-
PDF
- Split View
-
Views
-
Cite
Cite
Elisavet Stavropoulou, Pierre Monney, Georgios Tzimas, Nicoleta Ianculescu, Piergiorgio Tozzi, Matthias Kirsch, Benoit Guery, Matthaios Papadimitriou-Olivgeris, Predictors of Persistent Fever Among Patients With Suspected Infective Endocarditis: Think Outside the box, Clinical Infectious Diseases, Volume 80, Issue 4, 15 April 2025, Pages 795–803, https://doi.org/10.1093/cid/ciae588
Close - Share Icon Share
Abstract
Fever is common in infective endocarditis (IE), yet little is known about fever duration in such patients. We aim to identify predictors of persistent fever in patients with suspected IE.
This study was conducted at the Lausanne University Hospital, Switzerland, from January 2014 to June 2023. All patients with suspected IE being febrile upon presentation were included. Fever (>38°C) was considered persistent if it continued for at least 96 hours from antimicrobial treatment initiation. A case was classified as IE by the Endocarditis Team.
Among 1399 episodes with suspected IE, persistent fever was observed in 260 (19%) episodes. IE was diagnosed in 536 (41%) episodes, of which 82 (15%) had persistent fever. Among episodes with suspected IE, persistent bacteremia/candidemia for 96 hours (P < .001), spondylodiscitis (P = .039), intrabdominal infection (P = .001) were associated with persistent fever. Conversely, bacteremia by streptococci (P = .049), or enterococci (P = .001), source control performed withing 96 hours (P = .015) and appropriate antimicrobial treatment within 48 hours (P = .018) were associated with early defervescence. No association between persistent fever and infective endocarditis was found (P = .207). Among 536 IE episodes, persistent bacteremia/candidemia for 96 hours (P < .001), and native bone and joint infection (P = .020) were associated with persistent fever. Conversely, bacteremia by streptococci or enterococci (P = .001; adjusted odds ratio [aOR] 0.25, 95% confidence interval [CI] .11–.58) were associated with early defervescence.
In episodes with suspected IE, persistent fever was associated with spondylodiscitis, inappropriate antimicrobial treatment and absence of source control interventions. Among IE patients, persistent fever was associated with native bone and joint infections.
Fever is the most prevalent clinical sign among the many clinical manifestations of infective endocarditis (IE) [1, 2]. Due to its high prevalence, fever was included as a minor criterion in the original Duke criteria for diagnosing IE and remains part of subsequent updates [3–5].
In patients with suspected IE, fever prevalence is similar in both those who are ultimately diagnosed with IE and those who are not [6]. However, in certain contexts, such as Staphylococcus aureus bacteremia, persistent fever may indicate a more complicated disease and should raise the suspicion of IE [7, 8]. Despite this, a previous study from our institution found that persistent fever rates among patients with suspected IE did not significantly differ between those who were later confirmed to have IE and those who were not [9]. This indicates that in suspected cases of IE, persistent fever may be influenced by factors unrelated to IE.
The course of fever after the initiation of antimicrobial treatment among patients with diagnosed IE has not been adequately addressed. Retrospective studies conducted prior to 2000 found that persistent fever among IE patients, despite antimicrobial therapy, was associated factors to extensive cardiac involvement [10–12], and embolic events [12, 13]. Less common causes include extracardiac sources of infection, polymicrobial endocarditis, drug-induced fever, or nosocomial infections [10, 14]. Patients with persistent fever were significantly more likely to have life-threatening complications, such as paravalvular invasion, and large vegetations, leading to poorer clinical outcomes [10, 11, 13, 15–17].
Despite advancements in the diagnosis and management of IE, contemporary data on the duration and persistence of fever in patients with suspected or confirmed IE remain limited. This study aims to assess whether persistent fever is associated with IE in patients with suspected IE and to identify predictors of persistent fever in patients who are diagnosed with IE.
METHODS
Study Design
This single-center study was conducted at Lausanne University Hospital, Switzerland, from January 2014 to June 2023 (2014–17: retrospective cohort of IE patients; 2018 onward: prospective cohort of patients with suspected IE). The Ethics Committee of the Canton of Vaud has approved the study (CER-VD 2017-02137, CER-VD 2021-02516).
Patients
All adult patients (≥18 years old) with suspected IE (patients who had blood cultures drawn and an echocardiography performed specifically for the research of IE) and fever upon presentation were included. Exclusion criteria were absence of documentation of temperature measurements or death within 96 hours from antimicrobial treatment initiation without 24 hours of defervescence. Additional exclusion criteria were for the retrospective cohort refusal to use their data and for the prospective cohort the absence of written consent.
Demographic, clinical, microbiological, radiological, and surgical data were retrieved through patient's electronic health charts. Per internal guidelines, all suspected IE cases underwent infectious diseases (ID) consultation with a thorough physical examination. Follow-up blood cultures were taken every 24–48 hours until clearance. IE diagnosis was made by the Endocarditis Team based on clinical, laboratory, microbiological, imaging, surgical, and histopathological results. The determination of other foci of infection was based on the assessment by the ID consultant, taking into account clinical, radiological, microbiological, and operative findings. In case of fever, patients received paracetamol as needed.
Definitions
Fever was defined according to Duke criteria as a temperature superior to 38°C (>100.4°F). Persistent fever was defined as continuous fever for at least 96 hours from antimicrobial treatment initiation. Persistent bacteremia/candidemia was defined as continued positive blood cultures for at least 96 hours from antimicrobial treatment initiation. Cases were classified as definite, possible, and rejected IE based on the 2023 International Society of Cardiovascular Infectious Diseases (ISCVID) version of the Duke criteria [5]. Sepsis and septic shock were defined as per the Sepsis-3 International Consensus [18]. Antimicrobial treatment was considered appropriate if the patient received within 48 hours at least 1 active antimicrobial agent for which the isolated pathogen was considered susceptible. Source control was considered warranted in the following situations: (1) removal of venous catheter in patients with catheter-related bacteremia or bacteremia of unknown origin with the presence of a venous catheter; (2) imaging-guided or surgical drainage of infected collections; (3) joint fluid drainage (arthrotomy, arthroscopy, needle aspiration); (4) cardiac surgery in endocarditis patients when indicated for heart failure [19]; (5) correction of urinary-tract obstruction.
Statistical Analyses
SPSS version 26.0 (SPSS, Chicago, Illinois, USA) software was used for data analyses. Patients were categorized according to fever duration as “no persistent fever” and “persistent fever.” Group differences were investigated using the Mann–Whitney U test for continuous variables and the χ2 [2] or Fisher exact test for categorical variables. Univariable analyses were performed with persistent fever as dependent variable. Covariates were tested for multi-collinearity through variance inflation factor assessment: those not collinear and clinically relevant were used in multivariate logistic regression analyses. The strength of any association was calculated as adjusted odds ratios (aORs) and 95% confidence intervals (CIs). All tests were 2-tailed, and P < .05 was considered statistically significant.
RESULTS
Cohort Composition
Among 2082 episodes with suspected IE, 1399 episodes were included (Figure 1); among different reasons of exclusion, 341 episodes of suspected IE were excluded due to absence of fever upon presentation (Figure 1). From 1399 included episodes, 536 (38%) had IE, and for 863 (62%) IE diagnosis was excluded. In total, 105 (8%) episodes had a non-infectious diagnosis (malignancy-related fever, auto-immune disease, fever of unknown origin, non-infective endocarditis, etc). Transthoracic, transesophageal echocardiography (TTE, TEE), 18F-Fluorodeoxyglucose positron emission tomography/computed tomography (CT), and cardiac CT were performed in 1339 (96%), 694 (50%), 293 (21%), and 54 (4%) episodes, respectively. The first echocardiography was performed within a median of 3 days (interquartile range [IQR]: 1–5 days) from presentation. Among patients with S. aureus bacteremia first echocardiography was performed within a median of 3 days (interquartile range; IQR: 1–4 days).
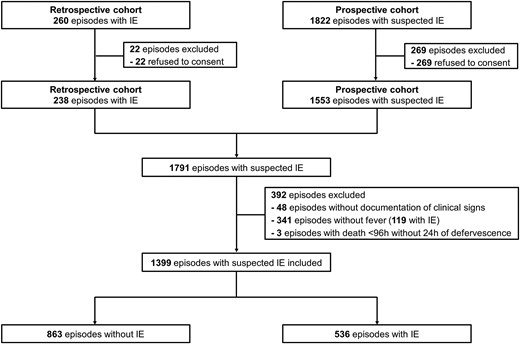
Flowchart of patients. Abbreviation: IE, infective endocarditis.
Persistent Fever Among Episodes With Suspected IE
Among 1399 episodes with suspected IE, persistent fever was observed in 260 (19%) episodes. Table 1 depicts predictors of persistent fever among patients with suspected IE. The median duration of fever was 1 day (IQR: 1–3 days) from antimicrobial treatment initiation. Spondylodiscitis (8% vs 5%; P = .050), intraabdominal infection (9% vs 3%; P = .001), persistent bacteremia/candidemia for 96 hours (15% vs 5%; P < .001) and fungemia (5% vs 3%; P = .029) were more prevalent among episodes with persistent fever, compared to those without. In contrast, IE was more prevalent among episodes without persistent fever compared to those with (40% vs 32%; P = .013). Bacteremia by either streptococci (19% vs 12%; P = .005) or enterococci (12% vs 5%; P =.001) were less prevalent among episodes with persistent fever, compared to those with early defervescence. Of the 1278 (out of 1399; 91%) episodes requiring antimicrobial treatment, a smaller proportion of cases with persistent fever received within 48 hours an appropriate therapy compared to those without persistent fever (91% vs 95%; P = .004). Source control procedures were necessary in 583 episodes (42%); these interventions were less frequently performed within 96 hours in episodes with persistent fever compared to those without (24% vs 59%; P = .015).
Predictors of Persistent Fever Among Patients With Suspected Infective Endocarditis
| . | No Persistent Fever (n = 1139) . | Persistent Fever (n = 260) . | P . |
|---|---|---|---|
| Demographics | |||
| Male sex | 819 (72) | 175 (67) | .150 |
| Age (y) | 68 (54–78) | 65 (55–75) | .022 |
| Age >60 y | 751 (66) | 158 (61) | .130 |
| Co-morbidities | |||
| Malignancy (solid organ or hematologic) | 222 ()20 | 77 (30) | .001 |
| Obesity | 289 (25) | 69 (27) | .694 |
| Immunosuppression | 367 (32) | 98 (38) | .094 |
| Manifestations | |||
| Sepsis | 431 (38) | 105 (40) | .480 |
| Septic shock | 125 (11) | 28 (11) | 1.000 |
| Cardiac predisposing factors | |||
| IV drug use | 69 (6) | 19 (7) | .479 |
| Prior endocarditis | 67 (6) | 10 (4) | .229 |
| Congenital disease | 82 (7) | 11 (4) | .097 |
| Prosthetic valve | 211 (19) | 34 (13) | .038 |
| Cardiac implantable electronic device | 152 (13) | 18 (7) | .003 |
| Non-cardiac prosthetic material | |||
| Vascular catheter (central or peripheral) | 440 (39) | 110 (42) | .291 |
| Prosthetic joint | 178 (16) | 45 (17) | .511 |
| Vascular graft | 93 (8) | 23 (9) | .709 |
| Final diagnosis | |||
| Non-infectious diagnosis | 74 (7) | 31 (12) | |
| Infectious diagnosis | 1065 (94) | 229 (88) | .004 |
| Bacteremia/candidemia of unknown origin | 96 (8) | 18 (7) | .530 |
| Catheter-related | 124 (11) | 28 (11) | 1.000 |
| Infective endocarditis | 454 (40) | 82 (32) | .013 |
| Skin and soft tissue infection | 112 (10) | 22 (9) | .560 |
| Bone and joint infection | 226 (20) | 62 (24) | .149 |
| Native bone and joint infection | 123 (11) | 39 (15) | .067 |
| Septic arthritis | 75 (7) | 22 (9) | .280 |
| Spondylodiscitis | 56 (5) | 21 (8) | .050 |
| Low-respiratory tract infection | 57 (5) | 17 (7) | .356 |
| Urinary tract infection | 49 (4) | 13 (5) | .617 |
| Intrabdominal infection | 39 (3) | 22 (9) | .001 |
| Other type of infection | 128 (11) | 25 (10) | .509 |
| Microbiological data | |||
| Bacteremia/candidemia | 930 (82) | 184 (71) | <.001 |
| S. aureus | 409 (36) | 99 (38) | .521 |
| Coagulase negative staphylococci | 71 (6) | 9 (4) | .102 |
| Streptococci | 215 (19) | 30 (12) | .005 |
| Enterococci | 137 (12) | 14 (5) | .001 |
| Other Gram-positive | 34 (3) | 6 (2) | .682 |
| HACEK | 13 (1) | 3 (1) | 1.000 |
| Other Gram-negative | 93 (8) | 22 (9) | .900 |
| Fungi | 30 (3) | 14 (5) | .029 |
| Polymicrobial bacteremia/candidemia | 80 (7) | 11 (4) | .124 |
| Persistent bacteremia/candidemia for 96h | 56 (5) | 99 (15) | <.001 |
| Source control warranted | 469 (41) | 114 (44) | .471 |
| Source control performed within 96ha | 275 (59) | 52 (24) | .015 |
| Antimicrobial treatment warranted | 1053 (93) | 225 (88) | .003 |
| Appropriate antimicrobial treatment within 48hb | 1005 (95) | 204 (91) | .004 |
| . | No Persistent Fever (n = 1139) . | Persistent Fever (n = 260) . | P . |
|---|---|---|---|
| Demographics | |||
| Male sex | 819 (72) | 175 (67) | .150 |
| Age (y) | 68 (54–78) | 65 (55–75) | .022 |
| Age >60 y | 751 (66) | 158 (61) | .130 |
| Co-morbidities | |||
| Malignancy (solid organ or hematologic) | 222 ()20 | 77 (30) | .001 |
| Obesity | 289 (25) | 69 (27) | .694 |
| Immunosuppression | 367 (32) | 98 (38) | .094 |
| Manifestations | |||
| Sepsis | 431 (38) | 105 (40) | .480 |
| Septic shock | 125 (11) | 28 (11) | 1.000 |
| Cardiac predisposing factors | |||
| IV drug use | 69 (6) | 19 (7) | .479 |
| Prior endocarditis | 67 (6) | 10 (4) | .229 |
| Congenital disease | 82 (7) | 11 (4) | .097 |
| Prosthetic valve | 211 (19) | 34 (13) | .038 |
| Cardiac implantable electronic device | 152 (13) | 18 (7) | .003 |
| Non-cardiac prosthetic material | |||
| Vascular catheter (central or peripheral) | 440 (39) | 110 (42) | .291 |
| Prosthetic joint | 178 (16) | 45 (17) | .511 |
| Vascular graft | 93 (8) | 23 (9) | .709 |
| Final diagnosis | |||
| Non-infectious diagnosis | 74 (7) | 31 (12) | |
| Infectious diagnosis | 1065 (94) | 229 (88) | .004 |
| Bacteremia/candidemia of unknown origin | 96 (8) | 18 (7) | .530 |
| Catheter-related | 124 (11) | 28 (11) | 1.000 |
| Infective endocarditis | 454 (40) | 82 (32) | .013 |
| Skin and soft tissue infection | 112 (10) | 22 (9) | .560 |
| Bone and joint infection | 226 (20) | 62 (24) | .149 |
| Native bone and joint infection | 123 (11) | 39 (15) | .067 |
| Septic arthritis | 75 (7) | 22 (9) | .280 |
| Spondylodiscitis | 56 (5) | 21 (8) | .050 |
| Low-respiratory tract infection | 57 (5) | 17 (7) | .356 |
| Urinary tract infection | 49 (4) | 13 (5) | .617 |
| Intrabdominal infection | 39 (3) | 22 (9) | .001 |
| Other type of infection | 128 (11) | 25 (10) | .509 |
| Microbiological data | |||
| Bacteremia/candidemia | 930 (82) | 184 (71) | <.001 |
| S. aureus | 409 (36) | 99 (38) | .521 |
| Coagulase negative staphylococci | 71 (6) | 9 (4) | .102 |
| Streptococci | 215 (19) | 30 (12) | .005 |
| Enterococci | 137 (12) | 14 (5) | .001 |
| Other Gram-positive | 34 (3) | 6 (2) | .682 |
| HACEK | 13 (1) | 3 (1) | 1.000 |
| Other Gram-negative | 93 (8) | 22 (9) | .900 |
| Fungi | 30 (3) | 14 (5) | .029 |
| Polymicrobial bacteremia/candidemia | 80 (7) | 11 (4) | .124 |
| Persistent bacteremia/candidemia for 96h | 56 (5) | 99 (15) | <.001 |
| Source control warranted | 469 (41) | 114 (44) | .471 |
| Source control performed within 96ha | 275 (59) | 52 (24) | .015 |
| Antimicrobial treatment warranted | 1053 (93) | 225 (88) | .003 |
| Appropriate antimicrobial treatment within 48hb | 1005 (95) | 204 (91) | .004 |
Data are depicted as number (percentage) or median (interquartile range).
Abbreviation: HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IV, intravenous; S. aureus, Staphylococcus aureus.
aAmong 583 episodes.
bAmong 1278 episodes.
Predictors of Persistent Fever Among Patients With Suspected Infective Endocarditis
| . | No Persistent Fever (n = 1139) . | Persistent Fever (n = 260) . | P . |
|---|---|---|---|
| Demographics | |||
| Male sex | 819 (72) | 175 (67) | .150 |
| Age (y) | 68 (54–78) | 65 (55–75) | .022 |
| Age >60 y | 751 (66) | 158 (61) | .130 |
| Co-morbidities | |||
| Malignancy (solid organ or hematologic) | 222 ()20 | 77 (30) | .001 |
| Obesity | 289 (25) | 69 (27) | .694 |
| Immunosuppression | 367 (32) | 98 (38) | .094 |
| Manifestations | |||
| Sepsis | 431 (38) | 105 (40) | .480 |
| Septic shock | 125 (11) | 28 (11) | 1.000 |
| Cardiac predisposing factors | |||
| IV drug use | 69 (6) | 19 (7) | .479 |
| Prior endocarditis | 67 (6) | 10 (4) | .229 |
| Congenital disease | 82 (7) | 11 (4) | .097 |
| Prosthetic valve | 211 (19) | 34 (13) | .038 |
| Cardiac implantable electronic device | 152 (13) | 18 (7) | .003 |
| Non-cardiac prosthetic material | |||
| Vascular catheter (central or peripheral) | 440 (39) | 110 (42) | .291 |
| Prosthetic joint | 178 (16) | 45 (17) | .511 |
| Vascular graft | 93 (8) | 23 (9) | .709 |
| Final diagnosis | |||
| Non-infectious diagnosis | 74 (7) | 31 (12) | |
| Infectious diagnosis | 1065 (94) | 229 (88) | .004 |
| Bacteremia/candidemia of unknown origin | 96 (8) | 18 (7) | .530 |
| Catheter-related | 124 (11) | 28 (11) | 1.000 |
| Infective endocarditis | 454 (40) | 82 (32) | .013 |
| Skin and soft tissue infection | 112 (10) | 22 (9) | .560 |
| Bone and joint infection | 226 (20) | 62 (24) | .149 |
| Native bone and joint infection | 123 (11) | 39 (15) | .067 |
| Septic arthritis | 75 (7) | 22 (9) | .280 |
| Spondylodiscitis | 56 (5) | 21 (8) | .050 |
| Low-respiratory tract infection | 57 (5) | 17 (7) | .356 |
| Urinary tract infection | 49 (4) | 13 (5) | .617 |
| Intrabdominal infection | 39 (3) | 22 (9) | .001 |
| Other type of infection | 128 (11) | 25 (10) | .509 |
| Microbiological data | |||
| Bacteremia/candidemia | 930 (82) | 184 (71) | <.001 |
| S. aureus | 409 (36) | 99 (38) | .521 |
| Coagulase negative staphylococci | 71 (6) | 9 (4) | .102 |
| Streptococci | 215 (19) | 30 (12) | .005 |
| Enterococci | 137 (12) | 14 (5) | .001 |
| Other Gram-positive | 34 (3) | 6 (2) | .682 |
| HACEK | 13 (1) | 3 (1) | 1.000 |
| Other Gram-negative | 93 (8) | 22 (9) | .900 |
| Fungi | 30 (3) | 14 (5) | .029 |
| Polymicrobial bacteremia/candidemia | 80 (7) | 11 (4) | .124 |
| Persistent bacteremia/candidemia for 96h | 56 (5) | 99 (15) | <.001 |
| Source control warranted | 469 (41) | 114 (44) | .471 |
| Source control performed within 96ha | 275 (59) | 52 (24) | .015 |
| Antimicrobial treatment warranted | 1053 (93) | 225 (88) | .003 |
| Appropriate antimicrobial treatment within 48hb | 1005 (95) | 204 (91) | .004 |
| . | No Persistent Fever (n = 1139) . | Persistent Fever (n = 260) . | P . |
|---|---|---|---|
| Demographics | |||
| Male sex | 819 (72) | 175 (67) | .150 |
| Age (y) | 68 (54–78) | 65 (55–75) | .022 |
| Age >60 y | 751 (66) | 158 (61) | .130 |
| Co-morbidities | |||
| Malignancy (solid organ or hematologic) | 222 ()20 | 77 (30) | .001 |
| Obesity | 289 (25) | 69 (27) | .694 |
| Immunosuppression | 367 (32) | 98 (38) | .094 |
| Manifestations | |||
| Sepsis | 431 (38) | 105 (40) | .480 |
| Septic shock | 125 (11) | 28 (11) | 1.000 |
| Cardiac predisposing factors | |||
| IV drug use | 69 (6) | 19 (7) | .479 |
| Prior endocarditis | 67 (6) | 10 (4) | .229 |
| Congenital disease | 82 (7) | 11 (4) | .097 |
| Prosthetic valve | 211 (19) | 34 (13) | .038 |
| Cardiac implantable electronic device | 152 (13) | 18 (7) | .003 |
| Non-cardiac prosthetic material | |||
| Vascular catheter (central or peripheral) | 440 (39) | 110 (42) | .291 |
| Prosthetic joint | 178 (16) | 45 (17) | .511 |
| Vascular graft | 93 (8) | 23 (9) | .709 |
| Final diagnosis | |||
| Non-infectious diagnosis | 74 (7) | 31 (12) | |
| Infectious diagnosis | 1065 (94) | 229 (88) | .004 |
| Bacteremia/candidemia of unknown origin | 96 (8) | 18 (7) | .530 |
| Catheter-related | 124 (11) | 28 (11) | 1.000 |
| Infective endocarditis | 454 (40) | 82 (32) | .013 |
| Skin and soft tissue infection | 112 (10) | 22 (9) | .560 |
| Bone and joint infection | 226 (20) | 62 (24) | .149 |
| Native bone and joint infection | 123 (11) | 39 (15) | .067 |
| Septic arthritis | 75 (7) | 22 (9) | .280 |
| Spondylodiscitis | 56 (5) | 21 (8) | .050 |
| Low-respiratory tract infection | 57 (5) | 17 (7) | .356 |
| Urinary tract infection | 49 (4) | 13 (5) | .617 |
| Intrabdominal infection | 39 (3) | 22 (9) | .001 |
| Other type of infection | 128 (11) | 25 (10) | .509 |
| Microbiological data | |||
| Bacteremia/candidemia | 930 (82) | 184 (71) | <.001 |
| S. aureus | 409 (36) | 99 (38) | .521 |
| Coagulase negative staphylococci | 71 (6) | 9 (4) | .102 |
| Streptococci | 215 (19) | 30 (12) | .005 |
| Enterococci | 137 (12) | 14 (5) | .001 |
| Other Gram-positive | 34 (3) | 6 (2) | .682 |
| HACEK | 13 (1) | 3 (1) | 1.000 |
| Other Gram-negative | 93 (8) | 22 (9) | .900 |
| Fungi | 30 (3) | 14 (5) | .029 |
| Polymicrobial bacteremia/candidemia | 80 (7) | 11 (4) | .124 |
| Persistent bacteremia/candidemia for 96h | 56 (5) | 99 (15) | <.001 |
| Source control warranted | 469 (41) | 114 (44) | .471 |
| Source control performed within 96ha | 275 (59) | 52 (24) | .015 |
| Antimicrobial treatment warranted | 1053 (93) | 225 (88) | .003 |
| Appropriate antimicrobial treatment within 48hb | 1005 (95) | 204 (91) | .004 |
Data are depicted as number (percentage) or median (interquartile range).
Abbreviation: HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IV, intravenous; S. aureus, Staphylococcus aureus.
aAmong 583 episodes.
bAmong 1278 episodes.
Multivariable logistic regression among the 1399 episodes with suspected IE (Table 2) identified malignancy, either solid organ or hematological (P = .012; aOR 1.89, 95% CI: 1.15–3.12), persistent bacteremia/candidemia for 96 hours (P < .001; aOR 2.55, 95% CI: 1.46–3.49), spondylodiscitis (P = .039; aOR 1.79, 95% CI: 1.03–3.12), intrabdominal infection (P = .001; aOR 2.86, 95% CI: 1.55–5.27) as factors associated with persistent fever. Conversely, bacteremia by streptococci (P = .049; aOR 064, 95% CI .41–.99), or enterococci (P = .001; aOR 0.37, 95% CI: .20–.69), source control performed within 96 hours (P = .015; aOR 0.58, 95% CI: .38–.90) and appropriate antimicrobial treatment within 48 hours (P = .018; aOR 0.60, 95% CI: .39–.92) were associated with early defervescence.
Bivariate and Multivariable Analyses of Predictors of Persistent Fever Among Patients With Suspected Infective Endocarditis
| . | Bivariate Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| . | P . | OR (95% CI) . | P . | aOR (95% CI) . |
| Malignancy (solid organ or hematologic) | .001 | 1.74 (1.28–2.36) | .012 | 1.89 (1.15–3.12) |
| Immunosuppression | .094 | 1.27 (.96–1.68) | .309 | .79 (.49–1.25) |
| Bacteremia by streptococci | .005 | .56 (.37–.84) | .049 | .64 (.41–.99) |
| Bacteremia by enterococci | .001 | .42 (.24–.73) | .001 | .37 (.20–.69) |
| Fungemia | .029 | 2.10 (1.10–4.03) | .242 | 1.52 (.75–3.07) |
| Persistent bacteremia/candidemia for 96ha | <.001 | 2.49 (1.69–3.69) | <.001 | 2.55 (1.46–3.49) |
| Spondylodiscitis | .050 | 1.70 (1.01–2.86) | .039 | 1.79 (1.03–3.12) |
| Infective endocarditis | .013 | .70 (.52–.93) | .207 | .80 (.57–1.13) |
| Intrabdominal infection | .001 | 2.61 (1.52–4.48) | .001 | 2.86 (1.55–5.27) |
| Source control | ||||
| Warranted; not performed within 96h | reference | reference | reference | reference |
| Warranted; performed within 96h | .015 | .59 (.39–.89) | .015 | .58 (.38–.90) |
| Not warranted | .030 | .68 (.49–.96) | .122 | .75 (.52–1.09) |
| Antimicrobial treatment | ||||
| Warranted; not appropriate within 48ha | reference | reference | reference | reference |
| Warranted; appropriate within 48h | .008 | .46 (.27–.79) | .018 | .60 (.39–.92) |
| Not warranted | 1.000 | .99 (.51–1.94) | .917 | 1.04 (.51–2.12) |
| . | Bivariate Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| . | P . | OR (95% CI) . | P . | aOR (95% CI) . |
| Malignancy (solid organ or hematologic) | .001 | 1.74 (1.28–2.36) | .012 | 1.89 (1.15–3.12) |
| Immunosuppression | .094 | 1.27 (.96–1.68) | .309 | .79 (.49–1.25) |
| Bacteremia by streptococci | .005 | .56 (.37–.84) | .049 | .64 (.41–.99) |
| Bacteremia by enterococci | .001 | .42 (.24–.73) | .001 | .37 (.20–.69) |
| Fungemia | .029 | 2.10 (1.10–4.03) | .242 | 1.52 (.75–3.07) |
| Persistent bacteremia/candidemia for 96ha | <.001 | 2.49 (1.69–3.69) | <.001 | 2.55 (1.46–3.49) |
| Spondylodiscitis | .050 | 1.70 (1.01–2.86) | .039 | 1.79 (1.03–3.12) |
| Infective endocarditis | .013 | .70 (.52–.93) | .207 | .80 (.57–1.13) |
| Intrabdominal infection | .001 | 2.61 (1.52–4.48) | .001 | 2.86 (1.55–5.27) |
| Source control | ||||
| Warranted; not performed within 96h | reference | reference | reference | reference |
| Warranted; performed within 96h | .015 | .59 (.39–.89) | .015 | .58 (.38–.90) |
| Not warranted | .030 | .68 (.49–.96) | .122 | .75 (.52–1.09) |
| Antimicrobial treatment | ||||
| Warranted; not appropriate within 48ha | reference | reference | reference | reference |
| Warranted; appropriate within 48h | .008 | .46 (.27–.79) | .018 | .60 (.39–.92) |
| Not warranted | 1.000 | .99 (.51–1.94) | .917 | 1.04 (.51–2.12) |
Abbreviations: aOR, adjusted odds ratios; CI, confidence interval; OR, odds ratio.
Bivariate and Multivariable Analyses of Predictors of Persistent Fever Among Patients With Suspected Infective Endocarditis
| . | Bivariate Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| . | P . | OR (95% CI) . | P . | aOR (95% CI) . |
| Malignancy (solid organ or hematologic) | .001 | 1.74 (1.28–2.36) | .012 | 1.89 (1.15–3.12) |
| Immunosuppression | .094 | 1.27 (.96–1.68) | .309 | .79 (.49–1.25) |
| Bacteremia by streptococci | .005 | .56 (.37–.84) | .049 | .64 (.41–.99) |
| Bacteremia by enterococci | .001 | .42 (.24–.73) | .001 | .37 (.20–.69) |
| Fungemia | .029 | 2.10 (1.10–4.03) | .242 | 1.52 (.75–3.07) |
| Persistent bacteremia/candidemia for 96ha | <.001 | 2.49 (1.69–3.69) | <.001 | 2.55 (1.46–3.49) |
| Spondylodiscitis | .050 | 1.70 (1.01–2.86) | .039 | 1.79 (1.03–3.12) |
| Infective endocarditis | .013 | .70 (.52–.93) | .207 | .80 (.57–1.13) |
| Intrabdominal infection | .001 | 2.61 (1.52–4.48) | .001 | 2.86 (1.55–5.27) |
| Source control | ||||
| Warranted; not performed within 96h | reference | reference | reference | reference |
| Warranted; performed within 96h | .015 | .59 (.39–.89) | .015 | .58 (.38–.90) |
| Not warranted | .030 | .68 (.49–.96) | .122 | .75 (.52–1.09) |
| Antimicrobial treatment | ||||
| Warranted; not appropriate within 48ha | reference | reference | reference | reference |
| Warranted; appropriate within 48h | .008 | .46 (.27–.79) | .018 | .60 (.39–.92) |
| Not warranted | 1.000 | .99 (.51–1.94) | .917 | 1.04 (.51–2.12) |
| . | Bivariate Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| . | P . | OR (95% CI) . | P . | aOR (95% CI) . |
| Malignancy (solid organ or hematologic) | .001 | 1.74 (1.28–2.36) | .012 | 1.89 (1.15–3.12) |
| Immunosuppression | .094 | 1.27 (.96–1.68) | .309 | .79 (.49–1.25) |
| Bacteremia by streptococci | .005 | .56 (.37–.84) | .049 | .64 (.41–.99) |
| Bacteremia by enterococci | .001 | .42 (.24–.73) | .001 | .37 (.20–.69) |
| Fungemia | .029 | 2.10 (1.10–4.03) | .242 | 1.52 (.75–3.07) |
| Persistent bacteremia/candidemia for 96ha | <.001 | 2.49 (1.69–3.69) | <.001 | 2.55 (1.46–3.49) |
| Spondylodiscitis | .050 | 1.70 (1.01–2.86) | .039 | 1.79 (1.03–3.12) |
| Infective endocarditis | .013 | .70 (.52–.93) | .207 | .80 (.57–1.13) |
| Intrabdominal infection | .001 | 2.61 (1.52–4.48) | .001 | 2.86 (1.55–5.27) |
| Source control | ||||
| Warranted; not performed within 96h | reference | reference | reference | reference |
| Warranted; performed within 96h | .015 | .59 (.39–.89) | .015 | .58 (.38–.90) |
| Not warranted | .030 | .68 (.49–.96) | .122 | .75 (.52–1.09) |
| Antimicrobial treatment | ||||
| Warranted; not appropriate within 48ha | reference | reference | reference | reference |
| Warranted; appropriate within 48h | .008 | .46 (.27–.79) | .018 | .60 (.39–.92) |
| Not warranted | 1.000 | .99 (.51–1.94) | .917 | 1.04 (.51–2.12) |
Abbreviations: aOR, adjusted odds ratios; CI, confidence interval; OR, odds ratio.
Persistent Fever Among Episodes With IE
Among 655 IE episodes with documented temperature upon presentation, 536 episodes were included, although 119 (18%) episodes were excluded due to absence of fever at presentation. Of the 536 included IE episodes, 82 (15%) exhibited persistent fever. The median duration of fever among IE episodes was 1 day (IQR: 1–3 days) from antimicrobial treatment initiation (Supplementary Table 1). Native bone and joint infections (27% vs 14%; P = .008), embolic events (51% vs 37%; P = .020), persistent bacteremia/candidemia for 96 hours (39% vs 11%; P < .001) and bacteremia by S. aureus (67% vs 38%; P < .001) were more prevalent among episodes with persistent fever, compared to those without (Table 3). Bacteremia by either streptococci or enterococci (45% vs 12%; P < .001) was less prevalent among episodes with persistent fever, compared to those with early defervescence. Cardiac lesions (vegetations ≥10 mm, intracardiac abscess) or type of valve (native or prosthetic) were not associated with persistent fever. The median duration of fever among episodes with either vegetation ≥10 mm, or intracardiac abscess was 1 day (IQR: 1–3 days) (Supplementary Table 1); even by excluding the episodes that had cardiac surgery within 4 days from antimicrobial treatment initiation the duration of fever for patients with vegetation ≥10 mm was 1 day (IQR: 1–3 days), and for intracardiac abscess 1 day (IQR: 1–4 days).
Characteristics of Patients With and With no Persistent Fever Among Patients With Infective Endocarditis
| . | No Persistent Fever (n = 454) . | Persistent Fever (n = 82) . | P . |
|---|---|---|---|
| Demographics | |||
| Male sex | 348 (77) | 60 (73) | .485 |
| Age (y) | 68 (54–77) | 66 (51–76) | .257 |
| Age >60 y | 301 (66) | 50 (61) | .378 |
| Comorbidities | |||
| Congestive heart failure | 53 (12) | 6 (7) | .337 |
| Chronic obstructive pulmonary disease | 48 (11) | 14 (17) | .094 |
| Cirrhosis | 27 (6) | 6 (7) | .619 |
| Diabetes mellitus | 108 (24) | 23 (28) | .405 |
| Chronic kidney disease (moderate or severe) | 362 (20) | 15 (18) | .765 |
| Malignancy (solid organ or hematologic) | 54 (12) | 9 (11) | 1.000 |
| Obesity | 113 (25) | 21 (26) | .890 |
| Immunosuppression | 91 (20) | 19 (23) | .553 |
| Cardiac predisposing factors | 291 (64) | 45 (55) | .136 |
| Microbiological data | |||
| S. aureus | 173 (38) | 55 (67) | <.001 |
| Coagulase negative staphylococci | 30 (7) | 4 (5) | .805 |
| Streptococci | 134 (30) | 10 (12) | .001 |
| Enterococci | 71 (16) | 0 (0) | <.001 |
| Other Gram-positive | 13 (3) | 1 (1) | .706 |
| HACEK | 12 (3) | 3 (4) | .713 |
| Other Gram-negative | 13 (3) | 3 (4) | .722 |
| Intracellular bacteria | 4 (.9) | 0 (0) | 1.000 |
| Fungi | 5 (1) | 4 (5) | .035 |
| Persistent bacteremia/candidemia for 96h | 50 (11) | 32 (39) | <.001 |
| Manifestations | |||
| Sepsis | 204 (45) | 49 (60) | .016 |
| Septic shock | 77 (17) | 19 (23) | .210 |
| Embolic events | 170 (37) | 42 (51) | .020 |
| Cerebral | 92 (20) | 19 (23) | .555 |
| Non-cerebral | 124 (27) | 32 (39) | .035 |
| Immunologic phenomena | 33 (7) | 12 (15) | .048 |
| Native bone and joint infection | 65 (14) | 22 (27) | .008 |
| Septic arthritis | 42 (9) | 14 (17) | .047 |
| Spondylodiscitis | 27 (6) | 11 (13) | .031 |
| Type of diagnosisa | |||
| Definite IE | 300 (66) | 59 (72) | .311 |
| Possible IE | 154 (34) | 23 (28) | |
| Site of infection | |||
| Aortic valve | 237 (52) | 40 (49) | .631 |
| Mitral valve | 182 (40) | 36 (44) | .543 |
| Tricuspid valve | 34 (8) | 11 (13) | .084 |
| Pulmonary valve | 9 (2) | 2 (2) | .679 |
| Multivalvular | 47 (10) | 11 (13) | .439 |
| CIED-IE | 61 (13) | 6 (7) | .147 |
| Other site of infection | 5 (1) | 2 (2) | .292 |
| Type of valve | |||
| Native | 299 (66) | 55 (67) | .899 |
| Prosthetic | 121 (27) | 24 (29) | .685 |
| Intracardiac lesions | |||
| Vegetation | 304 (67) | 55 (67) | 1.000 |
| Vegetation ≥10mm | 165 (36) | 33 (40) | .535 |
| Abscess | 83 (18) | 18 (22) | .444 |
| Other lesionsb | 79 (17) | 14 (17) | 1.000 |
| Source control warranted | 172 (38) | 40 (49) | .067 |
| Source control performed within 96hc | 76 (17) | 15 (18) | .482 |
| Appropriate antimicrobial treatment within 48h | 429 (95) | 75 (92) | .309 |
| . | No Persistent Fever (n = 454) . | Persistent Fever (n = 82) . | P . |
|---|---|---|---|
| Demographics | |||
| Male sex | 348 (77) | 60 (73) | .485 |
| Age (y) | 68 (54–77) | 66 (51–76) | .257 |
| Age >60 y | 301 (66) | 50 (61) | .378 |
| Comorbidities | |||
| Congestive heart failure | 53 (12) | 6 (7) | .337 |
| Chronic obstructive pulmonary disease | 48 (11) | 14 (17) | .094 |
| Cirrhosis | 27 (6) | 6 (7) | .619 |
| Diabetes mellitus | 108 (24) | 23 (28) | .405 |
| Chronic kidney disease (moderate or severe) | 362 (20) | 15 (18) | .765 |
| Malignancy (solid organ or hematologic) | 54 (12) | 9 (11) | 1.000 |
| Obesity | 113 (25) | 21 (26) | .890 |
| Immunosuppression | 91 (20) | 19 (23) | .553 |
| Cardiac predisposing factors | 291 (64) | 45 (55) | .136 |
| Microbiological data | |||
| S. aureus | 173 (38) | 55 (67) | <.001 |
| Coagulase negative staphylococci | 30 (7) | 4 (5) | .805 |
| Streptococci | 134 (30) | 10 (12) | .001 |
| Enterococci | 71 (16) | 0 (0) | <.001 |
| Other Gram-positive | 13 (3) | 1 (1) | .706 |
| HACEK | 12 (3) | 3 (4) | .713 |
| Other Gram-negative | 13 (3) | 3 (4) | .722 |
| Intracellular bacteria | 4 (.9) | 0 (0) | 1.000 |
| Fungi | 5 (1) | 4 (5) | .035 |
| Persistent bacteremia/candidemia for 96h | 50 (11) | 32 (39) | <.001 |
| Manifestations | |||
| Sepsis | 204 (45) | 49 (60) | .016 |
| Septic shock | 77 (17) | 19 (23) | .210 |
| Embolic events | 170 (37) | 42 (51) | .020 |
| Cerebral | 92 (20) | 19 (23) | .555 |
| Non-cerebral | 124 (27) | 32 (39) | .035 |
| Immunologic phenomena | 33 (7) | 12 (15) | .048 |
| Native bone and joint infection | 65 (14) | 22 (27) | .008 |
| Septic arthritis | 42 (9) | 14 (17) | .047 |
| Spondylodiscitis | 27 (6) | 11 (13) | .031 |
| Type of diagnosisa | |||
| Definite IE | 300 (66) | 59 (72) | .311 |
| Possible IE | 154 (34) | 23 (28) | |
| Site of infection | |||
| Aortic valve | 237 (52) | 40 (49) | .631 |
| Mitral valve | 182 (40) | 36 (44) | .543 |
| Tricuspid valve | 34 (8) | 11 (13) | .084 |
| Pulmonary valve | 9 (2) | 2 (2) | .679 |
| Multivalvular | 47 (10) | 11 (13) | .439 |
| CIED-IE | 61 (13) | 6 (7) | .147 |
| Other site of infection | 5 (1) | 2 (2) | .292 |
| Type of valve | |||
| Native | 299 (66) | 55 (67) | .899 |
| Prosthetic | 121 (27) | 24 (29) | .685 |
| Intracardiac lesions | |||
| Vegetation | 304 (67) | 55 (67) | 1.000 |
| Vegetation ≥10mm | 165 (36) | 33 (40) | .535 |
| Abscess | 83 (18) | 18 (22) | .444 |
| Other lesionsb | 79 (17) | 14 (17) | 1.000 |
| Source control warranted | 172 (38) | 40 (49) | .067 |
| Source control performed within 96hc | 76 (17) | 15 (18) | .482 |
| Appropriate antimicrobial treatment within 48h | 429 (95) | 75 (92) | .309 |
Data are depicted as number (percentage) or median (interquartile range).
Abbreviations: CIED, cardiac implantable electronic devices; HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IE, infective endocarditis; ISCVID, International Society of Cardiovascular Infectious Diseases; S. aureus, Staphylococcus aureus.
aAccording to the 2023 ISCVID version of the Duke criteria.
bPerforation, dehiscence of prosthetic valve, fistula, pseudoaneurysm, aneurysm.
CAmong 212 episodes.
Characteristics of Patients With and With no Persistent Fever Among Patients With Infective Endocarditis
| . | No Persistent Fever (n = 454) . | Persistent Fever (n = 82) . | P . |
|---|---|---|---|
| Demographics | |||
| Male sex | 348 (77) | 60 (73) | .485 |
| Age (y) | 68 (54–77) | 66 (51–76) | .257 |
| Age >60 y | 301 (66) | 50 (61) | .378 |
| Comorbidities | |||
| Congestive heart failure | 53 (12) | 6 (7) | .337 |
| Chronic obstructive pulmonary disease | 48 (11) | 14 (17) | .094 |
| Cirrhosis | 27 (6) | 6 (7) | .619 |
| Diabetes mellitus | 108 (24) | 23 (28) | .405 |
| Chronic kidney disease (moderate or severe) | 362 (20) | 15 (18) | .765 |
| Malignancy (solid organ or hematologic) | 54 (12) | 9 (11) | 1.000 |
| Obesity | 113 (25) | 21 (26) | .890 |
| Immunosuppression | 91 (20) | 19 (23) | .553 |
| Cardiac predisposing factors | 291 (64) | 45 (55) | .136 |
| Microbiological data | |||
| S. aureus | 173 (38) | 55 (67) | <.001 |
| Coagulase negative staphylococci | 30 (7) | 4 (5) | .805 |
| Streptococci | 134 (30) | 10 (12) | .001 |
| Enterococci | 71 (16) | 0 (0) | <.001 |
| Other Gram-positive | 13 (3) | 1 (1) | .706 |
| HACEK | 12 (3) | 3 (4) | .713 |
| Other Gram-negative | 13 (3) | 3 (4) | .722 |
| Intracellular bacteria | 4 (.9) | 0 (0) | 1.000 |
| Fungi | 5 (1) | 4 (5) | .035 |
| Persistent bacteremia/candidemia for 96h | 50 (11) | 32 (39) | <.001 |
| Manifestations | |||
| Sepsis | 204 (45) | 49 (60) | .016 |
| Septic shock | 77 (17) | 19 (23) | .210 |
| Embolic events | 170 (37) | 42 (51) | .020 |
| Cerebral | 92 (20) | 19 (23) | .555 |
| Non-cerebral | 124 (27) | 32 (39) | .035 |
| Immunologic phenomena | 33 (7) | 12 (15) | .048 |
| Native bone and joint infection | 65 (14) | 22 (27) | .008 |
| Septic arthritis | 42 (9) | 14 (17) | .047 |
| Spondylodiscitis | 27 (6) | 11 (13) | .031 |
| Type of diagnosisa | |||
| Definite IE | 300 (66) | 59 (72) | .311 |
| Possible IE | 154 (34) | 23 (28) | |
| Site of infection | |||
| Aortic valve | 237 (52) | 40 (49) | .631 |
| Mitral valve | 182 (40) | 36 (44) | .543 |
| Tricuspid valve | 34 (8) | 11 (13) | .084 |
| Pulmonary valve | 9 (2) | 2 (2) | .679 |
| Multivalvular | 47 (10) | 11 (13) | .439 |
| CIED-IE | 61 (13) | 6 (7) | .147 |
| Other site of infection | 5 (1) | 2 (2) | .292 |
| Type of valve | |||
| Native | 299 (66) | 55 (67) | .899 |
| Prosthetic | 121 (27) | 24 (29) | .685 |
| Intracardiac lesions | |||
| Vegetation | 304 (67) | 55 (67) | 1.000 |
| Vegetation ≥10mm | 165 (36) | 33 (40) | .535 |
| Abscess | 83 (18) | 18 (22) | .444 |
| Other lesionsb | 79 (17) | 14 (17) | 1.000 |
| Source control warranted | 172 (38) | 40 (49) | .067 |
| Source control performed within 96hc | 76 (17) | 15 (18) | .482 |
| Appropriate antimicrobial treatment within 48h | 429 (95) | 75 (92) | .309 |
| . | No Persistent Fever (n = 454) . | Persistent Fever (n = 82) . | P . |
|---|---|---|---|
| Demographics | |||
| Male sex | 348 (77) | 60 (73) | .485 |
| Age (y) | 68 (54–77) | 66 (51–76) | .257 |
| Age >60 y | 301 (66) | 50 (61) | .378 |
| Comorbidities | |||
| Congestive heart failure | 53 (12) | 6 (7) | .337 |
| Chronic obstructive pulmonary disease | 48 (11) | 14 (17) | .094 |
| Cirrhosis | 27 (6) | 6 (7) | .619 |
| Diabetes mellitus | 108 (24) | 23 (28) | .405 |
| Chronic kidney disease (moderate or severe) | 362 (20) | 15 (18) | .765 |
| Malignancy (solid organ or hematologic) | 54 (12) | 9 (11) | 1.000 |
| Obesity | 113 (25) | 21 (26) | .890 |
| Immunosuppression | 91 (20) | 19 (23) | .553 |
| Cardiac predisposing factors | 291 (64) | 45 (55) | .136 |
| Microbiological data | |||
| S. aureus | 173 (38) | 55 (67) | <.001 |
| Coagulase negative staphylococci | 30 (7) | 4 (5) | .805 |
| Streptococci | 134 (30) | 10 (12) | .001 |
| Enterococci | 71 (16) | 0 (0) | <.001 |
| Other Gram-positive | 13 (3) | 1 (1) | .706 |
| HACEK | 12 (3) | 3 (4) | .713 |
| Other Gram-negative | 13 (3) | 3 (4) | .722 |
| Intracellular bacteria | 4 (.9) | 0 (0) | 1.000 |
| Fungi | 5 (1) | 4 (5) | .035 |
| Persistent bacteremia/candidemia for 96h | 50 (11) | 32 (39) | <.001 |
| Manifestations | |||
| Sepsis | 204 (45) | 49 (60) | .016 |
| Septic shock | 77 (17) | 19 (23) | .210 |
| Embolic events | 170 (37) | 42 (51) | .020 |
| Cerebral | 92 (20) | 19 (23) | .555 |
| Non-cerebral | 124 (27) | 32 (39) | .035 |
| Immunologic phenomena | 33 (7) | 12 (15) | .048 |
| Native bone and joint infection | 65 (14) | 22 (27) | .008 |
| Septic arthritis | 42 (9) | 14 (17) | .047 |
| Spondylodiscitis | 27 (6) | 11 (13) | .031 |
| Type of diagnosisa | |||
| Definite IE | 300 (66) | 59 (72) | .311 |
| Possible IE | 154 (34) | 23 (28) | |
| Site of infection | |||
| Aortic valve | 237 (52) | 40 (49) | .631 |
| Mitral valve | 182 (40) | 36 (44) | .543 |
| Tricuspid valve | 34 (8) | 11 (13) | .084 |
| Pulmonary valve | 9 (2) | 2 (2) | .679 |
| Multivalvular | 47 (10) | 11 (13) | .439 |
| CIED-IE | 61 (13) | 6 (7) | .147 |
| Other site of infection | 5 (1) | 2 (2) | .292 |
| Type of valve | |||
| Native | 299 (66) | 55 (67) | .899 |
| Prosthetic | 121 (27) | 24 (29) | .685 |
| Intracardiac lesions | |||
| Vegetation | 304 (67) | 55 (67) | 1.000 |
| Vegetation ≥10mm | 165 (36) | 33 (40) | .535 |
| Abscess | 83 (18) | 18 (22) | .444 |
| Other lesionsb | 79 (17) | 14 (17) | 1.000 |
| Source control warranted | 172 (38) | 40 (49) | .067 |
| Source control performed within 96hc | 76 (17) | 15 (18) | .482 |
| Appropriate antimicrobial treatment within 48h | 429 (95) | 75 (92) | .309 |
Data are depicted as number (percentage) or median (interquartile range).
Abbreviations: CIED, cardiac implantable electronic devices; HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IE, infective endocarditis; ISCVID, International Society of Cardiovascular Infectious Diseases; S. aureus, Staphylococcus aureus.
aAccording to the 2023 ISCVID version of the Duke criteria.
bPerforation, dehiscence of prosthetic valve, fistula, pseudoaneurysm, aneurysm.
CAmong 212 episodes.
In multivariable logistic regression (Table 4), persistent bacteremia/candidemia for 96 hours (P < .001; aOR 2.71, 95% CI: 1.54–4.77), and native bone and joint infections (P = .020; aOR 2.07, 95% CI: 1.12–3.83) were associated with persistent fever among IE episodes. Conversely, bacteremia by streptococci or enterococci (P = .001; aOR 0.25, 95% CI: .11–.58) were associated with early defervescence.
Bivariate and Multivariable Analyses of Predictors of Persistent Fever Among Patients With Infective Endocarditis
| . | Bivariate Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| . | P . | OR (95% CI) . | P . | aOR (95% CI) . |
| Bacteremia by S. aureus | <.001 | 3.31 (2.01–5.44) | .890 | .96 (.50–1.84) |
| Bacteremia by streptococci or enterococci | <.001 | .17 (.09–.34) | .001 | .25 (.11–.58) |
| Persistent bacteremia/candidemia for 96h | <.001 | 5.17 (3.04–8.81) | <.001 | 2.71 (1.54–4.77) |
| Sepsis | .016 | 1.81 (.12–2.93) | .343 | 1.30 (.76–2.21) |
| Embolic events | .020 | 1.75 (1.09–2.82) | .120 | 1.54 (.89–2.67) |
| Native bone and joint infection | .008 | 2.19 (1.26–3.82) | .020 | 2.07 (1.12–3.83) |
| Immunological phenomena | .048 | 2.19 (1.08–4.44) | .331 | 1.52 (.66–3.52) |
| Tricuspid valve infective endocarditis | .084 | 1.91 (.93–3.95) | .996 | .99 (.45–2.23) |
| . | Bivariate Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| . | P . | OR (95% CI) . | P . | aOR (95% CI) . |
| Bacteremia by S. aureus | <.001 | 3.31 (2.01–5.44) | .890 | .96 (.50–1.84) |
| Bacteremia by streptococci or enterococci | <.001 | .17 (.09–.34) | .001 | .25 (.11–.58) |
| Persistent bacteremia/candidemia for 96h | <.001 | 5.17 (3.04–8.81) | <.001 | 2.71 (1.54–4.77) |
| Sepsis | .016 | 1.81 (.12–2.93) | .343 | 1.30 (.76–2.21) |
| Embolic events | .020 | 1.75 (1.09–2.82) | .120 | 1.54 (.89–2.67) |
| Native bone and joint infection | .008 | 2.19 (1.26–3.82) | .020 | 2.07 (1.12–3.83) |
| Immunological phenomena | .048 | 2.19 (1.08–4.44) | .331 | 1.52 (.66–3.52) |
| Tricuspid valve infective endocarditis | .084 | 1.91 (.93–3.95) | .996 | .99 (.45–2.23) |
Abbreviations: aOR, adjusted odds ratios; CI, confidence interval; OR, odds ratio; S. aureus, Staphylococcus aureus.
Bivariate and Multivariable Analyses of Predictors of Persistent Fever Among Patients With Infective Endocarditis
| . | Bivariate Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| . | P . | OR (95% CI) . | P . | aOR (95% CI) . |
| Bacteremia by S. aureus | <.001 | 3.31 (2.01–5.44) | .890 | .96 (.50–1.84) |
| Bacteremia by streptococci or enterococci | <.001 | .17 (.09–.34) | .001 | .25 (.11–.58) |
| Persistent bacteremia/candidemia for 96h | <.001 | 5.17 (3.04–8.81) | <.001 | 2.71 (1.54–4.77) |
| Sepsis | .016 | 1.81 (.12–2.93) | .343 | 1.30 (.76–2.21) |
| Embolic events | .020 | 1.75 (1.09–2.82) | .120 | 1.54 (.89–2.67) |
| Native bone and joint infection | .008 | 2.19 (1.26–3.82) | .020 | 2.07 (1.12–3.83) |
| Immunological phenomena | .048 | 2.19 (1.08–4.44) | .331 | 1.52 (.66–3.52) |
| Tricuspid valve infective endocarditis | .084 | 1.91 (.93–3.95) | .996 | .99 (.45–2.23) |
| . | Bivariate Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| . | P . | OR (95% CI) . | P . | aOR (95% CI) . |
| Bacteremia by S. aureus | <.001 | 3.31 (2.01–5.44) | .890 | .96 (.50–1.84) |
| Bacteremia by streptococci or enterococci | <.001 | .17 (.09–.34) | .001 | .25 (.11–.58) |
| Persistent bacteremia/candidemia for 96h | <.001 | 5.17 (3.04–8.81) | <.001 | 2.71 (1.54–4.77) |
| Sepsis | .016 | 1.81 (.12–2.93) | .343 | 1.30 (.76–2.21) |
| Embolic events | .020 | 1.75 (1.09–2.82) | .120 | 1.54 (.89–2.67) |
| Native bone and joint infection | .008 | 2.19 (1.26–3.82) | .020 | 2.07 (1.12–3.83) |
| Immunological phenomena | .048 | 2.19 (1.08–4.44) | .331 | 1.52 (.66–3.52) |
| Tricuspid valve infective endocarditis | .084 | 1.91 (.93–3.95) | .996 | .99 (.45–2.23) |
Abbreviations: aOR, adjusted odds ratios; CI, confidence interval; OR, odds ratio; S. aureus, Staphylococcus aureus.
DISCUSSION
In our cohort of episodes with suspected IE, persistent fever was observed in 19% of episodes, with no association between IE and persistent fever.
As previously reported, fever was a common clinical sign of IE [1, 2]. In our study, 81% of episodes with suspected IE and 82% of IE episodes presented with fever, consistent with findings from 2 large IE studies; 78% of IE cases had fever in the EURO-ENDO registry [1] and 96% in the International Collaboration on Endocarditis-Prospective Cohort Study [2]. Although fever is a minor Duke criterion for diagnosing IE [3–5], a study of 3127 episodes with suspected IE found the prevalence of fever to be similar among patients with confirmed IE (78%) and those whose suspicion of IE was ultimately ruled out (79%), indicating that fever alone lacks the discriminatory power to distinguish IE from non-IE cases [6].
As expected, among episodes with suspected IE in general and those with IE, persistent fever was associated with persistent bacteremia/candidemia, as both are hallmarks of uncontrolled infection. Successful management of infection relies on two core components: appropriate antimicrobial treatment and timely source control. In our study, most episodes with suspected IE requiring antimicrobial therapy received appropriate treatment within 48 hours, which was associated with early resolution of fever. Similarly, among episodes with suspected IE that necessitated source control, performing these interventions promptly was linked to early defervescence. As previously demonstrated in studies on S. aureus bacteremia, timely source control not only accelerated the clearance of bacteremia but also reduced mortality [20, 21]. This emphasizes the clinical importance of early fever resolution as an indicator of effective infection management. In cases of S. aureus bacteremia, fever persistence beyond 72 hours is a marker of complicated infection, along with persistent bacteremia and lack of source control. The presence of any of these criteria necessitates extending antibiotic therapy from 2 weeks to 4–6 weeks [7].
In suspected IE cases, certain infections, such as spondylodiscitis, were also linked to persistent fever in IE patients. Spondylodiscitis often coincides with paravertebral or epidural abscesses, which are typically small and do not cause progressive neurological deficits, spinal instability, or significant deformity, and therefore do not always require surgical intervention [22]. However, even small abscesses can continue to act as a source of bacterial spread due to reduced antimicrobial penetration. Persistent bacteremia despite appropriate antimicrobial therapy indicates the need for source control in patients with spondylodiscitis [22]. These findings align with the established association between spondylodiscitis and IE [23], as reflected in risk prediction models for S. aureus bacteremia (VIRSTA and LAUSTAPHEN scores) [24, 25].
In cases with diagnosed IE, few studies have examined the course of persistent fever, with none conducted in the past two decades. Historically, Douglas et al reported that 36% of 83 IE cases had persistent fever (>7 days), although they used a lower temperature threshold (37.1°C or >98.8°F) [10]. Other retrospective studies reported that fever persisted for more than 7 days in 28% of 123 and 36% of 193 IE cases [12, 13]. However, in the present study, only 15% of IE episodes presented with persistent fever (≥4 days). It is worth noting that in most of these earlier studies, IE was diagnosed using the von Reyn criteria, and the definition of persistent fever varied in terms of temperature cut-off and duration [10, 12, 13]. Despite these methodological differences, the duration of fever in all prior studies was significantly longer than in the present study, where the median duration was only 1 day. Interestingly, in contrast to older studies, large vegetations (≥10 mm), intracardiac abscesses, and embolic events were not associated with prolonged fever in our cohort, even after excluding patients that had early valve surgery [10, 11, 13]. For example, the median duration of fever was 1 day for patients with vegetations (IQR: 1–3 days), intracardiac abscesses (IQR: 1–3 days), and those with embolic events (IQR: 1–3 days). This is in stark contrast to the study by Lederman et al, which reported fever lasting 10 days in patients with vegetations and 9 days in those with embolic events [13]. Thus, early defervescence or early clearance of blood cultures, especially in non-staphylococcal IE cases [26], should not rule out the presence of intracardiac complications. A TEE should be performed on all patients with IE, or high suspicion of IE, even if there is rapid clinical improvement following the initiation of antimicrobial treatment [19]. The discrepancies in the duration of fever in the present study compared to those conducted previously could be explained by an advancements in diagnostic tools (microbiological and radiological) over the past three decades, allowing for earlier identification of patients with less advanced intracardiac invasion. In our study, patients with S. aureus bacteremia had the first echocardiography within 3 days of the first positive blood culture, compared to 5.7 days in a study conducted more than 20 years ago [27]. Earlier identification of IE patients has led to improved outcomes and reduced mortality by enabling earlier management of such patients [28].
Moreover, IE episodes caused by S. aureus were more likely to exhibit persistent fever, consistent with earlier findings [10, 12, 13], although this association was not confirmed in multivariable logistic regression. This suggests that other factors, such as persistent bacteremia or native bone and joint infections like spondylodiscitis, may mediate the relationship [24, 25]. Conversely, bacteremia caused by streptococci and enterococci was associated with a lower likelihood of persistent fever in both suspected IE and confirmed IE cases. Previous studies also demonstrated that fever duration was shorter in IE caused by enterococci and streptococci compared to S. aureus IE or culture negative IE [12, 13, 29].
This study has several limitations. First, it is a single-center study; however, to our knowledge, it is the largest study to date that explores persistent fever in patients with suspected IE and the only one conducted in the last 2 decades. Second, because this study was performed in a Swiss university hospital where ID specialists evaluate all suspected IE cases, with access to advanced imaging such as 18F-FDG PET/CT and cardiac CT for valvular and paravalvular lesions, as well as comprehensive imaging for embolic events, the findings may have limited generalizability to other healthcare systems. Third, due to the lack of a definitive gold standard for diagnosing IE, the Endocarditis Team's evaluation was used as the reference standard. Fourth, we did not account for medications such as paracetamol, anti-inflammatory drugs, or corticosteroids, which can affect body temperature. Fifth, we did not collect data on subfebrile temperatures (37.5–38°C), which in our study were considered afebrile. Therefore, we cannot exclude the possibility that patients with cardiac complications may have remained subfebrile for a longer duration than those without such complications.
In conclusion, our study challenges the traditional association between persistent fever and IE, because no association between persistent fever and IE among patients with suspected IE was found, with most patients achieving defervescence withing 4 days of initiating antimicrobial treatment. Among episodes with suspected IE, persistent fever was linked to inappropriate antimicrobial treatment, lack of source control interventions, and spondylodiscitis, whereas among IE patients, it was associated with native bone and joint infections. Notably, in these patients, large vegetations (≥10 mm), intracardiac abscess, or embolic events were not associated with persistent fever. These findings underscore the importance of reassessing patients with persistent fever despite appropriate antimicrobial therapy, which may require repeated imaging and consideration of source control procedures. Identifying these predictors can guide necessary investigations and optimize intervention timing, ultimately improving patient outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. M. P. O., P. M., and B. G. conceived the idea. E. S., P. M., G. T., N. I., P. T., M. K., B. G., and M. P. O. collected the patients' data. M. P. O. supervised the project. M. P. O. performed the analysis and interpreted the results. E. S. wrote the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
References
Author notes
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.




