abita Creek Flatwoods Preserve, and five at Lake Ramsay WMA. Plots were separated by at least 250 m; all plots were in different fire-management units and were always separated by a depression forested with hardwoods or a road. Therefore, home ranges of individual birds did not overlap multiple study plots.
Henslow's Sparrows were surveyed by being flush-netted in survey plots as described by Bechtoldt and Stouffer (2005). This procedure involved a line of five to ten people walking about 3 m apart to systematically cover the plot and flush secretive ground-dwelling birds out of the tall grasses. When a Henslow's Sparrow was flushed, we quickly surrounded where the bird landed and chased it into a 6-m mist net. If a bird was not captured, we noted where it was last seen to minimize double counting. Because we actively corralled birds into nets, our sampling should be free from heterogeneity in trapping success and detection probability due to variation in habitat, behavior, age, and sex (Tucker and Robinson 2003), unlike passive mist netting and other common sampling techniques. Even so, we tested for these possible biases (see Methods: Analysis). We captured 68% (n = 614) of the birds positively identified as Henslow's Sparrows, and variation in capture rate was not affected by years since fire or sampling period (unpubl. data). Swamp Sparrows (Melospiza georgiana) and Sedge Wrens (Cistothorus platensis) were also commonly encountered; they were easily identified by their shape, flight behavior, and call notes when plumage details were not apparent. Le Conte's Sparrows (Ammodramus leconteii) and Grasshopper Sparrows (A. savannarum) presented the greatest identification problem but could be identified in flight by their overall pale color. We were initially interested in these species, but because of their low densities (together they represented only 4% [n = 432] of all captures), we were unable to analyze these data effectively, and we expect any possible issues resulting from misidentifications to be insignificant. We report Henslow's Sparrow density as the number of identified birds per hectare, recognizing that this is a relative measure based on our sampling technique.
We sampled each plot seven times in 2003–04 and five times in 2004–05 between 1 November and 15 March. We also conducted additional weekly or biweekly flush-netting in October and from late March through April for a subset of three or four plots, except in October 2003, when one plot was sampled once. Because sampling effort in the two years differed and because the exact timing of migration can vary from year to year (Butler 2003, Marra et al. 2005), we present data for each year separately.
Captured Henslow's Sparrows were banded with a uniquely numbered aluminum U.S. Fish and Wildlife Service band. We aged birds according to plumage characteristics and skull pneumatization (Pyle 1997). For simplicity we refer to age classes as “first winter,” “adult,” or “unknown.” Locations from which captured birds were flushed were marked with a Global Positioning System (GPS); these locations were used to determine whether the bird was captured in the central 1 ha of the plot or in the peripheral 1.25 ha (see Methods: Analysis). Because the sex of Henslow's Sparrows cannot be determined in winter from external characters (Pyle 1997), we either collected blood from the brachial vein or collected a single tail feather for genetic sex determination. We isolated DNA from whole blood (Sambrook and Russell 2001) or from a feather calamus (Qiagen DNeasy Blood and Tissue Kit, Valencia, CA). Isolated DNA was amplified with CHD-P2 (Griffiths et al. 1996) and CHD-P8 primers (Griffiths et al. 1998) and the polymerase chain reaction according to Long and Stouffer (2003). Ultimately, this technique determined the presence or absence of the female-specific W chromosome (Griffiths et al. 1998).
Analysis
We used two approaches to determine the relative prevalence of bird movements. First, we calculated the proportion of birds in each sampling period that were not captured during any other sampling period. A sampling period in which most individuals were not captured during other sampling periods would suggest substantial movement beyond the scale of the plots. In this analysis we include only the subset of individuals marked at least once within the 1-ha core area centered within the 2.25-ha plot, thus avoiding confusion between permanent and temporary emigration of birds captured on the plot's edge.
Second, we considered results from a mark—recapture analysis that compared model-averaged estimates of apparent survival over the winter to the fall arrival period. Because previous work has shown that Henslow's Sparrows are site faithful during the over-winter period (Bechtoldt 2002, Thatcher et al. 2006; see also Results) we assumed that apparent survival estimates from the over-winter period were unbiased by emigration and therefore reflect actual survival probabilities. We then compare these “real” survival estimates to those generated for the fall arrival period to examine the relative importance of movements, assuming mortality between the arrival and over-winter periods is relatively constant (see Discussion). For this analysis, we used capture data from 1 November to 15 March and excluded data from October, late March, and April because their inclusion would create incomplete capture histories; this dataset includes 92% (n = 415) of all Henslow's Sparrow captures during the study. We analyzed each year separately because the number of occasions of sampling differed.
Using a Cormack—Jolly—Seber (CJS) model (Lebreton et al. 1992), we analyzed the encounter histories of captured birds with the “sin link” function in the program MARK (White and Burnham 1999). The global model considered the effects of years since fire (g: year 0 and year 1), time (t: sampling periods), and their interaction on apparent survival (φ) and recapture (p) probabilities ( Appendix 1). We modeled separate parameters for birds occupying the 1-ha core of sampling plots and for birds along plot edges. Birds captured within the 1-ha plot core would be expected to have home ranges mostly or completely within the 2.25-ha plot (Bechtoldt 2002, Thatcher et al. 2006). Birds captured outside this core likely used areas beyond the plot, which could be considered as temporary emigration (Crespin et al. 2008). We used the program U-CARE to assess the goodness of fit (GOF) of the global model, to estimate ĉ, a measure of over- or underdispersion in our data, and to test for capture heterogeneity among groups (Choquet et al. 2005). We then created 15 additional reduced-parameter models by considering all combinations of time-dependent and time-independent effects of years since fire on φ and p. The 16 candidate models were ranked according to lowest Akaike's information criterion (AIC) adjusted for ĉ and small sample size (QAICc). We calculated weights for each model by taking the likelihood of that model and dividing it by the sum of all models' likelihoods. We considered models with ΔQAICc < 2 to be equally parsimonious but those with ΔQAICc > 4 to have considerably less support (Burnham and Anderson 2002).
Before we addressed variation in bird density due to habitat or season, we first considered the problem of detection probability. We did this by using the mark-recapture approach described above and examined recapture probabilities and heterogeneity in capture success. We found that the CJS assumption of equal capture heterogeneity among groups was not violated (see Results). Therefore, for each sampling year separately, we analyzed the effect of years since fire on log-transformed observed bird densities by using a mixed-model analysis of variance (ANOVA) with repeated measures (i.e., sampling period) and plot as random effects. We chose a variance—covariance structure for this model by ranking eight possible variance—covariance structures in an information-theoretic approach (Littell et al. 2000); the top-ranking model assumed a structure of compound symmetry covariance (unpubl. data), which we used in the repeated-measures ANOVA. We report untransformed least-square means ± SE and set statistical significance at α = 0.05. We used a Tukey—Kramer test for post hoc pairwise comparisons of bird density by sampling period. Because only a subset of plots was sampled during October, late March, and April, bird densities during these periods were not compared statistically to bird densities between 1 November and 15 March.
After examining patterns of movements and changes in density from fall arrival through spring departure, we determined the dates of three periods: arrival, over-winter, and departure. We created contingency tables of age and sex classes by period with each cell representing the number of captured individuals. Age and sex ratios of birds during each period were tested for their statistical deviance from 50:50 by means of the binomial probability function. We hypothesized that during the arrival period age and sex ratios in the two categories of time since fire would not differ, but if individuals redistribute themselves between the fire categories as a result of class-mediated social interactions, skewed age and sex ratios would be apparent in the over-winter period (Marra 2000). We tested this by using Pearson chi-squared tests. All statistics were calculated with SAS 9.1.3 (SAS Institute 2003).
Results
In total, we identified 614 Henslow's Sparrows and captured 247 individuals 415 times. Of these, we determined the age of 86% and the sex of 97%.
Spatial and Temporal Patterns in Bird Density
The global mark—recapture model fit CJS assumptions (GOF2003–04: χ2= 13.3, df= 19, P = 0.82; GOF2004–05: χ2 = 5.1, df = 12, P = 0.96); we adjusted the models' rankings because of evidence of underdispersion in our data (ĉ2003–04 = 0.70; ĉ2004–05 = 0.42). Models with ΔQAICc < 4.0 with a combined model weight of 0.75 in 2003–04 and ΔQAICc < 3.0 with a combined model weight of 0.72 in 2004–05 did not detect variation in p due to season or years since fire. In addition, we did not violate the assumption of capture heterogeneity among groups, so we report unadjusted observed relative bird densities. We recognize that density may be biased low because < 1.0 (model-averaged pcore2003–04 = 0.34 ± 0.04, pedge2003–04 = 0.10 ± 0.02, pcore2004–05 = 0.55 ± 0.14, pedge2003–04 = 0.11 ± 0.05). Our calculation of bird density partially accommodates p < 1.0 by including birds that were positively identified but not captured.
We observed differences in Henslow's Sparrow densities resulting from the effects of years since fire (Fig. 1). Mean densities in year-0 savannas were higher than in year-1 savannas, significantly so in 2004–05 (2003–04: F1,8 = 1.5, P = 0.26; 2004–05: F1,8 = 15.1, P = 0.01). There was no interaction between years since fire and sampling period (2003–04: F6,47 = 1.5, P = 0.20; 2004–05: F4,32 = 1.6, P = 0.21).
We found temporal differences in relative bird density (Fig. 1). The first birds arrived in October, and densities continued to increase through November and into December. Our only October sample in 2003 was on the 23rd, at which time several Henslow's Sparrows were already present (Fig. 1a). In 2004, we began sampling on 2 October but did not detect the first Henslow's Sparrow until 17 October (Fig. 1b). Bird densities then increased dramatically, peaking on 6 December in 2003 and 15 December in 2004, followed by a gradual decline through March. Pairwise comparisons of bird densities differed significantly only between December and March in 2003–04 (2003–04: F6,47 = 2.5, P = 0.04; 2004–05: F4,32 = 1.6, P = 0.21); all other pairwise comparisons for densities estimated between 1 November and 15 March were not significant (P < 0.05). Bird densities remained >1 ha-1 at the beginning of April, but all Henslow's Sparrows had departed by 24 April.
Bird Movements and Demographic Patterns
For sampling periods after mid-November, 70% or more of individual birds captured during any given sampling period were recaptured during another sampling period (Fig. 2). In contrast, none of the birds captured in October and 40% of the birds captured in early November were recaptured later in the winter, suggesting they were transients. This pattern of transience during arrival and residency during winter was reflected in apparent mortality estimates; the top-ranking models (i.e., ΔQAIC < 4.0) included variation in φ over time ( Appendix 1), suggesting a temporal change in survival, movements, or both. In early to mid November, apparent mortality was estimated as 76 ± 12% in 2003 and 48 ± 8% in 2004, vastly higher than the 9 ± 6% and 8 ± 4% from late November to 15 March for 2003–04 and 2004–05, respectively. These large differences in apparent survival over <1 month must be largely or entirely driven by emigration from study plots (see also Discussion).
The presence of transients in October and early November was not related to years since fire because 59 % (n = 22) of birds disappeared from year-0 savannas and 67% (n = 9) disappeared from year-1 savannas (χ21 = 0.1, P = 0.76). Our small sample suggests that transience was not related to age or sex: 56%) (n = 9) of first-winter birds and 50% (n = 4) of adults disappeared (χ21 = 0.4, P = 0.55); 50% (n = 5) of males and 56% (n = 18) of females disappeared (χ21 = 0.1, P = 0.78).
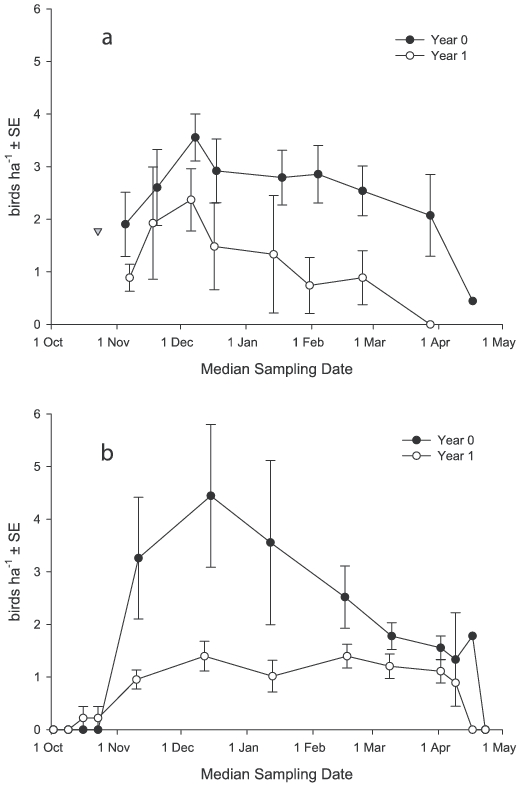
Relative abundance of Henslow's Sparrow in southeastern Louisiana during 2003–04 (a) and 2004–05 (b). The triangle in la represents a 2.25-ha year-0 plot that was never sampled again.
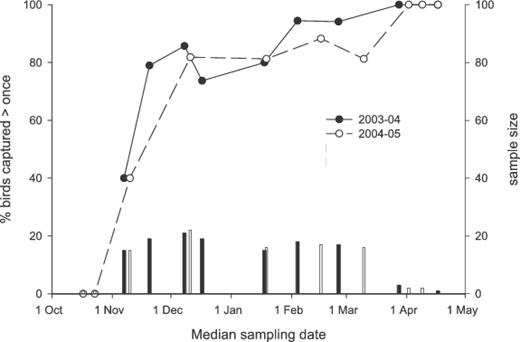
Percentage of Henslow's Sparrows captured in each sampling period that were recaptured during another sampling period (previous or successive) in southeastern Louisiana, 2003–05 (lines, left-hand axis). The sample size for each sampling period is represented by vertical bars and the right-hand axis.
During the arrival, over-winter, and departure periods the age ratio was significantly skewed toward first-winter birds (binomial probability function, arrival : p=0.006 [n=28]; over-winter: P < 0.001 [n = 192]; departure: P = 0.002 [n = 19]; Table 1). The age ratio in year-0 and year-1 savannas during the arrival and over-winter periods did not differ (arrival: χ21 = 1.6, P = 0.21; over-winter: χ21= 0.1, P = 0.73). We could not test differences in age ratios as a result of years since fire during the departure period because of low sample sizes.
Sex ratios were not skewed during the arrival or over-winter periods but were biased toward females during the departure period (binomial probability function, arrival: P = 0.21 [n = 64]; over-winter: P = 0.64 [n = 205]; departure: P = 0.004 [n = 18]; Table 1). During the arrival and over-winter periods, sex ratios in year-0 savannas did not differ from sex ratios in year-1 savannas (arrival: χ21= 1.2, P = 0.27; over-winter: χ21 = 0.9, P = 0.33; Table 1). We could not test differences in sex ratios as a result of years since fire during the departure period because of low sample sizes.
Discussion
Patterns of Arrival on Wintering Grounds
Like many passerines that over-winter in southern Louisiana, Henslow's Sparrows begin to arrive in October, following the first cold fronts to reach the gulf coast (Able 1972, Lowery 1974). In 2004 we began intensive surveys for Henslow's Sparrows on 1 October, but the first birds were not found until 17 October. The majority of Henslow's Sparrows arrived during a 1- to 2-week period in the first half of November. We do not know when the first birds arrived in 2003, but density again increased through November. In both years density peaked in early to mid December.
Henslow's Sparrow has been described as having high site fidelity within a winter and maintaining a small (<1 ha) home range (Plentovich et al. 1998, Thatcher 2003, Bechtoldt and Stouffer 2005), but we show that many individuals do not become established in winter territories until mid to late November. There was substantial turnover of individuals at our study sites in October and early November. Because our study sites lie along the southern edge of the species' wintering range (Herkert et al. 2002), we doubt that these movements in October and November represent birds still in passage to more distant wintering grounds. Rather, we suggest that they indicate local post-migration movements.
Percent (and number) of Henslow's Sparrows captured according to age and sex during arrival, over-winter, and departure periods in southeastern Louisiana, 2003–2005. See Results for statistical comparisons.
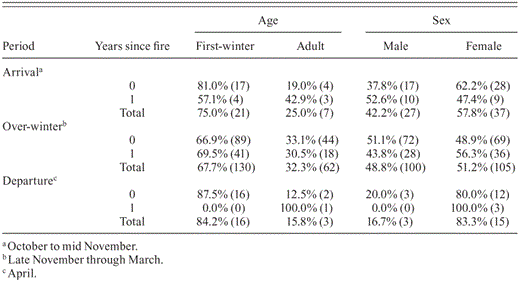
Percent (and number) of Henslow's Sparrows captured according to age and sex during arrival, over-winter, and departure periods in southeastern Louisiana, 2003–2005. See Results for statistical comparisons.

Post-Migration Movements
The mark—recapture analysis examined the rate of turnover of individuals and suggested that many birds emigrate from study plots in early November but not during the rest of the winter. Mortality could yield a similar pattern, but it would have to be at a rate 5–10 times higher than our estimate from later in the winter to account for the difference. We do not expect relatively high mortality during the arrival period to be a satisfactory explanation of the turnover for several reasons. First, although unfamiliarity with a site may increase mortality risk (Metzgar 1967, Yoder et al. 2004), we suspect this factor is minimal because Henslow's Sparrows are extremely wary, and their escape mechanism is to flush, fly a short distance, and take cover in dense grass or shrubs, which are plentiful and easy to locate in these open savannas. Second, seed resources are at their peak as Henslow's Sparrows are arriving (Johnson 2006), buffering some effects of site unfamiliarity. Finally, the abrupt change in apparent mortality between early and late November is temporally associated with a rapidly increasing population density. Therefore, the most parsimonious explanation is that the turnover in October and November is due to transients. Very rarely did we capture a bird in a plot other than that in which it was marked, so movements were difficult to identify empirically; however, we did recapture one bird that moved 800 m between November and February from a year-0 to year-1 savanna, providing supporting anecdotal evidence for post-migration movement.
Henslow's Sparrows disperse from year-0 and year-1 savannas at similar rates. Unfortunately, we do not know the origin or fate of transients or the spatial scale at which post-migration movements occur, except that movements are not influenced by age, sex, or years since fire. Recent evidence has shown that space use by Henslow's Sparrows is not homogeneous, suggesting that even within fire regimes there is variation in habitat suitability, possibly due to a site's history, edaphic properties, and local variation in fire intensity (Champlin 2007, Palasz 2008).
To understand the strategy behind post-migration movements and how they might lead to the observed pattern of higher bird densities in recently burned savannas, we examined spatial and temporal differences in age and sex ratios. These ratios did not differ between year-0 and year-1 savannas during the arrival or over-winter periods despite a reorganization of individuals among habitats. Therefore, the costs associated with occupying low-quality patches of savannas, rather than social interactions, probably induce post-migration movements. Like Henslow's Sparrow, the American Redstart (Setophaga ruticilla) also has age and sex ratios unbiased by habitat quality early in winter; however, in the case of the redstart, by mid-winter competitive social interactions lead to age and sex ratios differing by habitat (Marra 2000). Like several other monomorphic species (Brown et al. 2002, Brown and Sherry 2006) but unlike some sexually dimorphic species (e.g., Lynch et al. 1985, Parrish and Sherry 1994, Wunderle 1995, Latta and Faaborg 2002) Henslow's Sparrows appear to lack this class-mediated dominance hierarchy during the winter. A female can enhance her status during social encounters by concealing her sex (Langmore and Bennett 1999), suggesting that plumage polymorphism may be a trait useful for predicting the importance of winter class-mediated hierarchies (Saetre and Slagsvold 1996, Rainey and Grether 2007).
Spring Departure
After bird density peaks in December, it steadily declines until all birds have departed northward by late April. The rate of decreasing bird density through the winter is consistent with estimates of Henslow's Sparrow's winter mortality (Thatcher et al. 2006), which makes it difficult to distinguish mortality from the onset of spring migration. Apparently there is no increase in local movements in late winter, as we did not see an influx of unbanded birds. It is likely that the first Henslow's Sparrows begin departing in March, leading to skewed sex ratios by April due to earlier departure by males. This pattern of males migrating earlier than females has been observed in many migratory species (Cristol et al. 1999). Our last observations of Henslow's Sparrows were of four first-winter females on 17 April. These same plots were vacant on 24 April, although one unaged female was captured outside the plots on this date. This represents the latest date on which the species has been documented in Louisiana; a 15 May 1919 observation in Lowery (1974) is unsubstantiated (J. V Remsen, unpubl. data). These results provide an additional rationale for delaying burning until at least April, after most Henslow's Sparrows have departed the wintering grounds.
Detection Probability
We recognize that our density estimates are not measures of true density but instead reflect relative differences in density between habitats and through time. Because our data were already somewhat underdispersed (although they fit the simple assumptions of the CJS model well), they were problematic to use in more complex models that incorporated capture probability with density. Even so, our sampling technique of flush-netting should eliminate many of the issues related to detection probability. By actively pursuing birds into nets, we not only standardized their behavior (i.e., escape) but also reduced capture heterogeneity due to age, sex, habitat, and season (Tucker and Robinson 2003). This assumption is at least partially supported by two observations. First, although not all birds were captured, the proportion of birds captured was unaffected by years since fire and season (unpubl. data). Second, recapture probabilities were unaffected by habitat or time, and we did not detect capture heterogeneity among groups, at least during the period of site fidelity.
Conclusion
It is important to note that our data describe post-migration movements in relatively high-quality savannas (i.e., long-term management with fire and only one or two growing seasons since fire). Although settlement patterns may be quite different in suboptimal habitats, the plots we sampled probably represent the types of habitats used by the majority of wintering Henslow's Sparrows (Bechtoldt and Stouffer 2005). It would be difficult to test explicitly for transients in Henslow's Sparrow during the brief arrival period with a mark-recapture approach because of the logistics involved in flush-netting, but with multiple sampling periods during the movement period one might quantify transients by means of time-since-marking models (Pradel et al. 1997). With radio-telemetry, future studies could also focus on comparing the scale of post-migration movements in large areas of suitable habitat with that in smaller patches, such as edaphic prairies or pitcher-plant bogs (Tucker and Robinson 2003, Holimon et al. 2008).
Acknowledgments
Funding was provided by the Louisiana Department of Wildlife and Fisheries, J. Bennett Johnston Science Foundation, and the Louisiana Office of Environmental Education. We thank the Nature Conservancy, Louisiana Department of Wildlife and Fisheries, and the Girl Scouts of America for allowing access to their properties and offering logistical support. We also thank L. Duda for her help at the onset of this study and F. Benham, H. Conkerton, and R. Villani for help in the field. M. Stine kindly allowed us to use his laboratory to sex birds, and M. Bowen was essential to this process. B. Sandercock and E. Janney provided valuable guidance in the mark-recapture analysis. We offer a special thanks to the >100 volunteers who were the core of our bird flushing teams, especially K. Hackman and his students from Madison Central High School, Madison, Mississippi. Comments provided by M. Chamberlain, D. Fox, D. Johnson, R. Steidl, M. Patten, and two anonymous reviewers improved the quality of this manuscript. G. Bravo kindly provided the Spanish translation of the abstract. Capturing, banding, and collecting blood were conducted with approval of the Louisiana State University Institutional Animal Care and Use Committee, (permit AEO314), Louisiana Department of Wildlife and Fisheries,(LNHP-05-059), and U.S. Fish and Wildlife Service (MB0959180 and 22648). The manuscript was approved for publication by the director of the Louisiana Agricultural Center Experiment Station as number 2009-241-3722.
Literature Cited
Appendix 1
Model rankings for 16 candidate models that estimated apparent survival (φ) and recapture probability (p) parameters for Henslow's Sparrows in southeastern Louisiana in 2003–04 and 2004–05. Symbols follow White and Burnham (1999); (.) indicates that neither fire nor time showed variation in parameter estimates; (g) indicates that parameters were different by number of years since fire; (t) indicates that parameters differed through time. Bold-faced models are those with QAICc < 3.0.
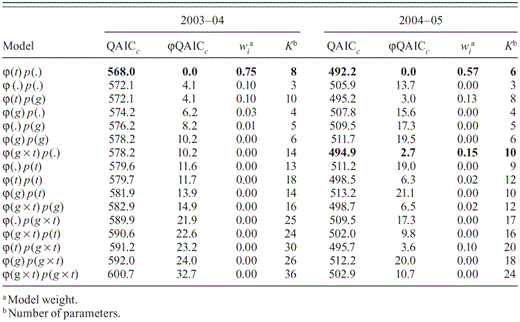
Model rankings for 16 candidate models that estimated apparent survival (φ) and recapture probability (p) parameters for Henslow's Sparrows in southeastern Louisiana in 2003–04 and 2004–05. Symbols follow White and Burnham (1999); (.) indicates that neither fire nor time showed variation in parameter estimates; (g) indicates that parameters were different by number of years since fire; (t) indicates that parameters differed through time. Bold-faced models are those with QAICc < 3.0.




