-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher E. Filardi, Sievert Rohwer, Life History Implications of Complete and Incomplete Primary Molts in Pelagic Cormorants, The Condor: Ornithological Applications, Volume 103, Issue 3, 1 August 2001, Pages 555–569, https://doi.org/10.1093/condor/103.3.555
Close - Share Icon Share
Abstract
We describe the rules of primary flight-feather replacement for Pelagic Cormorants (Phalacrocorax pelagicus), and contrast the completeness of primary replacement in individuals from Asia and North America. In adult Pelagic Cormorants primary replacement is stepwise, with multiple waves of molt, each initiated at the innermost primary (P1), proceeding simultaneously toward the tip of the wing. Shugart and Rohwer's (1996) ontogenetic model for generating and maintaining stepwise primary replacement depended upon incomplete molts. In each new episode of molt, waves of primary replacement were thought to be initiated at P1 and at each arrested wave that had failed to replace all old feathers in the preceding molt. Because most adult Pelagic Cormorants from North America completely replace their primaries but maintain stepwise primary molts, the latter assumption must be relaxed. In contrast to the present-day situation in North America, Pelagic Cormorants from northeastern Asia have incomplete molts of their primaries, and may be forced to skip breeding in some years to clear their wings of overworn primaries. Young birds from Asia start the replacement of their juvenile primaries later than North American birds and replace more feathers simultaneously.
Implicancias de la Muda Primaria Completa e Incompleta en la Historia de Vida de Phalacrocorax pelagicus
Resumen. Describimos las reglas de reemplazo de plumas primarias para Phalacrocorax pelagicus y contrastamos la totalización del reemplazo de primarias entre individuos de Asia y América del Norte. En individuos adultos, el reemplazo de primarias ocurre en varias etapas, con múltiples secuencias de muda cada una iniciada en la primaria más interna (P1), procediendo simultáneamente hacia la punta del ala. El modelo ontogenético de Shugart y Rohwer (1996) para la generación y mantenimiento del reemplazo en etapas de las plumas primarias depende de mudas incompletas. Se pensaba que en cada nuevo episodio de muda las secuencias de reemplazo de primarias eran iniciadas en P1 y en cada punto de interrupción de la muda precedente que hubiera impedido el reemplazo de todas las plumas viejas. Debido a que la mayoría de los individuos adultos de P. pelagicus de Norteamérica reeemplazan completamente sus primarias pero aún lo hacen en etapas, la última suposición debe ser re-evaluada. En contraste con la situación actual en Norteamérica, individuos del noreste de Asia tienen mudas incompletas de sus primarias y pueden verse forzados a no reproducirse en algunos años para despojarse de la presencia de primarias desgastadas. Las aves juveniles de Asia comienzan el reemplazo de sus primarias más tarde y reemplazan más plumas simultáneamente que las aves de Norteamérica.
Introduction
Molt and breeding are critical, time-consuming elements in the annual cycles of large birds. In some large birds, such as seabirds, waterfowl, and raptors, molt and breeding seldom co-occur, and time allocated to one of these activities may constrain the other (Pietiäinen et al. 1984, Langston and Rohwer 1996). Similarly, for many passerine species the scheduling of molt may have major effects on future reproduction and survival (Jenni and Winkler 1994, Nilsson and Svensson 1996, Voelker and Rohwer 1998). For large birds that maintain the ability to fly while molting, the duration of primary replacement is a useful index of the time constraint that molt may place on other activities in the annual cycle. This time demand is a function of the growth rate of individual primaries. Surprisingly, the rate at which primaries grow is remarkably constant across all groups of birds, despite differences in wing size that span several orders of magnitude (Rohwer 1999). Published primary growth rates range from means of 3.2 mm day−1 for small passerines to only 6.3 mm day−1 for large birds such as albatrosses and condors, whose wings may have five to ten times more total primary length than small birds (Prevost 1983, Rohwer 1999). For this reason, large birds require much more time than small birds to replace all of their primaries (Prevost 1983, Edelstam 1984, Langston and Rohwer 1996).
Large birds show three evolutionary solutions to the long time required for primary replacement. First, molt and breeding can overlap, thereby extending the time available for molting in large birds (Thompson and Slack 1983, Monteiro and Furness 1996). In small birds, overlapping molt and breeding also extends the time available for breeding (Foster 1974, Svensson and Nilsson 1997, Hemborg 1999). Second, the simultaneous molt of flight feathers reduces the time needed to replace these feathers to the time required to grow the longest primary. Waterfowl, grebes, loons, and some alcids can forage and avoid predation without flying, and become flightless by simultaneously losing and regrowing all of their flight feathers. Finally, many large birds that cannot efficiently forage and avoid predation without the ability to fly simultaneously replace flight feathers at multiple points in the wing. In such cases, these molt foci are staggered across the wing, creating relatively small gaps between feathers (compared to larger gaps if foci were adjacent to one another), presumably to minimize loss of flight performance (Ashmole 1968, Rohwer 1999). When molts fail to replace all primaries annually the resulting accumulation of worn primaries decreases aerodynamic efficiency (Langston and Rohwer 1996, Shugart and Rohwer 1996).
Although most small birds replace their primaries in a single wave of molt, many large birds that fly while molting replace their primaries in one of two more complex patterns. First, at least five groups of birds split their primaries into two separate molt series. In some albatrosses (Langston and Rohwer 1996), many parrots (Forshaw and Cooper 1989), some owls (Forsman 1981, Pietiäinen et al. 1984, Hörnfeldt et al. 1988), some kingfishers (Hanmer 1980), and apparently all falcons (Stresemann and Stresemann 1960, 1966), a group of inner primaries is molted separately, and, in some cases, in a different direction from the outer primaries. An advantage of dividing the primaries into two groups of feathers that can be molted independently of one another is that the outer primaries, which receive extreme wear, can be replaced more frequently than the inner primaries (Langston and Rohwer 1996). Second, many large birds replace their primaries in two or more waves of molt that proceed distally through the wing, a pattern known as “stepwise” or “serial-descendent” molt (Ashmole 1963, 1968, Stresemann and Stresemann 1966). In this case, the primaries constitute a single molt series because waves that start at the innermost primary (P1) may arrest and later restart at any primary position (Shugart and Rohwer 1996, Rohwer 1999). Large birds with stepwise primary molts typically do not molt throughout the nonbreeding season, and most fail to replace all of their primaries each year (Dorward 1962, Edelstam 1984, Shugart and Rohwer 1996). These incomplete molts, separated by arrests in feather replacement, are thought to be the developmental source of multiple waves of growing feathers (Shugart and Rohwer 1996). However, many cormorants (Phalacrocoracidae) can molt throughout the nonbreeding season and sometimes have complete molts as adults (Turbott 1956, Berry 1976, Cooper 1985), yet maintain multiple waves of primary replacement (Potts 1971, Rasmussen 1987, 1988).
This raises two basic questions. First, how do cormorants maintain stepwise molt patterns without incomplete molts? This is a problem because the only model for the development of stepwise primary molts relies upon incomplete molts (Rasmussen 1988, Shugart and Rohwer 1996). Second, why do cormorants maintain such complex molt patterns if they can molt throughout much of the year? In this paper, we analyze primary flight-feather molt in a large series of Pelagic Cormorant (Phalacrocorax pelagicus) specimens from the north Pacific coasts of Asia and North America, and present further evidence suggesting that stepwise primary molt can be maintained in the absence of incomplete molts.
Differing winter conditions on the two sides of the Pacific present an opportunity to compare stepwise primary molts in populations of cormorants with contrasting life-history regimes. These comparisons enable us to begin interpreting the adaptive significance of stepwise molt in Pelagic Cormorants.
Natural History of Pelagic Cormorants
Pelagic Cormorants are predominantly benthic foragers that breed along the Asian and North American shores of the North Pacific Ocean. On the western coastlines of the Pacific, populations breed from northern Chucotka, south through Kamchatka and the Kuril Islands, to northern Japan. On eastern coastlines, breeding occurs from northern Alaska south to central Baja California. Within the breeding range, loose colonies are distributed throughout inner and outer coastal areas that provide suitable cliffs, caves, or islets. In North American populations seasonal movements between breeding and nonbreeding areas are poorly described but, where known, are generally short-distance and facultative except north of the Bering Strait (Palmer 1962, Campbell et al. 1990, Siegel-Causey and Litvinenko 1993, Hobson 1997). In contrast, populations in northern Asia migrate between northern breeding areas and southern wintering areas in response to freezing seawater (Dement'ev et al. 1966, Siegel-Causey and Litvinenko 1993). Egg dates for the coasts of Alaska and British Columbia range from 25 April to 30 August with 52% recorded between 19 June and 8 July (Campbell et al. 1990). Fledging dates for the same area range from 18 June to 20 October with 51% recorded between 15 July and 13 August (Campbell et al. 1990). Most birds first breed during their third independent summer, although some individuals have been documented breeding during the second (Palmer 1962). Both breeding and nonbreeding birds move to and from the breeding and wintering areas throughout life despite delayed breeding in this species (Palmer 1962, Dement'ev et al. 1966, Campbell et al. 1990).
Methods
We examined 180 Pelagic Cormorant specimens from the west coast of North America, and 62 specimens from the Pacific coast of Asia. A majority (152) of the North American specimens that we examined were salvaged as casualties of the Exxon Valdez oil spill in Prince William Sound, Alaska (24 March 1989). Because these specimens were salvaged between March and August, they provide only limited information about annual molt cycles. Therefore we supplemented this series with Pelagic Cormorants collected from Puget Sound, Washington, from December 1996 through May 1997, and additional specimens borrowed from museums (see Acknowledgments). Most of the modern specimens (e.g., Exxon Valdez casualties) were preserved with one wing fully extended, facilitating detailed molt work.
Ageing Birds
Use of museum specimens to study the ontogeny and timing of feather replacement depends upon ageing birds correctly. Birds were aged using a combination of collection date, body plumage, flight feather retention, soft-part colors, and bursa measurements. The bursa of Fabricius is a fleshy dorsal diverticulum of the cloaca that has an immunological function in young birds and is resorbed as sex steroids become active (Glick 1983, Broughton 1994). When age could not be determined otherwise, we used the presence, size, and character of the bursa as evidence of pre-breeding status. All birds were placed into one of four age classes that assign birds to calendar years after birth (Table 1).
Criteria used to assign Pelagic Cormorants to age classes and molt episodes. Molt episodes are defined as first, second, and adult (third and subsequent) as described in the text. The arrest in molt indicated for SY birds during May–August is followed by a (?) because it is unclear whether all birds actually arrest their molts around the breeding season during juvenile feather replacement
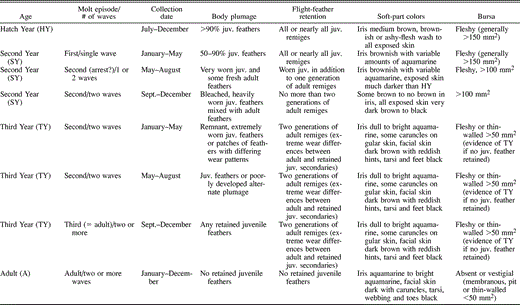
Criteria used to assign Pelagic Cormorants to age classes and molt episodes. Molt episodes are defined as first, second, and adult (third and subsequent) as described in the text. The arrest in molt indicated for SY birds during May–August is followed by a (?) because it is unclear whether all birds actually arrest their molts around the breeding season during juvenile feather replacement

Episodes of Molt
Following Shugart and Rohwer (1996), we use the informal “episode of primary molt” to refer to periods when birds are molting primaries. We depart from the terminology of Humphrey and Parkes (1959) because, unlike many species, the primary molts of Pelagic Cormorants overlap both prebasic and prealternate molts of body plumage, confounding the usefulness of this distinction in dividing molting birds into chronological groupings. Furthermore, the rules of primary replacement and the tradeoffs between flight-feather molt and breeding do not require identifying these molts as prebasic or prealternate. The summer breeding season of Pelagic Cormorants (approximately May through August) appears to be the only time that North American birds are not molting (Palmer 1962, see Results). Therefore, Pelagic Cormorants can be categorized by long episodes of molt that correspond to the number of summers an individual has lived through.
Based upon our ageing criteria and patterns of feather replacement, we divided specimens into first, second, and adult (third and subsequent) episodes of primary molt (see Table 1). The first episode of molt almost always involves “second-year” birds undergoing their first primary molt. (First-year birds rarely initiate their first episode of primary molt in December, at the very end of their hatching year.) The second episode of molt typically begins in the late summer or fall of a bird's second year and runs through winter and spring, thus including individuals in the first half of their third calendar year. The third episode of molt typically begins in late summer or fall in third-year birds, and continues until the following spring. Because fall third-year birds often cannot be distinguished from adults undergoing a subsequent episode of molt we did not attempt to separate third and subsequent episodes of primary molt.
A technically difficult issue is that August specimens replacing juvenile primaries can be undergoing either their first or their second episode of molt. Some second-year birds delay the initiation of their first molt of primaries until the summer after they were born, presumably because they fledged late or are in poor condition. However, other second-year birds begin their first replacement of primaries in the winter before their second summer. These birds appear to arrest this molt in late spring; thus when they reinitiate molt in summer they are starting their second episode of molt.
Scoring Molt and Determining the Age of Primaries
Primaries are numbered from the most proximal (P1) to the most distal (P11, which is vestigial and was excluded from our analyses). For each primary that was growing at the time of collection, we assigned a decimal fraction (recorded as 0.1 to 0.9) to indicate its stage of development (e.g., 0.1 = 0–10% grown). Empty follicles were assigned a score of 0.01 to indicate that the feather was lost near the time of collection. Newly replaced feathers that appeared to be fully grown were checked at their bases and assigned a growth score of 0.99 if flakes of sheathing indicated that they had just completed growth.
We assigned ages for all fully grown feathers as follows: Newly replaced feathers were given an age of 0 indicating replacement during the most recent episode of molt. Feathers of this age were nearly black with extensive iridescence and unfrayed barbs at the tip of the feather. Feathers replaced in the episode of molt completed prior to the collection of the bird were assigned an age of 1, although these feathers are rarely one year old. They were generally less iridescent, slightly brownish in color, and often slightly bleached and subtly frayed at their tips. Feathers replaced in the episode of molt completed two episodes prior to the collection of the bird were assigned an age of 2. These extensively bleached and extremely worn feathers had likely been retained through two summers or retained through one breeding season and a considerable portion of a nonbreeding season. Because the molt episodes of Pelagic Cormorants are long, it is sometimes difficult to distinguish feathers replaced early and late in the same episode of molt from feathers replaced in different episodes. We noted these ambiguities, but report here only our best estimates of feather age.
Determining Rules for Feather Replacement
A set of remiges replaced according to a single set of rules is termed a molt series (Langston and Rohwer 1995). Conceptually, the feathers of a single molt series show high pairwise correlations in the timing of feather to feather replacement, while those correlations from any pair of feathers from separate molt series will be low. In practice, molt series can be identified using this highly correlated timing, the direction of feather replacement, and the identification of feathers that mark the beginning and end of a series (Underhill 1986, Yuri and Rohwer 1997). Ontogeny is also a great help in identifying molt series. In species with stepwise molts, adults may have several simultaneous waves of primary replacement. A wave of primary molt is defined as a group of feathers that begins replacement at a single point of initiation (such as P1) with molt proceeding in one direction, sequentially along the wing. Unlike adults, young birds in species with stepwise molts never initiate their first molt of primaries at multiple points or “foci” along the wing, suggesting all the primaries constitute a single molt series (Table 3 in Rohwer 1999). In contrast, species with multiple molt series in their primaries will show multiple points of initiation when they replace their juvenile primaries. Table 2 defines the four categories we used to characterize each growing (focal) primary feather (Yuri and Rohwer 1997). As we show below, these categorizations are sufficient to demonstrate that all of the primaries of Pelagic Cormorants constitute a single molt series.
Definitions of the four focal feather categories used to determine the rules of feather replacement in Pelagic Cormorants; information obtained from each focal feather type indicates how we used them in our analyses
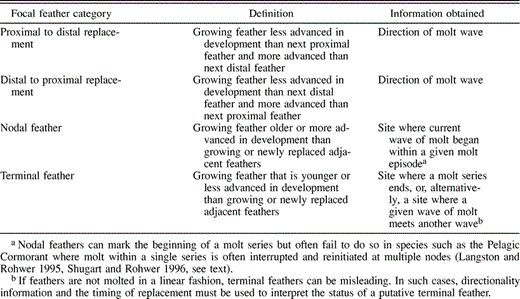
Definitions of the four focal feather categories used to determine the rules of feather replacement in Pelagic Cormorants; information obtained from each focal feather type indicates how we used them in our analyses

Estimating the Duration and the Date of Initiation of Primary Molt
Growth bands are alternating light and dark bands that cross the vane of the feather. Each pair of bands represents the amount of feather grown in a day (Michener and Michener 1938, Grubb et al. 1991). Therefore, we use growth bands to estimate the rate of primary growth. We measured the length of a minimum of 5 adjacent pairs of bands and divided this by the number of band-pairs to estimate the per-day rate of feather growth.
The summed length of the primaries for each sex was used to estimate the average time required to complete the primary molt, given the average number of feathers growing per wave and the average number of waves of primary replacement. The length of each primary for 10 adult Pelagic Cormorants of each sex was measured from its estimated attachment to the manus to the feather tip. These measurements were then averaged and summed for each feather position to compute the summed length of all the primaries for each sex. To use the growth band estimates of the rate of primary growth to calculate and compare overall rates of feather replacement, the average number of feathers growing per wave and the average number of waves of molt were measured for juveniles in their first molt of primaries and for older birds in molt that had no retained juvenile primaries.
For this measure of the rate of feather replacement, individuals that have just initiated the molt will be growing so few feathers that they will cause us to underestimate the rate of feather replacement. To avoid this bias, we excluded all specimens that had grown only P1 and any specimens that were growing two feathers if P1 was less than 80% of its total length. With this revised sample we could calculate the average time required to complete the primary molt for juvenile feather replacement and for subsequent (adult) episodes of molt.
We evaluated our growth-band estimate of the time required to replace the primaries by regressing date of collection (in Julian days) for each adult in active molt on the summed length of primary feather grown to obtain an independent estimate of molt duration (see Pimm 1976, Langston and Rohwer 1996, Thompson et al. 1998). The summed length of primary grown was calculated by multiplying the decimal score for growing and newly replaced feathers (newly replaced feathers = 1, indicating completed growth; growing feathers = 0.1, 0.2, etc.) by the average length for that feather for a given sex. We then summed the values for all newly replaced and growing primaries to obtain the length of feather grown for each bird. This regression validated the growth-band estimates, enabling us to increase the number of birds in analyses of initiation dates by using the feather growth rate estimated from growth bands to extrapolate starting dates for birds collected early in their primary molt (P1 less than fully grown).
The Valdez specimens are poorly suited for assessing the completeness of feather replacement by adults because most of these birds were oiled before their molt was completed, and oiling may have caused many birds to arrest their molts prematurely. Thus, we evaluated the completeness of the annual molt in adults using specimens that had been collected, and not salvaged, during the breeding season (between May and August).
Statistical Analyses
All statistical tests were performed using StatView 5.0 (SAS Institute 1998). Comparisons of the timing of the initiation of molt, and the number of feathers per wave of molt were evaluated using Mann-Whitney U-tests. Differences in the proportion of adult birds completing their primary molts in Asia and North America were tested using a one-tailed Fisher's exact test, and a one-way ANOVA was used to test differences between male and female summed primary lengths. Means are expressed ± SE except as indicated otherwise in the text. We considered results significant if P < 0.05.
Results
None of the six hatch-year (HY) birds we examined were replacing juvenile primaries; four of these specimens were collected late in December, when they were presumably about five months old. On six collecting trips during November, December, and January in Puget Sound, we observed many other HY Pelagic Cormorants, none of which had gaps in their primaries. Because other cormorants are also reported to delay their first molt of primaries (Potts 1971, Rasmussen 1988), and because none of the HY specimens we examined had begun the first episode of primary molt, we excluded HY specimens from further analyses.
Primary Replacement in Juveniles
Timing
The mean date for second-year (SY) birds from North America to initiate their primary molt was 11 March (Fig. 1a; SD = 36.7 days, n = 21). In Asia, the mean date for the initiation of primary molt for SY birds was 11 April (Fig. 1b; SD = 30.1 days, n = 10). Most SY birds began replacement of their juvenile primaries between February and April (Fig. 1), before adults on either side of the Pacific started nesting.
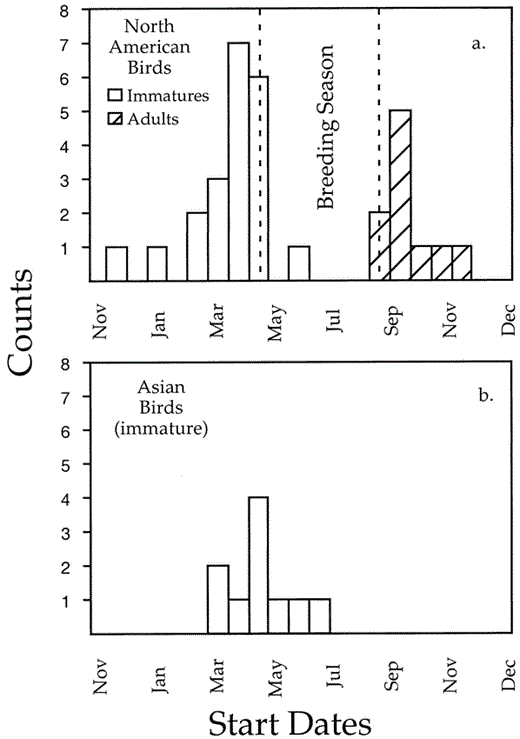
Start dates (a) for juvenile primary replacement and adult primary molt in North American Pelagic Cormorants and (b) for juvenile primary replacement in Asian Pelagic Cormorants
Rules of primary replacement
The direction of replacement for the juvenile primaries was proximal to distal (Table 3). For the 26 Asian and North American birds collected early in their first molt of primaries, 24 feathers suggested the direction of primary replacement was proximal to distal. An additional 8 SY birds were collected after the second wave of juvenile primary replacement had been established; all 13 of their focal feathers (five in the distal primaries) also showed proximal to distal replacement (Table 3).
Frequency distribution of focal (growing) feathers in a study of Pelagic Cormorants from North America and Asia, divided into three feather categories: nodal feathers, terminal feathers and feathers indicating direction of molt (see Table 2)
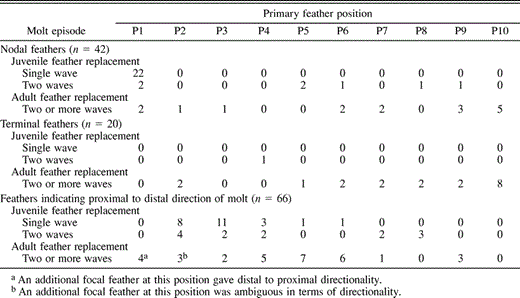
Frequency distribution of focal (growing) feathers in a study of Pelagic Cormorants from North America and Asia, divided into three feather categories: nodal feathers, terminal feathers and feathers indicating direction of molt (see Table 2)

The distribution of nodal feathers indicated that two waves of primary molt are established before the juvenile primaries are fully replaced. P1 was unambiguously the node of initiation for the first wave of juvenile primary molt in 22 of 26 SY birds that were collected early in their replacement of juvenile primaries (prior to P2 being fully grown; Table 3). None of these 22 birds showed other points of initiation in the more distal primaries. The second wave of primary replacement is established later in the molt, possibly following an arrest that may occur during the spring or early summer when young birds move with the adults to summer breeding colonies. Three of 10 SY birds collected late in summer had two waves of primary replacement: one wave in the distal primaries, and another that began again at P1. A fourth had a wave of replacement initiating at P6 but had not reinitiated at P1. Initiation points for the outer waves of feather replacement in these SY birds are presented in Table 3.
The three SY specimens that had reinitiated a wave at P1 suggest that the second wave of molt initiates after a brief arrest that occurs when these birds are about a year old. Each of the three birds showed two distinct age classes of post-juvenile primaries. One bird, collected in western Washington in July (UWBM 45099), had only a single wave of primary replacement in the outer part of the wing. However, the growing primaries were so much brighter in appearance than P1–5 that the bird must have arrested its juvenile primary molt at P5, and begun again at P6 (Table 4). This specimen demonstrates that the reinitiation of molt at P1 may not be inextricably linked to the reinitiation of an interrupted wave of molt in the outer primaries (though molt at P1 might have begun a short time later had the bird survived). The other two specimens, both collected in western Washington in September (PSM 12787 and PSM 12831), showed a similar age break between P4 and P5, but each also had initiated a new wave of molt at P1 (Table 4). The extrapolated initiation dates for the outer waves of molt in these 3 birds are 9, 12, and 13 June. Just two additional specimens that we examined showed nodal feathers in the outer primaries, one at P8, the other at P9 (Table 3). Direct evidence that the second replacement of the primaries begins at P1 is limited to the 2 specimens from the Slater Museum. However, none of the specimens we inspected in more advanced stages of the second molt of primaries contradict this pattern.
Primary feather ages for selected Pelagic Cormorant specimens from North America and Asia
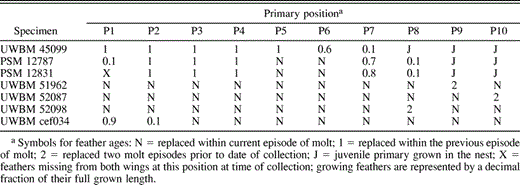
Primary feather ages for selected Pelagic Cormorant specimens from North America and Asia

Primary Replacement in Adults
Timing
We extrapolated the starting dates for a wave of molt only if there were no more than four growing or fully replaced primaries in that wave. With more than four new or growing feathers in a series of molting feathers, there is an increasing possibility that two molt waves might have merged, in which case an extrapolated estimate of the date of molt initiation would be spuriously early. Starting dates of molt for the earliest waves of primary replacement in the wings of 10 adults were concentrated in August and September (mean = 3 September ± 7.8 days), suggesting that molt begins following the breeding season (Fig. 1a).
Rules of primary replacement
Points at which primary replacement was initiated or terminated in adults were spread throughout the 10 primaries (Table 3). For all but 2 of the 33 focal feathers where the direction of primary molt could be inferred, replacement was proximal to distal (Table 3).
Seventeen of 33 molting adults had one or more sets of new or growing primaries separated by older feathers, showing clear evidence of multiple waves of molt. Ten of the remaining 16 birds were collected so early in the molt that multiple waves of primary replacement may not have had sufficient time to develop. The other 6 were birds taken near the end of their primary molt, when molt from several waves may have merged.
We were able to extend our data on the distribution of initiation points by using all growing feathers (not just nodal feathers) in adults that were replacing primaries. For waves of molt that did not include a nodal feather, we assumed that the initiation point for a set of adjacent, growing primaries was the most proximal growing or newly replaced primary in that set. This set of all 33 molting adults shows that waves of primary replacement begin at all feather positions except P4 (Fig. 2). Note that this procedure will underestimate the number of initiation points in primaries beyond P1 when waves have merged, something that may explain the lack of waves starting at P4. The patterns of modality in these data suggest that adjacent waves of primary replacement are typically separated by at least three feathers (Fig. 2). Interestingly, of the five adults with multiple waves of active molt, adjacent waves were separated by a mean of 3.4 ± 1.1 (SD) primaries.
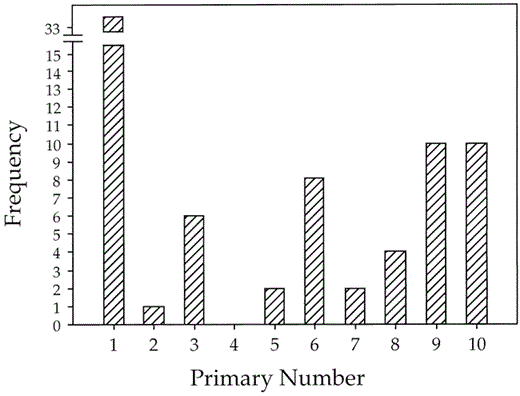
Initiation points for primary molt in 33 adult Pelagic Cormorants
Because no middle primaries are consistent points of the initiation or termination of waves of molt (Table 3), these data suggest that the primaries of adults constitute a single molt series, usually characterized by proximal to distal feather replacement.
A comparison between the probability of replacement of juvenile and adult primaries in non-molting North American birds (Fig. 3) reveals three important points. First, all birds probably replace P1 in every molt. Second, although there is a slight reduction in the probability of adult primaries being replaced as one moves distally from P1, most adults replace all of their primaries. Third, immature birds are less likely than adults to have replaced all of their primaries and much less likely to replace P10.
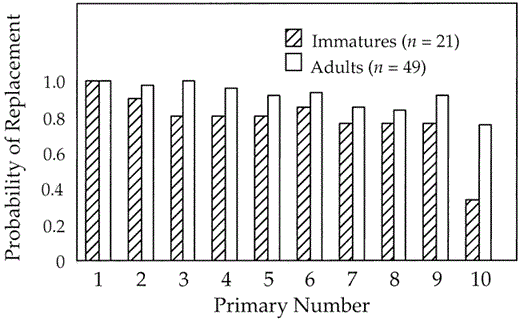
Probability of individual primaries being recently replaced in adult and immature Pelagic Cormorants collected during the breeding-season arrest in molt
Time Available to Molt
For birds from the Pacific coast of North America, we estimated the time available to molt by recording the percentage of adult specimens that were molting in each month of the year (Fig. 4). Small sample sizes for February and March reflect general collecting biases toward breeding birds. We excluded Valdez specimens from this analysis to avoid possible biases due to effects of the spill on molting. The high frequency of adults molting in August probably represents failed breeders. However, most chicks have fledged by September, so these data suggest that successful breeders have from September through April (36 weeks) available for molting because adults molt throughout the winter (Fig. 4). Not all birds molt continuously throughout this 36-week period (Fig. 4), but we do not know if some birds arrest and restart the molt or if there is a great deal of variability in the initiation or completion of adult primary molts.
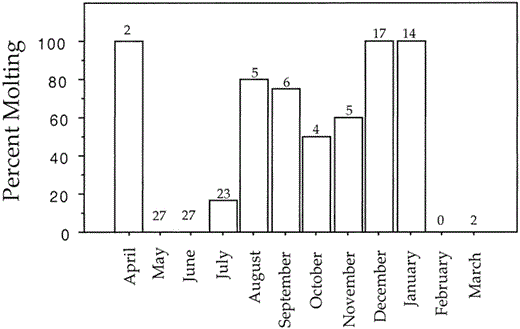
Monthly percentage of adult Pelagic Cormorants in molt from the Pacific coast of North America; sample sizes are above each bar (total n = 105)
Time available for primary replacement may be especially limited for Pelagic Cormorants from the Asian Pacific coast, where severe winter conditions cause northern birds to migrate relatively long distances. Five results suggest that time constraints are more severe along the Asian Pacific coast than they are in North America. First, young birds from Asia initiated their first molt of primaries on average 31 days later than young birds from North America (Fig. 1b; U = 53.5, df = 2, P < 0.03). Second, the number of feathers simultaneously growing in the wave of molt that replaces the juvenile primaries was significantly higher (U = 23, P < 0.001) for Asian birds (mean = 3, range 2–5, n = 9) than for North American birds (mean = 1.4, range 1–3, n = 27). Third, all of the Asian birds (n = 9) began replacing secondaries at a stage in the molt when only one of 27 North American birds had commenced secondary replacement. Fourth, 83% (38 of 46) of breeding adults from the Pacific coast of North America replaced all of their primaries, while not one of 13 Asian adults had done so (Fisher's exact, df = 1, P < 0.001). And, finally, the 13 Asian birds with incomplete molts retained more primaries (range 2–5) than the 8 North American birds with incomplete molts (range 1–2, U = 11.5, P < 0.005).
Time Required to Replace Primaries
For 24 individuals from North America, the mean rate of primary growth for the sexes combined was 4.6 ± 0.5 mm day−1 (range = 3.7–5.5 mm), a value similar to that for other large birds (Prevost 1983, Rohwer 1999). On average, North American adults have 2.9 simultaneously growing primaries (1.2 ± 0.3 [SD] feathers wave−1 × 2.42 ± 0.5 [SD] waves wing−1; n = 17). While some adults have three waves and grow four or more primaries simultaneously, most adults grow only a single feather in each wave of primary replacement. Although juveniles on average grew more feathers per wave (1.3 ± 0.6 [SD]) than adults, this difference was not significant. The average summed length of male primaries was 1921.7 ± 7.6 mm, which is significantly longer than that for females (1765.5 ± 8.3 mm; F1,8 = 193.1, P < 0.001).
According to these values, a Pelagic Cormorant growing only one primary at a time would need an average of 58 weeks (60 weeks for males, 55 weeks for females) to replace all 10 primaries (Fig. 5), considerably more time than the 36 weeks we estimated to be available for molting. Because of multiple waves, however, the average adult grows 2.9 primaries simultaneously, facilitating complete replacement of all primaries in 21 weeks, well within the time available.
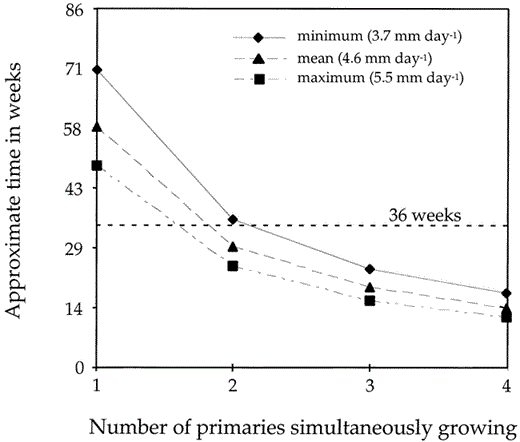
Differences in estimated time needed to grow all ten primaries for minimum, maximum, and mean growth rates plotted against the number of simultaneously growing feathers; the hatched line indicates the estimated 36-week period available to molt for adult Pelagic Cormorants from North America. Estimated time available to molt is based upon the monthly distribution of molting adults in the specimens we examined
Our regression of date of collection on length of primary grown gave an independent estimate of 29 weeks to replace all primaries (Fig. 6, r2 = 0.81, P < 0.001). That this regression estimate is 8 weeks longer than the growth-band estimates suggests that adults periodically arrest or slow their rate of feather replacement during periods of winter stress, as has been suggested for other cormorants (Potts 1971, Rasmussen 1988) and as seems evident in Figure 4.
![Regression of Julian date of collection on molt score (Y = 200.26 [19 July] + 0.11X; r2 = 0.81; n = 36; P < 0.001) to estimate the time necessary for Pelagic Cormorants to completely replace all 10 primaries (mean total primary length: males 1921.7 mm; females 1765.5 mm)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/condor/103/3/10.1093_condor_103.3.555/1/m_i0010-5422-103-3-555-f06.gif?Expires=1748270665&Signature=pGnkrhbeubu20KbF5fcVZwUZsy6q~pLi0IgGic-acG-22exEWQVuY3XgYxwBpCeNcSGColef3FK7odobcs8r8TRTVukM4313s4NRMwrPBYkykc8i5a6sV7u4aNdawy6Bn3s2m0m7QoOeXAZrJsfgh7mgb~8bhb5IjfRo15WYfEOWqRoq8A72LSes438JtoZo4VzhAwkItwB5sSgEks8KJw39PQPgffr-Djk1Lhl4PIWluywQmQrNMBTuIeAFypfY0jGO8nDT3Dm2fW8Cis-0rMgtaujJpYaGydIjxYQuX0d7ZNx41Qd6FA2qX-SoDLGX~lcBFErU~8mBGi22QhJzQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Regression of Julian date of collection on molt score (Y = 200.26 [19 July] + 0.11X; r2 = 0.81; n = 36; P < 0.001) to estimate the time necessary for Pelagic Cormorants to completely replace all 10 primaries (mean total primary length: males 1921.7 mm; females 1765.5 mm)
Discussion
Interpreting patterns of molt requires answering questions at two different levels. First, we need to understand the rules of feather replacement, and how orderly progressions of replacement are maintained at the physiological level. Studies such as this one are well suited to establish the rules of feather replacement, but they offer little insight into the physiological mechanisms underlying the orderly replacement of feathers. Second, we need to interpret the evolutionary origin and maintenance of the rules of feather replacement in a life-history context, informed by both physiological and phylogenetic constraints (Winkler 2000). Despite significant progress in this regard for some passerines (Holmgren and Hedenström 1995, Svensson and Hedenström 1999), comparative studies of large birds are in their infancy.
Rules for Feather Replacement
Pelagic Cormorants molt their primaries in a stepwise fashion (Table 3; Fig. 2), as do most other pelecaniforms that have been studied in detail (Dorward 1962, Rasmussen 1987, 1988). Furthermore, their first molt of primaries begins as just a single wave of feather replacement at P1, matching the ontogeny of multiple waves documented for species with stepwise primary molts (Rasmussen 1988, Rohwer 1999). The second wave of primary replacement also starts at P1, resulting in the inner primaries being replaced twice before the outermost juvenile primaries have been molted (Rohwer 1999; Table 3).
As Rasmussen (1988) and Rohwer (1999) have noted, this ontogeny of multiple waves has the disadvantage of leaving very worn primaries in the outer part of the wing. Curiously, some individuals in some species with stepwise primary molt skip the replacement of one or more of their outer primaries (Potts 1971, Rasmussen 1988, Shugart and Rohwer 1996). These so-called omissive molts, which we observed in some young Pelagic Cormorants, may be an adaptation to set up a molt wave in the outermost primaries, so that young birds will have replaced their longest primaries in the molt that precedes their first breeding attempt.
Generation of Multiple Waves Without Incomplete Molts
The only model proposed for the maintenance of multiple waves of feather replacement in adult birds is based on incomplete molts (Rasmussen 1988, Shugart and Rohwer 1996). Molts are assumed to reinitiate at each two-year-old primary immediately distal to the point of arrest and at P1. Shugart and Rohwer (1996) developed this model in detail for a population of Black-crowned Night-Herons in which adults often replace only about half of their primaries per year. However, Pelagic Cormorants from North America maintain multiple waves even though they typically replace all of their primaries each year. Furthermore, some young birds that start the replacement of juvenile primaries late in the year after they were born may generate multiple waves without an arrest. This result challenges two assumptions of the model proposed by Shugart and Rohwer (1996) for the maintenance of stepwise primary molts.
First, the assumption that each new wave of molt established at P1 is initiated following an arrest probably needs to be relaxed. We did find such an arrest in young birds that began their first molt of primaries early, and we suspect this arrest was associated with them moving to breeding areas with adults. However, other young birds that were late in initiating their first molt of primaries apparently did not arrest their molt. These juveniles also started a second wave of primary molt at P1 when the first wave of primary replacement reached the outer primaries, but we found no indication of an arrest in these late molters. Rasmussen (1988) and Potts (1971) observed similar patterns in King and Blue-eyed Shags (Phalacrocorax atriceps and P. albiventor) and in European Shags (Phalacrocorax aristotelis).
Second, Shugart and Rohwer (1996) assumed that molt would not reinitiate at points where a proximal wave of feather replacement had merged with a more distal wave initiated during the same episode of molt. This provides a mechanism that prevents the generation of excessive waves of molt when primary molts are usually incomplete. However, in Pelagic Cormorants, most adults complete their primary molts. Without reinitiation at merged waves, adults would have just single waves of primary replacement initiated at P1 in the next episode of molt. As we have shown above, adults would be unable to completely replace all their primaries with a single wave of molt unless they created larger gaps in their wings. Because adult Pelagic Cormorants show multiple waves of primary replacement and have complete primary molts, a “follicular memory” apparently allows them to reinitiate molt with the feather distal to the point of termination in the preceding molt, regardless of whether those waves merged. We know of no study that has elucidated the physiological mechanisms for generating the multiple waves of primary replacement that characterize stepwise molts.
Implications for Display and Aerodynamics
During their second winter, immature Pelagic Cormorants have two waves of primary replacement (Table 4). The distal wave is still replacing the juvenile primaries grown in the nest, while the proximal wave is replacing the inner primaries for a second time. Given that the first wave of molt would succeed in replacing all of the juvenile primaries by the end of their second winter, why do immature birds initiate a second wave? The second wave of molt may set up multiple waves of primary replacement that facilitate the annual replacement of all primaries in breeding adults at minimal aerodynamic cost. A compatible hypothesis is that redundant replacement of the inner primaries may reduce contrasts in fading and wear between the inner and outer primaries of young birds at the beginning of their second summer. Given that young birds are returning to the breeding colonies with adults (Palmer 1962, Campbell et al. 1991), a more recently replaced set of primaries may serve them in social competition.
One January adult from Puget Sound, Washington, and an Asian adult both reinitiated molt at P1 after replacing all of their primaries, suggesting the importance of a uniform set of primaries serving displays. If early-molting birds (failed or early breeders) continued to molt after replacing all of their primaries, their primaries would be especially iridescent at the beginning of the breeding season. Cormorants are well known for their spread-wing displays (Johnsgard 1983), and we assume that such displays would be less effective if the inner primaries were much more worn and faded than the outer primaries. Voelker (1997) makes a similar argument to explain the well-known redundant molts of inner primaries in most Sterna terns.
We found that on average, adult Pelagic Cormorants from North America simultaneously grew just 1.2 feathers per wave of molt, necessitating at least two waves to replace all primaries. However, if just two feathers grew simultaneously in a single wave, the primaries could be completely replaced between breeding seasons (Fig. 5). Two reasons may explain adults maintaining multiple waves of primary replacement. First, the aerodynamic costs of two or more small gaps in the primaries may be less than the aerodynamic costs of a single large gap (Rasmussen 1988). This seems reasonable because overlap between adjacent primaries reduces the gap created by a single missing primary to less than the area of that feather. Our results, like those of Rasmussen (1988), show that adults maintain more waves and, although not significant, have fewer feathers growing per wave than birds replacing juvenile primaries. Second, our impressions of the time available for molting come from birds living during an interglacial period. Historically, relatively severe winter conditions during glacial maxima may have necessitated longer migrations, which would have increased the time constraints on molting.
Life History Implications of Incomplete Primary Molts
Like most other cormorants, Pelagic Cormorants from the west coast of North America molt throughout the winter (Fig. 4; Potts 1971, Cooper 1985, Rasmussen 1988). Thus, most North American adults have time to breed successfully and to completely replace their primaries (Fig. 5). In contrast, all 13 of the adults we examined from northeastern Asia had failed to replace all primaries; some had retained four or five feathers. Young birds from northeast Asia initiate their first primary molt so late (Fig. 1b) that they are unlikely to replace all of their worn juvenile primaries by the beginning of their third summer, whereas many young birds from North America begin this molt early enough that they could be finished molting by the beginning of their third summer. These contrasts suggest that the initiation of breeding by young birds may be delayed an extra year in Asia, and that adults from Asia that breed successfully for two or three years in succession may be forced to skip breeding to clear worn flight feathers that have accumulated (Rohwer 1999).
The potency of specimen-based studies lies in their ability to elucidate patterns on geographic and temporal scales that are larger than can be accomplished in the field. A weakness of specimen-based studies is that they inform us only about mean differences between populations. Thus, while museum studies may suggest interesting life-history trade-offs between molt and breeding, they cannot directly test trade-off hypotheses. This molt study points to Asian, but not North American, birds as interesting to investigate for evidence of molt-breeding trade-offs. Testing the existence of molt-breeding trade-offs will require monitoring individual Pelagic Cormorants through several breeding seasons. We predict Asian adults that breed successfully will be unable to replace all their primaries and, further, Asian adults that have accumulated many worn flight feathers will skip breeding to molt throughout the summer. If this is true, Pelagic Cormorants would represent the fourth family of birds in which the accumulation of worn flight-feathers may constrain breeding (Pietiäinen et al. 1984, Langston and Rohwer 1996, Shugart and Rohwer 1996). While at least two field studies of owls have demonstrated that breeding success affects the number of flight feathers replaced (Pietiäinen et al. 1984, Petty 1994), no field study has shown that adults with many worn flight feathers skip breeding to undergo an extensive molt (Rohwer 1999). Exploring this suggestion will clarify our understanding of the role molt plays in constraining the breeding schedules of large birds.
Acknowledgments
We are particularly indebted to Catherine E. Smith for editorial assistance and help in the field. Thanks also to Gerald W. Scoville and Vanya Rohwer for field assistance and specimen preparation. Sergei Drovetski, Ann Edwards, Scott Pearson, Chris Thompson, Gary Voelker, and other members of the Rohwer lab group commented helpfully on the manuscript. Sasha Kitaysky provided valuable information and references on Asian populations and Sergei Drovetski translated Russian references. NSF grants BSR 9216534 and BSR 9316045 facilitated salvage and preparation of specimens from the Exxon Valdez oil spill. Most of the specimens used in this study are housed at the University of Washington Burke Museum (UWBM). Thanks to George Barrowclough, Mary LeCroy, and Paul Sweet at the American Museum of Natural History (AMNH), Gary Shugart at the University of Puget Sound Slater Museum (PSM), and Evgeny Koblik at Moscow State University Museum (MSUM) for help with access to collections at their institutions. Chris Wood provided storage and workspace at the Burke Museum. An Eddy Fellowship in Ornithology supported CEF during work on this project.
Literature Cited



