-
PDF
- Split View
-
Views
-
Cite
Cite
Marva Seifert, Donald G Catanzaro, Michael Gracia, Naomi Hillery, Sabira Tahseen, Faisal Masood, Alamdar Hussain, Uzma Majeed, Rebecca E Colman, Rehan R Syed, Antonino Catanzaro, Timothy Rodwell, Prospective Exploratory Evaluation of Cepheid Xpert Mycobacterium tuberculosis Host Response Cartridge: A Focus on Adolescents and Young Adults, Clinical Infectious Diseases, Volume 80, Issue 1, 15 January 2025, Pages 180–188, https://doi.org/10.1093/cid/ciae461
Close - Share Icon Share
Abstract
An accurate, rapid, non-sputum-based triage test for diagnosing tuberculosis (TB) is needed.
A prospective evaluation of the Cepheid GeneXpert Mycobacterium tuberculosis Host Response cartridge (Xpert-MTB-HR), a prototype blood-based host response mRNA signature assay, among individuals presenting with TB-like symptoms was performed in Pakistan and results were compared to 3 reference standards: Xpert MTB/RIF Ultra, bacteriological confirmation (Xpert MTB/RIF Ultra and/or culture positivity), and composite clinical diagnosis (clinician diagnosis, treatment initiation, Xpert MTB/RIF Ultra, and/or culture positivity). Analyses were conducted both for the entire study cohort and separately in the adolescent and young adult cohort (aged 10–24 years).
A total of 497 participants, aged 6–83 years, returned valid Xpert-MTB-HR results. When a diagnostic threshold was set for a sensitivity of >90%, specificity was 32% (95% confidence interval [CI], 28%–37%) compared to Xpert MTB/RIF Ultra, 29% (95% CI, 25%–34%) compared to a bacteriological confirmation, and 22% (95% CI, 18%–26%) compared to a composite clinical diagnosis. However, when evaluating only the adolescent and young adult cohort with a diagnostic threshold set for sensitivity of >90%, specificity was 82% (95% CI, 74%–89%) compared to Xpert MTB/RIF Ultra, 84% (95% CI, 75%–90%) compared to a bacteriological confirmation, and 54% (95% CI, 44%–64%) compared to a composite clinical diagnosis.
While the Xpert-MTB-HR does not meet World Health Organization minimum criteria in the general population, in our study it does meet the minimum sensitivity and specificity requirements for a non-sputum-based triage test among adolescents and young adults when compared to Xpert MTB/RIF Ultra or bacteriological confirmation.
Rapid and accurate detection of tuberculosis disease (TB) is critical for reducing ongoing transmission and improving treatment outcomes. Yet, despite recent advances in novel diagnostic technologies, in 2022 only 63% of all new cases of TB were bacteriologically confirmed [1]. This gap in diagnosis, which is driven in part by the variable detection capabilities of currently available diagnostics, has led the World Health Organization (WHO) to call for the development of novel non-sputum-based triage tests with a minimum sensitivity of 90% and specificity of 70% [1, 2]. In response, Cepheid developed a prototype blood-based cartridge, the Xpert-MTB-HR, which was designed to detect host response to TB by quantifying messenger RNA (mRNA) from 3 genes associated with TB in large population studies [3]. While most TB diagnostics are designed to detect Mycobacterium tuberculosis, the causative agent of disease, or its by-products in sputum or urine, the Xpert-MTB-HR was designed to identify a host mRNA signature associated with active TB disease.
This Xpert-MTB-HR mRNA signature or TBTBP score has been described previously and includes cycle threshold (Ct) values from 2 upregulated genes, GBP5 and DUSP3, and 1 downregulated gene, TBP [4]. GBP5 encodes a protein that plays a role in innate immunity and inflammation, DUSP3 encodes a protein associated with inactivation of proteins associated with cellular proliferation and differentiation, and TBP is associated with genetic transcription and is typically highly expressed in lymph node tissues [5]. While the Xpert-MTB-HR prototype yielded promising results in an initial study of individuals with TB-like symptoms, and produced an area under the curve (AUC) of 0.94 (95% confidence interval [CI], .88–.99) [6], variability in subsequent studies indicates the need for a more nuanced approach to clinical evaluation. When assessing the ability of the cartridge to discriminate between individuals with microbiologically confirmed TB and those with other respiratory diseases, 4 studies conducted among adults produced AUCs that ranged from 0.94 (95% CI, .91–.97) [7] and 0.94 (95% CI, .89–.98) [8] to 0.89 (95% CI, .86–.91) [4] and 0.80 (95% CI, .75–.85) [9]. A study among children aged 0–15 years produced an overall AUC of 0.85 (95% CI, .80–.89); however, in this study among a subset of children aged 10–14 years, the AUC was 0.88 [10]. This variability between studies and study cohorts warrants further investigation and we propose to evaluate the performance of the cartridge by age.
It is well known that the risk of TB infection and incidence rates change over the life course, yet the exact mechanisms that drive these differences are not well understood [11, 12]. Fluctuating sex hormones, differences in social contact patterns, and immune system changes are all thought to be factors that contribute to these changes in disease acquisition and presentation [13, 14]. In settings where TB is endemic, the risk of exposure to M. tuberculosis increases with age; however, the relationship between age and disease incidence is not linear [11, 15]. Among children <5 years of age, the risk of progression to extrapulmonary forms of the disease following infection is substantial [16]. However, as children age, the risk of developing disease decreases, and between the ages of 5 and 9 children have the lowest incidence of TB. The risk of disease rises again dramatically as children enter adolescence and young adulthood, posing significant concern as this age cohort accounts for a considerable proportion of the population in many countries where TB is endemic [11, 13]. Despite the growing recognition of the unique epidemiology of TB by age, the burden of disease among adolescents and young adults has historically not been well documented. Traditionally, TB incidence is reported for 2 major categories, children (14 years and younger) and adults (15 years and older). This stratification obscures the changing relationship between risk of infection and disease, and recent calls for more age-specific reporting have been made [13, 14]. A 2012 study estimated that 1.8 million new cases occur in adolescents and young adults annually, accounting for 18% of global TB incidence [17]. This adolescent and young adult (AYA) cohort, defined as individuals aged 10–24 years, corresponds to biological maturation and social exposure changes thought to be associated with TB [13, 14, 18]. Due to the currently evolving understanding of the unique risks of TB incidence in the AYA cohort, in this study we describe not only the diagnostic performance of the Xpert-MTB-HR in the entire study cohort, but we also perform a separate analysis among the 125 individuals in the AYA cohort.
MATERIALS AND METHODS
Overview
We conducted a prospective diagnostic performance study among patients seeking care in Pakistan at either Federal General Hospital in Islamabad or Rawalpindi Leprosy Hospital in Rawalpindi. Patients presenting at these facilities who indicated they had a cough for >2 weeks, or of any duration with symptoms consistent with TB (expectoration, fever, night sweats, loss of appetite), were entered into the respective hospital's presumptive TB register and referred for Xpert MTB/RIF testing by their treating clinician. Patients on the presumptive TB register who were aged 5 years or older, and not incarcerated or institutionalized, were eligible for the study. Patients were excluded if they had been treated for TB at any time within the previous 6 months or had already initiated treatment for TB when invited to enroll in the study. After completing the consent process, participants were interviewed by study personnel, asked to provide sputum and blood samples, and had additional data extracted from their medical records. Participants who were not diagnosed with TB initially were contacted 3 months postenrollment to determine if any additional TB testing had occurred and if the participant had initiated treatment for TB.
The protocol was reviewed and approved by the institutional review board at the University of California, San Diego and the National Bioethics Committee of Pakistan.
Procedures
A portion of raw sputa collected was evaluated using Xpert MTB/RIF Ultra (Cepheid) and the remainder was processed using N-acetyl-L-cysteine/sodium hydroxide (NALC/NaOH) decontamination according to the National TB Program protocol and used for standard testing. Venous blood samples were collected for human immunodeficiency virus (HIV) testing, QuantiFERON-TB Gold Plus (QFT-Plus, Qiagen, Venlo, Netherlands), and the Cepheid GeneXpert Mycobacterium tuberculosis Host Response cartridge (Xpert-MTB-HR). Approximately 50 µL of whole blood was used for HIV rapid testing using Abbot Determine HIV Early Detect (Abbot Diagnostics Medical Co. Ltd, Japan), 1 mL of blood was collected directly into each of the 4 QFT-Plus collection tubes and incubated at 37°C for antigenic stimulation, and 100 µL of whole blood was transferred via pipette into the sample chamber for Xpert-MTB-HR cartridge for analysis.
Reference Testing and Classification
Composite clinical diagnosis of TB included participants with a positive result on Xpert MTB/RIF Ultra (including trace results), those with a positive result on mycobacteria growth indicator tube liquid culture, or participants who were clinically diagnosed by a physician and/or placed on treatment either at enrollment or at 3-month follow-up. Participants with negative bacteriological tests and no clinical diagnosis, but who returned a positive result on QFT-Plus were classified as TB infected (TBI). Participants with a negative result on both bacteriological tests, who were not clinically diagnosed by a physician and/or placed on treatment either at enrollment or during follow-up, and who had a negative or indeterminate result on QFT-Plus were classified as not having TB.
Automated Xpert-MTB-HR Processing
All samples collected and analyzed in this study were venous ethylenediaminetetraacetic acid (EDTA) blood. The protocol allowed for storing the EDTA tube at −4°C for up to 24 hours before either initial testing or retesting. Blood was added directly to the cartridge, scanned, and placed into the GeneXpert instrument for testing. In cases where the first test yielded a nonvalid result, the protocol was to retest samples once by extracting an additional 100 µL of whole blood from the EDTA tube after thorough inversion.
Ct values for 3 target genes (GBP5, DUSP3, and TBP) were quantified and used to calculate a TBTBP score using the following formula: (GBP5 Ct + DUSP3 Ct)/2 – TBP Ct. This differs from the previous version of the Xpert-MTB-HR cartridge, which used KLF2 instead of TBP in the formula. This change was recommended by the manufacturer as TBP mRNA levels were thought to be more stable compared to KLF2 [4]. No prespecified cut-point value was provided by the manufacturer.
Statistical Analysis
Study data were collected and managed using a secure, web-based software platform designed to support data capture for research studies using Research Electronic Data Capture (REDCap) tools [19, 20]. All other analysis were conducted using Stata version 17 software (StataCorp LLC, College Station, Texas). The relationships between TB status and patient or disease characteristics were assessed using χ2, rank-sum, or t test. Graphs were used to visualize the distribution of the TBTBP score and the Kruskal-Wallis test was used to compare distribution differences between groups and the Dunn test for pairwise post hoc comparisons. AUC analysis was performed against 3 reference standards—(1) Xpert MTB/RIF Ultra diagnosis, (2) bacteriological confirmation (Xpert MTB/RIF Ultra positive and/or liquid culture positive), and (3) composite clinical TB diagnosis (physician diagnosis, a record of treatment initiation, and/or positive bacteriological confirmation)—using 3 optimization methods for cut-point determination for both the entire study cohort and the AYA cohort separately. These methods included the Youden method, which maximizes the sum of sensitivity and specificity; the Liu method, which maximizes the product of sensitivity and specificity; and by setting sensitivity at 90% and assessing the corresponding specificity. Results from these cut-point methods were presented as sensitivity and specificity with confidence intervals (CIs) constructed using the Wilson score method.
RESULTS
Clinical Characteristics
Between May 2022 and May 2023, a total of 1358 patients referred to TB testing at either facility were prescreened (Figure 1). Of the 500 Xpert-MTB-HR tests performed, 26 (5%) returned a nonvalid result and of the 24 retested, 23 yielded valid results, resulting in 497 Xpert-MTB-HR results for analysis. One hundred eighty-four (37%) samples were frozen and stored prior to processing. Ninety-seven participants (20%) were classified as having TB using the composite clinical TB diagnosis reference standard, 201 (40%) as TBI, and the remaining 199 (40%) as not diagnosed with TB. Among the 81 individuals diagnosed with biologically confirmed TB, 74 (91%) were Xpert MTB/RIF Ultra positive and 63 (78%) were culture positive. Of the remaining 16 individuals classified as having TB but who were not bacteriologically confirmed, 11 (69%) were clinically diagnosed at enrollment and 5 (31%) had initiated treatment for TB by the 3-month follow-up.
![Flowchart of patients included for final analysis (Standards for Reporting Diagnostic Accuracy Studies [STARD] flow diagram). Abbreviation: QFT, QuantiFERON; TB, tuberculosis.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/80/1/10.1093_cid_ciae461/2/m_ciae461f1.jpeg?Expires=1750262762&Signature=I2I-F2uDMYxMnd~4Az2nrTUdoX1K-XT7m3-DHaLCcAUPdCtJdretVVhASWhBebwnvsQ0-rdDbYxvllYNDvvy46PCxRtxd5c4iRfourZhiWT5nZlQ42~HrLEHb69-fgqT1Om1Qj-DiiC7Pd2Nb~Cup~GBeAIusiThhZNmkSNGTXWrWwCNe0U8zZ-uaA~gpHAJ2sczdtiYWU0wyo496ySyIutikAVPP~LxUsZFRloEPLig9BzvYatSV7vXYreJ26r4h3T2vGeaDk4gHS75carTB77G96RlIQqUwABFcOv0WBhOp24ffIS9HinDUPDgHTvSag8eROPVEZmaeN3RZXGFiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Flowchart of patients included for final analysis (Standards for Reporting Diagnostic Accuracy Studies [STARD] flow diagram). Abbreviation: QFT, QuantiFERON; TB, tuberculosis.
Median age of the study cohort was 36 years (interquartile range, 24–50 years) and 244 participants (49%) were female. One hundred sixteen (23%) participants reported at least 1 comorbid condition (Table 1), which was defined as having asthma, diabetes, hepatitis B, hepatitis C, chronic kidney disease, a history of transplant, and/or an immunosuppressive condition. Individuals classified as having TB were more likely to have reported unexplained weight loss and night sweats, and more likely to have X-ray findings with infiltrates and nodules compared to undiagnosed individuals or those with TBI (P < .05). The TBTBP score among the 497 valid Xpert-MTB-HR results varied significantly by TB diagnostic status, and participants with TB had lower TBTBP scores compared to TBI and not infected categories (P < .001).
Participant Demographics, Disease Characteristics, and Diagnostic Results by Tuberculosis Status
| Characteristic . | TB (n = 97) . | TBI (n = 201) . | Not TB/TBI (n = 199) . | Total (N = 497) . |
|---|---|---|---|---|
| Male sex | 47 (48.5) | 101 (50.2) | 105 (52.8) | 253 (50.9) |
| Age | ||||
| Years, median (IQR) | 38 (25–51) | 37 (26–51) | 35 (22–48) | 36 (24–50) |
| AYA | 24 (24.7) | 42 (20.9) | 59 (29.6) | 125 (25.2) |
| HIV infected | 1 (1.0) | 3 (1.5) | 1 (0.5) | 5 (1.0) |
| With comorbid condition(s) | 29 (29.9) | 41 (20.4) | 46 (23.1) | 116 (23.3) |
| Previous history of TB | ||||
| Yes | 23 (23.7) | 56 (27.9) | 29 (14.6) | 108 (21.7) |
| Symptoms | ||||
| Persistent cough | 85 (87.6) | 157 (78.1) | 170 (85.4) | 412 (82.9) |
| Fever | 90 (92.8) | 185 (92.0) | 189 (95.0) | 464 (93.4) |
| Fatigue | 83 (85.6) | 172 (85.6) | 146 (73.4) | 401 (80.7) |
| Coughing blood | 18 (18.6) | 24 (11.9) | 30 (15.1) | 72 (14.5) |
| Weight loss | 79 (81.4) | 119 (59.2) | 131 (65.8) | 329 (66.2) |
| Night sweats | 34 (35.1) | 42 (20.9) | 48 (24.1) | 124 (24.9) |
| Duration of symptoms, d, median (IQR) | 60 (30–90) | 30 (20–60) | 30 (18–90) | 30 (20–90) |
| QuantiFERON status | ||||
| Indeterminate | 11 (11.3) | 0 (0.0) | 51 (25.6) | 62 (12.5) |
| Positive | 70 (72.2) | 201 (100.0) | 0 (0.0) | 271 (54.5) |
| Negative | 16 (16.5) | 0 (0.0) | 148 (74.4) | 164 (33.0) |
| X-ray findings | ||||
| Infiltrates | 70 (72.2) | 72 (35.8) | 60 (30.2) | 202 (40.6) |
| Nodules | 18 (18.6) | 12 (6.0) | 14 (7.0) | 44 (8.9) |
| Lymph | 2 (2.1) | 16 (8.0) | 14 (7.0) | 32 (6.4) |
| Cavities | 5 (5.2) | 3 (1.5) | 4 (2.0) | 12 (2.4) |
| Pleural effusion | 6 (6.2) | 11 (5.5) | 4 (2.0) | 21 (4.2) |
| Smear status positive | 30 (30.9) | 0 (0.0) | 0 (0.0) | 30 (6.0) |
| TB culture status | ||||
| Contaminated | 5 (5.2) | 6 (3.0) | 3 (1.5) | 14 (2.8) |
| Negative | 29 (29.9) | 195 (97.0) | 196 (98.5) | 420 (84.5) |
| Positive | 63 (64.9) | 0 (0.0) | 0 (0.0) | 63 (12.7) |
| Xpert MTB/RIF Ultra | ||||
| Not detected | 23 (23.7) | 201 (100.0) | 199 (100.0) | 423 (85.1) |
| MTB trace | 12 (12.4) | 0 (0.0) | 0 (0.0) | 12 (2.4) |
| MTB very low | 10 (10.3) | 0 (0.0) | 0 (0.0) | 10 (2.0) |
| MTB low | 24 (24.7) | 0 (0.0) | 0 (0.0) | 24 (4.8) |
| MTB medium | 11 (11.3) | 0 (0.0) | 0 (0.0) | 11 (2.2) |
| MTB high | 17 (17.5) | 0 (0.0) | 0 (0.0) | 17 (3.4) |
| Xpert-MTB-HR | ||||
| TBTBP score, median (IQR) | −2.7 (−1.6 to −3.4) | −1.4 (−1.0 to −1.8) | −1.2 (−0.8 to −1.9) | −1.4 (−0.9 to −2.2) |
| Characteristic . | TB (n = 97) . | TBI (n = 201) . | Not TB/TBI (n = 199) . | Total (N = 497) . |
|---|---|---|---|---|
| Male sex | 47 (48.5) | 101 (50.2) | 105 (52.8) | 253 (50.9) |
| Age | ||||
| Years, median (IQR) | 38 (25–51) | 37 (26–51) | 35 (22–48) | 36 (24–50) |
| AYA | 24 (24.7) | 42 (20.9) | 59 (29.6) | 125 (25.2) |
| HIV infected | 1 (1.0) | 3 (1.5) | 1 (0.5) | 5 (1.0) |
| With comorbid condition(s) | 29 (29.9) | 41 (20.4) | 46 (23.1) | 116 (23.3) |
| Previous history of TB | ||||
| Yes | 23 (23.7) | 56 (27.9) | 29 (14.6) | 108 (21.7) |
| Symptoms | ||||
| Persistent cough | 85 (87.6) | 157 (78.1) | 170 (85.4) | 412 (82.9) |
| Fever | 90 (92.8) | 185 (92.0) | 189 (95.0) | 464 (93.4) |
| Fatigue | 83 (85.6) | 172 (85.6) | 146 (73.4) | 401 (80.7) |
| Coughing blood | 18 (18.6) | 24 (11.9) | 30 (15.1) | 72 (14.5) |
| Weight loss | 79 (81.4) | 119 (59.2) | 131 (65.8) | 329 (66.2) |
| Night sweats | 34 (35.1) | 42 (20.9) | 48 (24.1) | 124 (24.9) |
| Duration of symptoms, d, median (IQR) | 60 (30–90) | 30 (20–60) | 30 (18–90) | 30 (20–90) |
| QuantiFERON status | ||||
| Indeterminate | 11 (11.3) | 0 (0.0) | 51 (25.6) | 62 (12.5) |
| Positive | 70 (72.2) | 201 (100.0) | 0 (0.0) | 271 (54.5) |
| Negative | 16 (16.5) | 0 (0.0) | 148 (74.4) | 164 (33.0) |
| X-ray findings | ||||
| Infiltrates | 70 (72.2) | 72 (35.8) | 60 (30.2) | 202 (40.6) |
| Nodules | 18 (18.6) | 12 (6.0) | 14 (7.0) | 44 (8.9) |
| Lymph | 2 (2.1) | 16 (8.0) | 14 (7.0) | 32 (6.4) |
| Cavities | 5 (5.2) | 3 (1.5) | 4 (2.0) | 12 (2.4) |
| Pleural effusion | 6 (6.2) | 11 (5.5) | 4 (2.0) | 21 (4.2) |
| Smear status positive | 30 (30.9) | 0 (0.0) | 0 (0.0) | 30 (6.0) |
| TB culture status | ||||
| Contaminated | 5 (5.2) | 6 (3.0) | 3 (1.5) | 14 (2.8) |
| Negative | 29 (29.9) | 195 (97.0) | 196 (98.5) | 420 (84.5) |
| Positive | 63 (64.9) | 0 (0.0) | 0 (0.0) | 63 (12.7) |
| Xpert MTB/RIF Ultra | ||||
| Not detected | 23 (23.7) | 201 (100.0) | 199 (100.0) | 423 (85.1) |
| MTB trace | 12 (12.4) | 0 (0.0) | 0 (0.0) | 12 (2.4) |
| MTB very low | 10 (10.3) | 0 (0.0) | 0 (0.0) | 10 (2.0) |
| MTB low | 24 (24.7) | 0 (0.0) | 0 (0.0) | 24 (4.8) |
| MTB medium | 11 (11.3) | 0 (0.0) | 0 (0.0) | 11 (2.2) |
| MTB high | 17 (17.5) | 0 (0.0) | 0 (0.0) | 17 (3.4) |
| Xpert-MTB-HR | ||||
| TBTBP score, median (IQR) | −2.7 (−1.6 to −3.4) | −1.4 (−1.0 to −1.8) | −1.2 (−0.8 to −1.9) | −1.4 (−0.9 to −2.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AYA, adolescent and young adult; HIV, human immunodeficiency virus; IQR, interquartile range; MTB, Mycobacterium tuberculosis; TB, tuberculosis; TBI, tuberculosis infection; TBTBP, value derived using the following formula: (GBP5 Ct + DUSP3 Ct)/2 – TBP Ct); Xpert-MTB-HR, Cepheid GeneXpert Mycobacterium tuberculosis Host Response cartridge.
Participant Demographics, Disease Characteristics, and Diagnostic Results by Tuberculosis Status
| Characteristic . | TB (n = 97) . | TBI (n = 201) . | Not TB/TBI (n = 199) . | Total (N = 497) . |
|---|---|---|---|---|
| Male sex | 47 (48.5) | 101 (50.2) | 105 (52.8) | 253 (50.9) |
| Age | ||||
| Years, median (IQR) | 38 (25–51) | 37 (26–51) | 35 (22–48) | 36 (24–50) |
| AYA | 24 (24.7) | 42 (20.9) | 59 (29.6) | 125 (25.2) |
| HIV infected | 1 (1.0) | 3 (1.5) | 1 (0.5) | 5 (1.0) |
| With comorbid condition(s) | 29 (29.9) | 41 (20.4) | 46 (23.1) | 116 (23.3) |
| Previous history of TB | ||||
| Yes | 23 (23.7) | 56 (27.9) | 29 (14.6) | 108 (21.7) |
| Symptoms | ||||
| Persistent cough | 85 (87.6) | 157 (78.1) | 170 (85.4) | 412 (82.9) |
| Fever | 90 (92.8) | 185 (92.0) | 189 (95.0) | 464 (93.4) |
| Fatigue | 83 (85.6) | 172 (85.6) | 146 (73.4) | 401 (80.7) |
| Coughing blood | 18 (18.6) | 24 (11.9) | 30 (15.1) | 72 (14.5) |
| Weight loss | 79 (81.4) | 119 (59.2) | 131 (65.8) | 329 (66.2) |
| Night sweats | 34 (35.1) | 42 (20.9) | 48 (24.1) | 124 (24.9) |
| Duration of symptoms, d, median (IQR) | 60 (30–90) | 30 (20–60) | 30 (18–90) | 30 (20–90) |
| QuantiFERON status | ||||
| Indeterminate | 11 (11.3) | 0 (0.0) | 51 (25.6) | 62 (12.5) |
| Positive | 70 (72.2) | 201 (100.0) | 0 (0.0) | 271 (54.5) |
| Negative | 16 (16.5) | 0 (0.0) | 148 (74.4) | 164 (33.0) |
| X-ray findings | ||||
| Infiltrates | 70 (72.2) | 72 (35.8) | 60 (30.2) | 202 (40.6) |
| Nodules | 18 (18.6) | 12 (6.0) | 14 (7.0) | 44 (8.9) |
| Lymph | 2 (2.1) | 16 (8.0) | 14 (7.0) | 32 (6.4) |
| Cavities | 5 (5.2) | 3 (1.5) | 4 (2.0) | 12 (2.4) |
| Pleural effusion | 6 (6.2) | 11 (5.5) | 4 (2.0) | 21 (4.2) |
| Smear status positive | 30 (30.9) | 0 (0.0) | 0 (0.0) | 30 (6.0) |
| TB culture status | ||||
| Contaminated | 5 (5.2) | 6 (3.0) | 3 (1.5) | 14 (2.8) |
| Negative | 29 (29.9) | 195 (97.0) | 196 (98.5) | 420 (84.5) |
| Positive | 63 (64.9) | 0 (0.0) | 0 (0.0) | 63 (12.7) |
| Xpert MTB/RIF Ultra | ||||
| Not detected | 23 (23.7) | 201 (100.0) | 199 (100.0) | 423 (85.1) |
| MTB trace | 12 (12.4) | 0 (0.0) | 0 (0.0) | 12 (2.4) |
| MTB very low | 10 (10.3) | 0 (0.0) | 0 (0.0) | 10 (2.0) |
| MTB low | 24 (24.7) | 0 (0.0) | 0 (0.0) | 24 (4.8) |
| MTB medium | 11 (11.3) | 0 (0.0) | 0 (0.0) | 11 (2.2) |
| MTB high | 17 (17.5) | 0 (0.0) | 0 (0.0) | 17 (3.4) |
| Xpert-MTB-HR | ||||
| TBTBP score, median (IQR) | −2.7 (−1.6 to −3.4) | −1.4 (−1.0 to −1.8) | −1.2 (−0.8 to −1.9) | −1.4 (−0.9 to −2.2) |
| Characteristic . | TB (n = 97) . | TBI (n = 201) . | Not TB/TBI (n = 199) . | Total (N = 497) . |
|---|---|---|---|---|
| Male sex | 47 (48.5) | 101 (50.2) | 105 (52.8) | 253 (50.9) |
| Age | ||||
| Years, median (IQR) | 38 (25–51) | 37 (26–51) | 35 (22–48) | 36 (24–50) |
| AYA | 24 (24.7) | 42 (20.9) | 59 (29.6) | 125 (25.2) |
| HIV infected | 1 (1.0) | 3 (1.5) | 1 (0.5) | 5 (1.0) |
| With comorbid condition(s) | 29 (29.9) | 41 (20.4) | 46 (23.1) | 116 (23.3) |
| Previous history of TB | ||||
| Yes | 23 (23.7) | 56 (27.9) | 29 (14.6) | 108 (21.7) |
| Symptoms | ||||
| Persistent cough | 85 (87.6) | 157 (78.1) | 170 (85.4) | 412 (82.9) |
| Fever | 90 (92.8) | 185 (92.0) | 189 (95.0) | 464 (93.4) |
| Fatigue | 83 (85.6) | 172 (85.6) | 146 (73.4) | 401 (80.7) |
| Coughing blood | 18 (18.6) | 24 (11.9) | 30 (15.1) | 72 (14.5) |
| Weight loss | 79 (81.4) | 119 (59.2) | 131 (65.8) | 329 (66.2) |
| Night sweats | 34 (35.1) | 42 (20.9) | 48 (24.1) | 124 (24.9) |
| Duration of symptoms, d, median (IQR) | 60 (30–90) | 30 (20–60) | 30 (18–90) | 30 (20–90) |
| QuantiFERON status | ||||
| Indeterminate | 11 (11.3) | 0 (0.0) | 51 (25.6) | 62 (12.5) |
| Positive | 70 (72.2) | 201 (100.0) | 0 (0.0) | 271 (54.5) |
| Negative | 16 (16.5) | 0 (0.0) | 148 (74.4) | 164 (33.0) |
| X-ray findings | ||||
| Infiltrates | 70 (72.2) | 72 (35.8) | 60 (30.2) | 202 (40.6) |
| Nodules | 18 (18.6) | 12 (6.0) | 14 (7.0) | 44 (8.9) |
| Lymph | 2 (2.1) | 16 (8.0) | 14 (7.0) | 32 (6.4) |
| Cavities | 5 (5.2) | 3 (1.5) | 4 (2.0) | 12 (2.4) |
| Pleural effusion | 6 (6.2) | 11 (5.5) | 4 (2.0) | 21 (4.2) |
| Smear status positive | 30 (30.9) | 0 (0.0) | 0 (0.0) | 30 (6.0) |
| TB culture status | ||||
| Contaminated | 5 (5.2) | 6 (3.0) | 3 (1.5) | 14 (2.8) |
| Negative | 29 (29.9) | 195 (97.0) | 196 (98.5) | 420 (84.5) |
| Positive | 63 (64.9) | 0 (0.0) | 0 (0.0) | 63 (12.7) |
| Xpert MTB/RIF Ultra | ||||
| Not detected | 23 (23.7) | 201 (100.0) | 199 (100.0) | 423 (85.1) |
| MTB trace | 12 (12.4) | 0 (0.0) | 0 (0.0) | 12 (2.4) |
| MTB very low | 10 (10.3) | 0 (0.0) | 0 (0.0) | 10 (2.0) |
| MTB low | 24 (24.7) | 0 (0.0) | 0 (0.0) | 24 (4.8) |
| MTB medium | 11 (11.3) | 0 (0.0) | 0 (0.0) | 11 (2.2) |
| MTB high | 17 (17.5) | 0 (0.0) | 0 (0.0) | 17 (3.4) |
| Xpert-MTB-HR | ||||
| TBTBP score, median (IQR) | −2.7 (−1.6 to −3.4) | −1.4 (−1.0 to −1.8) | −1.2 (−0.8 to −1.9) | −1.4 (−0.9 to −2.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AYA, adolescent and young adult; HIV, human immunodeficiency virus; IQR, interquartile range; MTB, Mycobacterium tuberculosis; TB, tuberculosis; TBI, tuberculosis infection; TBTBP, value derived using the following formula: (GBP5 Ct + DUSP3 Ct)/2 – TBP Ct); Xpert-MTB-HR, Cepheid GeneXpert Mycobacterium tuberculosis Host Response cartridge.
To further understand differences in TB disease and phenotype by age cohort, we compared TBTBP score distributions, sputum bacterial burden proxies, and diagnosis method among individuals classified as having TB (Table 2). The method of diagnosis was similar between AYAs and adults, approximately 83% of both groups were bacteriologically diagnosed with TB, approximately 11% were clinically diagnosed, and 5% were on treatment at follow-up. Additionally, there was no significant difference (P > .05) by bacterial burden proxy including Xpert MTB/RIF Ultra, culture, or smear status, between AYAs and adults. However, there was a significant difference in TBTBP score, AYAs produced significantly lower scores compared to older individuals with TB (P < .001).
Tuberculosis Diagnostic Results Among Individuals Classified as Having Tuberculosis Using the Composite Clinical Diagnosis Method and Stratified by Age Category
| Characteristic . | Adolescent/Young Adult (Aged 10–24 y) (n = 24) . | Adult (Aged ≥25 y) (n = 73) . |
|---|---|---|
| Diagnostic method | ||
| Bacteriological confirmation | 20 (83.3) | 61 (83.6) |
| Clinical diagnosis (clinician diagnosed at enrollment and on treatment at follow-up) | 4 (16.7) | 12 (16.4) |
| Xpert MTB/RIF Ultra | ||
| Not detected | 7 (29.2) | 16 (21.9) |
| MTB trace | 0 (0.0) | 12 (16.4) |
| MTB very low | 3 (12.5) | 7 (9.6) |
| MTB low | 8 (33.3) | 16 (21.9) |
| MTB medium | 3 (12.5) | 8 (11.0) |
| MTB high | 3 (12.5) | 14 (19.2) |
| Culture status | ||
| Contaminated | 1 (4.2) | 4 (5.5) |
| Negative | 6 (25.0) | 23 (31.5) |
| Positive | 17 (70.8) | 46 (63.0) |
| Smear status | ||
| Positive | 7 (29.2) | 23 (31.5) |
| Comorbid condition | ||
| Yes | 4 (16.7) | 25 (34.2) |
| TBTBP score | ||
| Median (IQR) | −3.3 (−3.8 to −2.9) | −2.3 (−3.2 to −1.3) |
| Characteristic . | Adolescent/Young Adult (Aged 10–24 y) (n = 24) . | Adult (Aged ≥25 y) (n = 73) . |
|---|---|---|
| Diagnostic method | ||
| Bacteriological confirmation | 20 (83.3) | 61 (83.6) |
| Clinical diagnosis (clinician diagnosed at enrollment and on treatment at follow-up) | 4 (16.7) | 12 (16.4) |
| Xpert MTB/RIF Ultra | ||
| Not detected | 7 (29.2) | 16 (21.9) |
| MTB trace | 0 (0.0) | 12 (16.4) |
| MTB very low | 3 (12.5) | 7 (9.6) |
| MTB low | 8 (33.3) | 16 (21.9) |
| MTB medium | 3 (12.5) | 8 (11.0) |
| MTB high | 3 (12.5) | 14 (19.2) |
| Culture status | ||
| Contaminated | 1 (4.2) | 4 (5.5) |
| Negative | 6 (25.0) | 23 (31.5) |
| Positive | 17 (70.8) | 46 (63.0) |
| Smear status | ||
| Positive | 7 (29.2) | 23 (31.5) |
| Comorbid condition | ||
| Yes | 4 (16.7) | 25 (34.2) |
| TBTBP score | ||
| Median (IQR) | −3.3 (−3.8 to −2.9) | −2.3 (−3.2 to −1.3) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; MTB, Mycobacterium tuberculosis; TBTBP, value derived using the following formula: (GBP5 Ct + DUSP3 Ct)/2 – TBP Ct).
Tuberculosis Diagnostic Results Among Individuals Classified as Having Tuberculosis Using the Composite Clinical Diagnosis Method and Stratified by Age Category
| Characteristic . | Adolescent/Young Adult (Aged 10–24 y) (n = 24) . | Adult (Aged ≥25 y) (n = 73) . |
|---|---|---|
| Diagnostic method | ||
| Bacteriological confirmation | 20 (83.3) | 61 (83.6) |
| Clinical diagnosis (clinician diagnosed at enrollment and on treatment at follow-up) | 4 (16.7) | 12 (16.4) |
| Xpert MTB/RIF Ultra | ||
| Not detected | 7 (29.2) | 16 (21.9) |
| MTB trace | 0 (0.0) | 12 (16.4) |
| MTB very low | 3 (12.5) | 7 (9.6) |
| MTB low | 8 (33.3) | 16 (21.9) |
| MTB medium | 3 (12.5) | 8 (11.0) |
| MTB high | 3 (12.5) | 14 (19.2) |
| Culture status | ||
| Contaminated | 1 (4.2) | 4 (5.5) |
| Negative | 6 (25.0) | 23 (31.5) |
| Positive | 17 (70.8) | 46 (63.0) |
| Smear status | ||
| Positive | 7 (29.2) | 23 (31.5) |
| Comorbid condition | ||
| Yes | 4 (16.7) | 25 (34.2) |
| TBTBP score | ||
| Median (IQR) | −3.3 (−3.8 to −2.9) | −2.3 (−3.2 to −1.3) |
| Characteristic . | Adolescent/Young Adult (Aged 10–24 y) (n = 24) . | Adult (Aged ≥25 y) (n = 73) . |
|---|---|---|
| Diagnostic method | ||
| Bacteriological confirmation | 20 (83.3) | 61 (83.6) |
| Clinical diagnosis (clinician diagnosed at enrollment and on treatment at follow-up) | 4 (16.7) | 12 (16.4) |
| Xpert MTB/RIF Ultra | ||
| Not detected | 7 (29.2) | 16 (21.9) |
| MTB trace | 0 (0.0) | 12 (16.4) |
| MTB very low | 3 (12.5) | 7 (9.6) |
| MTB low | 8 (33.3) | 16 (21.9) |
| MTB medium | 3 (12.5) | 8 (11.0) |
| MTB high | 3 (12.5) | 14 (19.2) |
| Culture status | ||
| Contaminated | 1 (4.2) | 4 (5.5) |
| Negative | 6 (25.0) | 23 (31.5) |
| Positive | 17 (70.8) | 46 (63.0) |
| Smear status | ||
| Positive | 7 (29.2) | 23 (31.5) |
| Comorbid condition | ||
| Yes | 4 (16.7) | 25 (34.2) |
| TBTBP score | ||
| Median (IQR) | −3.3 (−3.8 to −2.9) | −2.3 (−3.2 to −1.3) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; MTB, Mycobacterium tuberculosis; TBTBP, value derived using the following formula: (GBP5 Ct + DUSP3 Ct)/2 – TBP Ct).
TBTBP score distribution was plotted against 2 proxy measures of sputum bacterial burden (culture status and semi-quantitative Xpert MTB/RIF Ultra values) and diagnostic method in Figure 2. TBTBP scores were lower among individuals with higher sputum bacterial burdens in the adult study cohort (Figure 2C), compared to the AYA cohort, where variations in bacterial burden did not appear to alter TBTBP score (Figure 2B). Additionally, TBTBP score distribution was assessed by processing method, and no significant difference in TBTBP score distribution was found between samples processed with a live workflow and those processed after frozen storage (P = .25; data not shown).
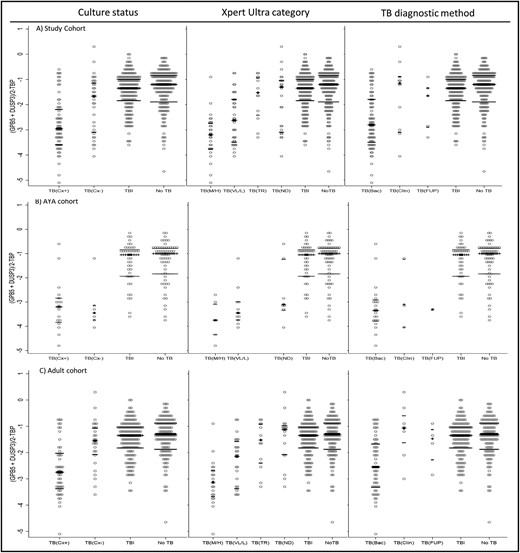
Performance of the Cepheid GeneXpert Mycobacterium tuberculosis Host Response cartridge (Xpert-MTB-HR) by age cohort: all study participants (A), adolescents and young adults aged 10–24 years (B), and adults aged ≥25 years (C). TBTBP score distributions are displayed by tuberculosis status and stratified by specific diagnostic method in each column: culture (culture postive vs culture negative or contaminated), Xpert MTB/RIF Ultra status (high or medium vs low or very low vs trace vs not detected), and diagnostic method (bacteriologic confirmation vs clincial diagnosis at enrollment vs treatement at follow-up). The center bar indicates median and the outer bars represent interquartile range for each category. Abbreviations: Bac, bacteriologic confirmation; Clin, clincial diagnosis; Cx+, culture postive; Cx–, culture negative; M/H, medium/high; ND, not detected; TB, tuberculosis; TBI, tuberculosis infected; TR, trace; VL/L, very low/low.
Next, we computed the AUC of the Xpert-MTB-HR using the 3 diagnostic reference categories (Table 3). Across the entire study cohort, the AUC was 0.82 (95% CI, .76–.87) when using Xpert MTB/RIF Ultra as the diagnostic reference, 0.80 (95% CI, .74–.86) when using bacteriological confirmation as the diagnostic reference, and 0.77 (95% CI, .71–.83) when using the composite clinical diagnosis as the diagnostic reference. In comparison, when evaluating only the AYA cohort, the AUC was 0.92 (95% CI, .87–.97) when using Xpert MTB/RIF Ultra as the diagnostic reference, 0.88 (95% CI, .89–.98) when using bacteriological confirmation as the diagnostic reference, and 0.89 (95% CI, .81–.91) when using the composite clinical diagnosis as the diagnostic reference (Figure 3). Again, among the entire study cohort when the diagnostic TBTBP score threshold was set for a sensitivity of >90%, the specificity was 32% (95% CI, 28%–37%) compared to Xpert MTB/RIF Ultra, 29% (95% CI, 25%–34%) compared to bacteriological confirmation diagnosis, and 22% (95% CI, 18%–26%) compared to a composite clinical diagnosis. In contrast, when evaluating only the AYA cohort, the specificity was 82% (95% CI, 74%–89%) compared to Xpert MTB/RIF Ultra, 84% (95% CI, 75%–90%) compared to a bacteriological confirmation diagnosis, and 54% (95% CI, 44%–64%) compared to a composite clinical diagnosis. When evaluating sensitivity and specificity results and using the cut-point method of optimizing sensitivity at >90% (TBTBP score cut point of −2.35), the Xpert-MTB-HR met WHO triage minimum standards only in the AYA cohort when using Ultra MTB/RIF or the bacteriological confirmation as diagnostic references.
Area Under the Curve, Sensitivity, and Specificity Estimates by Age Cohort for Each of the 3 Cut Point Optimization Strategies and Stratified by Diagnostic Reference Category
| Method . | Study Cohort (n = 497) . | AYA Subcohort (n = 125) . | ||||
|---|---|---|---|---|---|---|
| TBTBP Score Cut Point . | Sensitivity (95% CI) . | Specificity (95% CI) . | TBTBP Score Cut Point . | Sensitivity (95% CI) . | Specificity (95% CI) . | |
| Diagnostic reference: Xpert MTB/RIF Ultra Positive | ||||||
| AUC | 0.82 (95% CI, .76–.87) | 0.92 (95% CI, .87–.98) | ||||
| Youden | −2.300 | 0.68 (.56–.78) | 0.84 (.80–.87) | −2.300 | 0.94 (.69–1.00) | 0.79 (.70–.86) |
| Liu | −2.325 | 0.66 (.54–.77) | 0.86 (.82–.89) | −2.325 | 0.94 (.69–1.00) | 0.82 (.74–.89) |
| >90% sensitivity | −1.050 | 0.91 (.81–.96) | 0.32 (.28–.37) | −2.350 | 0.94 (.69–1.00) | 0.82 (.74–.89) |
| Diagnostic reference: Bacteriological confirmation (Xpert MTB/RIF Ultra and/or liquid culture positive) | ||||||
| AUC | 0.80 (95% CI, .74–.86) | 0.88 (95% CI, .79–.98) | ||||
| Youden | −2.450 | 0.63 (.51–.73) | 0.87 (.84–.90) | −2.450 | 0.85 (.61–.96) | 0.84 (.75–.90) |
| Liu | −1.925 | 0.73 (.62–.82) | 0.75 (.71–.79) | −2.325 | 0.90 (.67–.98) | 0.84 (.75–.90) |
| >90% sensitivity | −1.000 | 0.91 (.82–.96) | 0.29 (.25–.34) | −2.350 | 0.90 (.67–.98) | 0.84 (.75–.90) |
| Diagnostic reference: Composite clinical diagnosis (clinical diagnosis or bacteriologicial confirmation) | ||||||
| AUC | 0.77 (95% CI, .71–.83) | 0.89 (95% CI, .81–.98) | ||||
| Youden | −2.450 | 0.58 (.47–.68) | 0.88 (.84–.91) | −2.300 | 0.88 (.67–.97) | 0.82 (.73–.89) |
| Liu | −1.975 | 0.67 (.57–.76) | 0.78 (.73–.81) | −2.325 | 0.88 (.67–.97) | 0.86 (.77–.92) |
| >90% sensitivity | −0.850 | 0.93 (.85–.97) | 0.22 (.18–.26) | −1.200 | 0.96 (.77–1.00) | 0.54 (.44–.64) |
| Method . | Study Cohort (n = 497) . | AYA Subcohort (n = 125) . | ||||
|---|---|---|---|---|---|---|
| TBTBP Score Cut Point . | Sensitivity (95% CI) . | Specificity (95% CI) . | TBTBP Score Cut Point . | Sensitivity (95% CI) . | Specificity (95% CI) . | |
| Diagnostic reference: Xpert MTB/RIF Ultra Positive | ||||||
| AUC | 0.82 (95% CI, .76–.87) | 0.92 (95% CI, .87–.98) | ||||
| Youden | −2.300 | 0.68 (.56–.78) | 0.84 (.80–.87) | −2.300 | 0.94 (.69–1.00) | 0.79 (.70–.86) |
| Liu | −2.325 | 0.66 (.54–.77) | 0.86 (.82–.89) | −2.325 | 0.94 (.69–1.00) | 0.82 (.74–.89) |
| >90% sensitivity | −1.050 | 0.91 (.81–.96) | 0.32 (.28–.37) | −2.350 | 0.94 (.69–1.00) | 0.82 (.74–.89) |
| Diagnostic reference: Bacteriological confirmation (Xpert MTB/RIF Ultra and/or liquid culture positive) | ||||||
| AUC | 0.80 (95% CI, .74–.86) | 0.88 (95% CI, .79–.98) | ||||
| Youden | −2.450 | 0.63 (.51–.73) | 0.87 (.84–.90) | −2.450 | 0.85 (.61–.96) | 0.84 (.75–.90) |
| Liu | −1.925 | 0.73 (.62–.82) | 0.75 (.71–.79) | −2.325 | 0.90 (.67–.98) | 0.84 (.75–.90) |
| >90% sensitivity | −1.000 | 0.91 (.82–.96) | 0.29 (.25–.34) | −2.350 | 0.90 (.67–.98) | 0.84 (.75–.90) |
| Diagnostic reference: Composite clinical diagnosis (clinical diagnosis or bacteriologicial confirmation) | ||||||
| AUC | 0.77 (95% CI, .71–.83) | 0.89 (95% CI, .81–.98) | ||||
| Youden | −2.450 | 0.58 (.47–.68) | 0.88 (.84–.91) | −2.300 | 0.88 (.67–.97) | 0.82 (.73–.89) |
| Liu | −1.975 | 0.67 (.57–.76) | 0.78 (.73–.81) | −2.325 | 0.88 (.67–.97) | 0.86 (.77–.92) |
| >90% sensitivity | −0.850 | 0.93 (.85–.97) | 0.22 (.18–.26) | −1.200 | 0.96 (.77–1.00) | 0.54 (.44–.64) |
Abbreviations: AUC, area under the curve; AYA, adolescent and young adult; CI, confidence interval; TBTBP, value derived using the following formula: (GBP5 Ct + DUSP3 Ct)/2 – TBP Ct).
Area Under the Curve, Sensitivity, and Specificity Estimates by Age Cohort for Each of the 3 Cut Point Optimization Strategies and Stratified by Diagnostic Reference Category
| Method . | Study Cohort (n = 497) . | AYA Subcohort (n = 125) . | ||||
|---|---|---|---|---|---|---|
| TBTBP Score Cut Point . | Sensitivity (95% CI) . | Specificity (95% CI) . | TBTBP Score Cut Point . | Sensitivity (95% CI) . | Specificity (95% CI) . | |
| Diagnostic reference: Xpert MTB/RIF Ultra Positive | ||||||
| AUC | 0.82 (95% CI, .76–.87) | 0.92 (95% CI, .87–.98) | ||||
| Youden | −2.300 | 0.68 (.56–.78) | 0.84 (.80–.87) | −2.300 | 0.94 (.69–1.00) | 0.79 (.70–.86) |
| Liu | −2.325 | 0.66 (.54–.77) | 0.86 (.82–.89) | −2.325 | 0.94 (.69–1.00) | 0.82 (.74–.89) |
| >90% sensitivity | −1.050 | 0.91 (.81–.96) | 0.32 (.28–.37) | −2.350 | 0.94 (.69–1.00) | 0.82 (.74–.89) |
| Diagnostic reference: Bacteriological confirmation (Xpert MTB/RIF Ultra and/or liquid culture positive) | ||||||
| AUC | 0.80 (95% CI, .74–.86) | 0.88 (95% CI, .79–.98) | ||||
| Youden | −2.450 | 0.63 (.51–.73) | 0.87 (.84–.90) | −2.450 | 0.85 (.61–.96) | 0.84 (.75–.90) |
| Liu | −1.925 | 0.73 (.62–.82) | 0.75 (.71–.79) | −2.325 | 0.90 (.67–.98) | 0.84 (.75–.90) |
| >90% sensitivity | −1.000 | 0.91 (.82–.96) | 0.29 (.25–.34) | −2.350 | 0.90 (.67–.98) | 0.84 (.75–.90) |
| Diagnostic reference: Composite clinical diagnosis (clinical diagnosis or bacteriologicial confirmation) | ||||||
| AUC | 0.77 (95% CI, .71–.83) | 0.89 (95% CI, .81–.98) | ||||
| Youden | −2.450 | 0.58 (.47–.68) | 0.88 (.84–.91) | −2.300 | 0.88 (.67–.97) | 0.82 (.73–.89) |
| Liu | −1.975 | 0.67 (.57–.76) | 0.78 (.73–.81) | −2.325 | 0.88 (.67–.97) | 0.86 (.77–.92) |
| >90% sensitivity | −0.850 | 0.93 (.85–.97) | 0.22 (.18–.26) | −1.200 | 0.96 (.77–1.00) | 0.54 (.44–.64) |
| Method . | Study Cohort (n = 497) . | AYA Subcohort (n = 125) . | ||||
|---|---|---|---|---|---|---|
| TBTBP Score Cut Point . | Sensitivity (95% CI) . | Specificity (95% CI) . | TBTBP Score Cut Point . | Sensitivity (95% CI) . | Specificity (95% CI) . | |
| Diagnostic reference: Xpert MTB/RIF Ultra Positive | ||||||
| AUC | 0.82 (95% CI, .76–.87) | 0.92 (95% CI, .87–.98) | ||||
| Youden | −2.300 | 0.68 (.56–.78) | 0.84 (.80–.87) | −2.300 | 0.94 (.69–1.00) | 0.79 (.70–.86) |
| Liu | −2.325 | 0.66 (.54–.77) | 0.86 (.82–.89) | −2.325 | 0.94 (.69–1.00) | 0.82 (.74–.89) |
| >90% sensitivity | −1.050 | 0.91 (.81–.96) | 0.32 (.28–.37) | −2.350 | 0.94 (.69–1.00) | 0.82 (.74–.89) |
| Diagnostic reference: Bacteriological confirmation (Xpert MTB/RIF Ultra and/or liquid culture positive) | ||||||
| AUC | 0.80 (95% CI, .74–.86) | 0.88 (95% CI, .79–.98) | ||||
| Youden | −2.450 | 0.63 (.51–.73) | 0.87 (.84–.90) | −2.450 | 0.85 (.61–.96) | 0.84 (.75–.90) |
| Liu | −1.925 | 0.73 (.62–.82) | 0.75 (.71–.79) | −2.325 | 0.90 (.67–.98) | 0.84 (.75–.90) |
| >90% sensitivity | −1.000 | 0.91 (.82–.96) | 0.29 (.25–.34) | −2.350 | 0.90 (.67–.98) | 0.84 (.75–.90) |
| Diagnostic reference: Composite clinical diagnosis (clinical diagnosis or bacteriologicial confirmation) | ||||||
| AUC | 0.77 (95% CI, .71–.83) | 0.89 (95% CI, .81–.98) | ||||
| Youden | −2.450 | 0.58 (.47–.68) | 0.88 (.84–.91) | −2.300 | 0.88 (.67–.97) | 0.82 (.73–.89) |
| Liu | −1.975 | 0.67 (.57–.76) | 0.78 (.73–.81) | −2.325 | 0.88 (.67–.97) | 0.86 (.77–.92) |
| >90% sensitivity | −0.850 | 0.93 (.85–.97) | 0.22 (.18–.26) | −1.200 | 0.96 (.77–1.00) | 0.54 (.44–.64) |
Abbreviations: AUC, area under the curve; AYA, adolescent and young adult; CI, confidence interval; TBTBP, value derived using the following formula: (GBP5 Ct + DUSP3 Ct)/2 – TBP Ct).
DISCUSSION
Results presented in this study demonstrate that while the Xpert-MTB-HR performance did not meet the WHO minimum target product profile criteria for a non-sputum-based TB triage test regardless of diagnostic reference in the general study cohort, the Xpert-MTB-HR did perform considerably better in the AYA cohort and met WHO minimum standards for a non-sputum-based triage test when using bacteriological confirmation as a diagnostic reference.
This better than expected performance in the AYA cohort, AUC of 0.88 (95% CI, .79–.98), when using bacteriological confirmation as reference in our study, was similar to the reported performance of the Xpert-MTB-HR, AUC of 0.86 (95% CI, .77–.96), described in a nested case-control study designed to identify progression from TB infection to active disease among adolescents during the 6 months prior to sputum conversion [6]. Additionally, in a subanalysis of 82 participants between the ages of 10 and 14 years conducted by Olbrich and colleagues, an AUC of 0.88 was reported, again similar to the AYA AUC calculated in the present study when using biological confirmation as a reference standard [10]. The signal found by Olbrich and colleagues in their study's adolescent cohort contrasted with additional subanalyses they conducted in younger age cohorts that yielded lower AUC results, ranging from 0.77 to 0.86. Taken in context, these results indicate a potential utility of the Xpert-MTB-HR assay among a narrow but clinically important population of adolescents and young adults.
Our AYA findings did differ, however, from the original host response biomarker study, which found that within the pediatric datasets evaluated, culture status significantly impacted TBTBP score, but within the adult-only datasets evaluated, culture status did not affect TBTBP score, suggesting that culture and not age group may impact assay results [3]. In our study, however, the proportion of culture-positive participants was not significantly different between the AYA and adult cohorts, negating this possible explanation for our findings. One notable difference and possible explanation of these contrasting findings between the original study and the current study, is the use of the TBP gene in place of the KLF2 gene in the TBTBP score calculation.
It is well documented that the components of the immune system driving host response to M. tuberculosis (macrophage function, T-cell response, and interferon release) peak in early adulthood [16, 21, 22]. We hypothesize that this may explain, in part, the diagnostic performance of the Xpert-MTB-HR among AYAs. Furthermore, AYAs, by virtue of their age, are less likely to have been subject to a high environmental antigen stimulation or chronic disease, resulting in chronic or “background” inflammation when compared to older adults [21]. This may explain the overlap in TBTBP score between TB and sarcoidosis previously reported in adults—as these 2 disease states share similar immune pathways given their tendency to cause granulomatous inflammation [23, 24]. The increased sensitivity and specificity of the Xpert-MTB-HR among AYAs may be a “Goldilocks” phenomenon caused by the heightened ability of the immune system as it finalizes its development (resulting in its ability to regulate itself and produce a robust response to novel pathogens), combined with a lower degree of chronic inflammation and relative naivety to environmental antigens in that age group, as demonstrated in our study cohort (comorbidity prevalence of 34% among adults vs 17% in the AYA cohort; Table 2). We postulate that our findings in this AYA cohort—in terms of sensitivity and specificity independent of culture status—may be related to a myriad of interacting principles of immune development and previous pathogen exposure.
Study limitations include the evaluation of individuals only for pulmonary TB, thus excluding the possibility of evaluating this assay as a diagnostic for extrapulmonary disease; the limited number of participants in the AYA cohort, resulting in relatively wide CIs; and the single-country study design, reducing generalizability of the results. Additional studies among larger AYA cohorts to confirm findings are needed. Strengths of the study include the use of multiple and highly robust reference standards, including a 3-month follow-up, to ensure capture of incipient disease.
In conclusion, the Xpert-MTB-HR cartridge did not meet WHO minimum target product profile criteria for a non-sputum-based triage test for pulmonary TB in the general population; however, it did demonstrate potential utility among AYAs when evaluated against a bacteriological confirmation reference. Further evaluation is warranted.
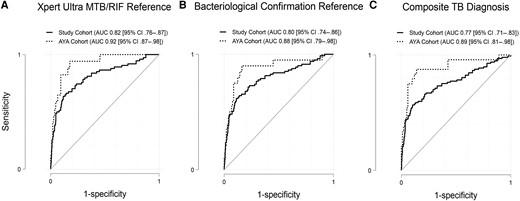
Receiver operating characteristic curves by complete study cohort and by the adolescent and young adult cohort. A, Xpert MTB/RIF Ultra reference. B, Bacteriological confirmation reference including culture and Xpert MTB/RIF Ultra results. C, Composite clinical tuberculosis diagnosis reference including clinical diagnosis and bacteriological confirmation. Abbreviations: AUC, area under the curve; AYA, adolescent and young adult; CI, confidence interval; TB, tuberculosis.
Notes
Acknowledgments. The authors acknowledge the individuals who participated in this study in Pakistan.
Financial support. Data reported in this study were collected and research was performed with the support of the US Department of Defense (award number W81XWH-18-1-0253) and National Institutes of Health (award number R01AI137681). M. G. and R. R. S. receive salary support from National Institutes of Health (training grant number T32AI007384). Xpert-MTB-HR cartridges were provided by the manufacturer, Cepheid.
References
Author notes
Potential conflicts of interest. A. C. and T. R. are co-founders, board members, and shareholders of Verus Diagnostics Inc, a company that was founded with the intent of developing TB diagnostic assays. A. C. and T. R. have not received any financial support from Verus Diagnostics nor has Verus Diagnostics supported the development of the assay evaluated in this manuscript in any way, financial or otherwise. The University of California, San Diego Conflict of Interest office has reviewed and approved their roles in Verus Diagnostics Inc. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




