-
PDF
- Split View
-
Views
-
Cite
Cite
John B Lynch, Perica Davitkov, Deverick J Anderson, Adarsh Bhimraj, Vincent Chi-Chung Cheng, Judith Guzman-Cottrill, Jasmine Dhindsa, Abhijit Duggal, Mamta K Jain, Grace M Lee, Stephen Y Liang, Allison McGeer, Valery Lavergne, M Hassan Murad, Reem A Mustafa, Rebecca L Morgan, Yngve Falck-Ytter, Shahnaz Sultan, Infectious Diseases Society of America Guidelines on Infection Prevention for Healthcare Personnel Caring for Patients With Suspected or Known COVID-19 (July 2020), Clinical Infectious Diseases, Volume 78, Issue 7, 15 June 2024, Pages e133–e149, https://doi.org/10.1093/cid/ciaa1063
Close - Share Icon Share
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible virus that can infect healthcare personnel (HCP) and patients in healthcare settings. Specific care activities, in particular, aerosol-generating procedures, may have a higher risk of transmission. The rapid emergence and global spread of SARS-CoV-2 has created significant challenges in healthcare facilities, particularly with severe shortages of personal protective equipment (PPE) used to protect HCP. Evidence-based recommendations for what PPE to use in conventional, contingency, and crisis standards of care are needed. Where evidence is lacking, the development of specific research questions can help direct funders and investigators.
Our objective was to develop evidence-based rapid guidelines intended to support HCP in their decisions about infection prevention when caring for patients with suspected or known coronavirus disease 2019 (COVID-19).
The Infectious Diseases Society of America (IDSA) formed a multidisciplinary guideline panel that included front-line clinicians, infectious diseases specialists, experts in infection control, and guideline methodologists with representation from the disciplines of preventive care, public health, medical microbiology, pediatrics, critical care medicine, and gastroenterology. The process followed a rapid recommendation checklist. The panel prioritized questions and outcomes. Then, a systematic review of the peer-reviewed and gray literature was conducted. The Grading of Recommendations Assessment, Development and Evaluation approach was used to assess the certainty of evidence and make recommendations.
The IDSA guideline panel agreed on 8 recommendations and provided narrative summaries of other interventions undergoing evaluations.
Using a combination of direct and indirect evidence, the panel was able to provide recommendations for 8 specific questions on the use of PPE for HCP who provide care for patients with suspected or known COVID-19. Where evidence was lacking, attempts were made to provide potential avenues for investigation. Significant gaps in the understanding of the transmission dynamics of SARS-CoV-2 remain, and PPE recommendations may need to be modified in response to new evidence.
INFECTIOUS DISEASES SOCIETY OF AMERICA LEGAL DISCLAIMER
It is important to realize that guidelines cannot always account for individual variation among patients. They are assessments of current scientific and clinical information provided as an educational service; are not continually updated and may not reflect the most recent evidence (new evidence may emerge between the time information is developed and when it is published or read); should not be considered inclusive of all proper treatment methods of care or as a statement of the standard of care; do not mandate any particular course of medical care; and are not intended to supplant physician judgment with respect to particular patients or special clinical situations. Whether and the extent to which to follow guidelines is voluntary, with the ultimate determination regarding their application to be made by the physician in the light of each patient’s individual circumstances. While the Infectious Diseases Society of America (IDSA) makes every effort to present accurate, complete, and reliable information, these guidelines are presented “as is” without any warranty, either express or implied. IDSA (and its officers, directors, members, employees, and agents) assume no responsibility for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with these guidelines or reliance on the information presented.
The guidelines represent the proprietary and copyrighted property of IDSA. Copyright 2020 Infectious Diseases Society of America. All rights reserved. No part of these guidelines may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of IDSA. Permission is granted to physicians and healthcare providers solely to copy and use the guidelines in their professional practices and clinical decision-making. No license or permission is granted to any person or entity, and prior written authorization by IDSA is required to sell, distribute, or modify the guidelines or to make derivative works of or incorporate the guidelines into any product, including but not limited to clinical decision-support software or any other software product. Except for the permission granted above, any person or entity desiring to use the guidelines in any way must contact IDSA for approval in accordance with the terms and conditions of third-party use, in particular, any use of the guidelines in any software product.
EXECUTIVE SUMMARY
Summarized below are the 8 recommendations for infection prevention among healthcare personnel (HCP) caring for suspected or known patients with coronavirus disease 2019 (COVID-19). A detailed description of background, methods, evidence summary, and rationale that support each recommendation and research needs can be found online in the full text. In brief, per Grading of Recommendations Assessment, Development and Evaluation methodology, recommendations are labeled as “strong” or “conditional.” The word “recommend” indicates strong recommendations, and “suggest” indicates conditional recommendations. In situations where the guideline panel judged there was insufficient evidence of benefit to support the use of specific personal protective equipment (PPE) with concerns for negatively impacting resources, the expert panel acknowledged the knowledge gap and made no recommendation, highlighting the need for more definitive evidence.
The Infectious Diseases Society of America guideline panel used the Crisis Standards of Care framework to develop its recommendations [1–3]. In the setting of a pandemic with documented shortages of PPE across various healthcare settings, the availability of supplies is an important driver of recommendations. Using the crisis capacity framework, separate recommendations were made for contingency or crisis capacity settings, acknowledging the limited availability of PPE (see Figure 1).
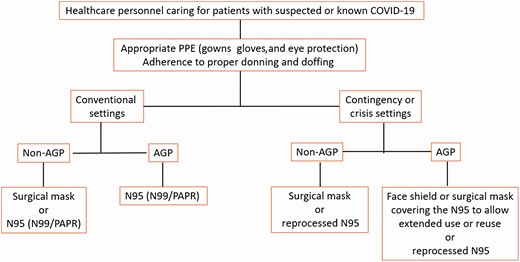
Infectious Diseases Society of America algorithm for appropriate PPE in conventional and contingency or crisis settings. Abbreviations: AGP, aerosol-generating procedures; COVID-19, coronavirus disease 2019; PAPR, powered air-purifying respirator; PPE, personal protective equipment.
For all recommendations listed below, the panel emphasizes the impact of conventional, contingency, and crisis standards of care on how PPE is used. It also is critical to emphasize the importance of “appropriate PPE” for the care of patients with suspected or known COVID-19, including gowns, gloves, and eye protection, as well as adherence to standards for donning and doffing, to minimize transmission. The panel recognizes the need to address the potential role of eye protection and masks as part of standard precautions, how to mitigate gown shortages (eg, use of garbage bags as a safe alternative), and if there is a role for hair covers to prevent severe acute respiratory syndrome coronavirus 2 transmission. In addition, the behaviors associated with how PPE is used, particularly while PPE is being removed, cannot be separated from the technical qualities of the equipment. The panel hopes to address these questions in subsequent updates.
ROUTINE PATIENT CARE
In Conventional Settings
Recommendation 1: The IDSA guideline panel recommends that HCP caring for patients with suspected or known COVID-19 use either a surgical mask or N95 (or N99 or powered air-purifying respirator) respirator as part of appropriate PPE.* Strong recommendation, moderate certainty of evidence.
In Contingency or Crisis Capacity Settings
Recommendation 2: While in contingency or crisis capacity settings (respirator shortages), the IDSA guideline panel recommends that HCP caring for patients with suspected or known COVID-19 use a surgical mask or reprocessed respirator instead of no mask as part of appropriate PPE.* Strong recommendation, moderate certainty of evidence.
In Conventional, Contingency, or Crisis Capacity Settings
Recommendation 3: The IDSA guideline panel makes no recommendation for the use of double gloves vs single gloves for healthcare PPE.* Knowledge gap.
Recommendation 4: The IDSA guideline panel makes no recommendation for the use of shoe covers vs no shoe covers for HCP caring for patients with suspected or known COVID-19 as part of appropriate PPE.* Knowledge gap.
AEROSOL-GENERATING PROCEDURES
In Conventional Settings
Recommendation 5: The IDSA guideline panel recommends that HCP involved with aerosol-generating procedures on suspected or known COVID-19 patients use an N95 (or N99 or powered air-purifying respirator) respirator instead of a surgical mask as part of appropriate PPE.* Strong recommendation, very low certainty of evidence. Comment: Despite the very low-quality and indirect evidence supporting this recommendation, the IDSA guideline panel placed a high value on avoiding serious harms to exposed HCP.
In Contingency or Crisis Capacity Settings
Recommendation 6: While in contingency or crisis capacity settings (respirator shortages), the IDSA guideline panel suggests that HCP involved with aerosol-generating procedures on suspected or known COVID-19 patients use a reprocessed N95 respirator for reuse instead of surgical masks as part of appropriate PPE.* Conditional recommendation, very low certainty evidence.
Recommendation 7: While in contingency or crisis settings (respirator shortages), the IDSA guideline panel recommends that HCP involved with aerosol-generating procedures on suspected or known COVID-19 patients add a face shield or surgical mask as a cover for the N95 respirator to allow for extended use as part of appropriate PPE.* Strong recommendation, very low certainty evidence. Comment: This recommendation assumes correct doffing sequence and hand hygiene are performed before and after removing the face shield or surgical mask covering the respirator.
Recommendation 8: While in contingency or crisis settings (respirator shortages), the IDSA guideline panel suggests that HCP involved with aerosol-generating procedures on suspected or known COVID-19 patients add a face shield or surgical mask as a cover for the N95 respirator to allow for reuse as part of appropriate PPE.* Conditional recommendation, very low certainty evidence. Comment: This recommendation assumes correct doffing sequence and hand hygiene are performed before and after removing the face shield or surgical mask covering the respirator.
*Appropriate PPE includes, in addition to a mask or respirator, eye protection, gown, and gloves.
BACKGROUND
The first cases of coronavirus disease 2019 (COVID-19) were reported from Wuhan, China, in early December 2019 [4], now known to be caused by a novel beta-coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Within a span of months, COVID-19 became a pandemic due to its transmissibility, spreading across continents with the number of cases and deaths rising daily [5]. Although more than 80% of infected individuals exhibit a mild illness, 14% have serious illness and 5% have critical illness [6].
It is increasingly clear that COVID-19 is primarily a community spread disease. Transmission occurs from persons incubating the disease before the onset of symptoms and from persons with mild illness and, possibly, from persons with asymptomatic infection. Although viral shedding appears to decrease over time, some patients shed viral RNA for prolonged periods. Throughout the course of infection, much remains unknown about the risk of transmission between patients and caregivers.
Accurately identifying the contribution to overall transmission of different modes of transmission of respiratory viruses has been, and remains, a challenge. The patterns of COVID-19 spread (eg, highest risk in households, absence of identified transmission on aircraft, absence of outbreaks in staff of COVID-19 treatment centers not using airborne precautions and N95 respirators) strongly suggest that SARS-CoV-2 is primarily spread by large respiratory droplets. However, substantial contamination of near-patient environments has been documented, and some, although not all, studies have identified viral RNA in air or air vents at a sufficient distance from patients to suggest that airborne transmission might be possible. Whether this viral RNA represents living virus remains unknown. Similarly, the infectious dose of SARS-CoV-2 is unknown. In an effort to find answers to these questions, there has been an expanding number of studies rapidly published online and in academic journals; however, some of these may be of limited quality and are prepublished without sufficient peer-review. Critical appraisal of the existing studies is needed to determine if the evidence is sufficient to support currently proposed management strategies.
Given the rapid global spread of SARS-CoV-2 and the difficulties faced by overburdened front-line healthcare personnel (HCP) and policymakers trying to remain up-to-date on emerging literature, the Infectious Diseases Society of America (IDSA) has recognized the necessity of developing a rapid guideline for infection prevention in healthcare settings. The guideline panel used a methodologically rigorous process to evaluate the best available evidence and provide treatment recommendations. A limited number of specific questions were chosen for review based on recommendations from the panel members, all of whom are currently working directly with patients with COVID-19 and/or on policies and protocols for the healthcare response.
This guideline on infection prevention complements IDSA’s additional guidelines on COVID-19 treatment and management (now available) and diagnostic testing (to be released soon). These guidelines will be frequently updated and questions added as substantive literature becomes available and will be made accessible on an easy-to-navigate web and device interface: http://www.idsociety.org/covid19guidelines/ip.
These recommendations are intended to inform patients, clinicians, and other HCP by providing the latest available evidence.
METHODS
This guideline was developed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for evidence assessment. In addition, given the need for an urgent response to a major public health crisis, the methodological approach was modified according to the GIN/McMaster checklist for the development of rapid recommendations [7].
Panel Composition
The panel was composed of 11 members including front-line clinicians, infectious diseases specialists, experts in infection control, and guideline methodologists. The panel also included experts in preventive care, public health, medical microbiology, pediatrics, critical care medicine, and gastroenterology. Organizational representatives were included on the panel from the Society for Healthcare Epidemiology of America (SHEA) and the Pediatric Infectious Diseases Society (PIDS). The Evidence Foundation provided technical support and guideline methodologists for the development of this guideline.
Disclosure and Management of Potential Conflicts of Interest
The conflicts of interest (COI) review group included 2 representatives from IDSA who were responsible for reviewing, evaluating, and approving all disclosures. All members of the expert panel complied with the COI process for reviewing and managing COIs, which required disclosure of any financial, intellectual, or other interest that might be construed as constituting an actual, potential, or apparent conflict, regardless of relevancy to the guideline topic. The assessment of disclosed relationships for possible COIs was based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The COI review group ensured that the majority of the panel and chair were without potential relevant (related to the topic) conflicts. The chair and all members of the technical team were determined to be unconflicted.
Question Generation
Clinical questions were developed into a PICO (population, intervention, comparison, outcomes) format [8] prior to the first panel meeting. In order for these guidelines to be implementable in various healthcare environments, the following 2 types of clinical settings were defined a priori to account for the availability of personal protective equipment (PPE): conventional settings (ie, no restriction on PPE availability) and contingency or crisis capacity settings (ie, limited availability of PPE). Panel members focused on the protective effect of PPE on HCP such as the prevention of healthcare-associated transmission of viral respiratory infections (RVIs) (either laboratory-confirmed infection or inferred by clinical compatible syndrome) and adverse events leading to discontinuation of PPE.
Search Strategy
With the help of an information specialist, OVID Medline and Embase were searched to identify all relevant English language studies from inception to 14 April 2020 related to COVID-19 using the newly developed MeSH term. In certain circumstances, searches were also conducted to identify relevant literature including Google Scholar, World Health Organization (WHO), and Centers for Disease Control and Prevention (CDC) websites. Horizon scans were performed daily during the evidence assessment and recommendation process to locate additional gray literature and manuscript preprints from Medrxiv. Reference lists and literature suggested by panelists were reviewed for inclusion. The reference lists of relevant articles were scanned for additional studies.
Indirect evidence related to severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), Ebola virus disease, and influenza was searched using the systematic review filter. When applicable, existing systematic reviews were used to inform the recommendations. Laboratory experiments were also reviewed to provide further indirect evidence of mechanistic explanations when appropriate.
Screening and Study Selection
Two reviewers independently screened titles and abstracts, as well as eligible full-text studies. When acceptable systematic reviews were found, no additional randomized, controlled trials were sought. Evidence from experimental or laboratory studies was included as sources of indirect evidence, and public health websites, such as the CDC and WHO, were also reviewed for additional literature. See Supplementary Figure 1 for the PRISMA flow diagram.
Data Collection and Analyses
Pairs of reviewers extracted relevant information into a standardized data extraction form. Reviewers assessed risk of bias with the Cochrane risk-of-bias tool for randomized clinical trials (RCTs) and using modified domains to assess confounding bias, selection bias, and bias due to misclassification of the nonrandomized studies. Existing systematic reviews also were reviewed for methodologic rigor [9]. When appropriate, specific subgroup analyses were conducted using Review Manager [10].
Certainty of Evidence
Evidence profile tables were used to display the summary estimates as well as the judgments about the overall certainty of the body of evidence for each clinical question across outcomes. GRADE evidence profile and summary of findings tables were developed in the GRADEpro Guideline Development Tool [11].
The certainty of evidence was assessed using the GRADE approach [12]. Within GRADE, the body of evidence across each outcome is assessed for domains that may reduce or increase one’s certainty in the evidence (Figure 2). Evidence from RCTs starts as high certainty of evidence, and observational studies start as low certainty of evidence. Factors that may reduce one’s certainty include risk of bias (study limitations), inconsistency (unexplained heterogeneity across study findings), indirectness (applicability or generalizability to the research question), imprecision (the confidence in the estimate of an effect to support a particular decision), or publication bias (selective publication of studies). One’s certainty in the evidence may be strengthened if the following considerations are present: large or very large magnitude of effect, evidence of a dose-response gradient, or opposing residual confounding. The certainty of evidence is categorized into 4 levels ranging from very low to high. For each recommendation, an overall judgment of certainty of evidence was made based on critical outcomes.
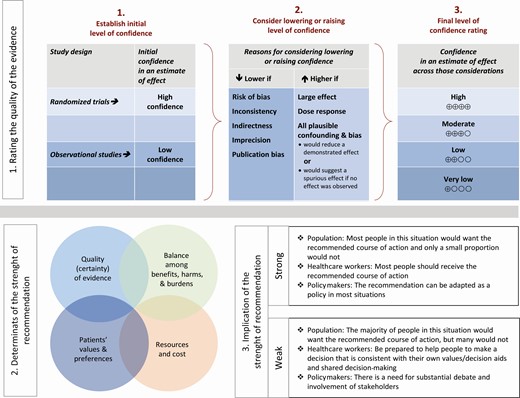
Approach and implications to rating the quality of evidence and strength of recommendations using the Grading of Recommendations Assessment, Development and Evaluation methodology. Unrestricted use of the figure granted by the US GRADE Network.
Evidence to Recommendations
The panel considered core elements of the GRADE evidence in the decision process, including certainty of evidence, balance between desirable and undesirable effects, assumption on values and preferences, and resource implications. Additional domains were acknowledged where applicable (feasibility, acceptability, and equity). The panel deliberated over the impact of resource limitations on the feasibility of and ability to implement these recommendations. Therefore, the panel’s recommendations addressed both “conventional” settings where there is no restriction on PPE availability and “contingency or crisis capacity” settings in which PPE availability is limited.
For all recommendations, the expert panelists reached consensus. Voting rules were agreed on prior to the panel meetings for situations when consensus could not be reached. As per GRADE methodology, recommendations are labeled as “strong” or “weak/conditional.” The words “we recommend” indicate strong recommendations, and the words “we suggest” indicate conditional recommendations. Figure 2 provides the suggested interpretation of strong and weak/conditional recommendations for patients, clinicians, and healthcare policymakers. In some situations where the evidence was judged insufficient to provide a clear direction “for” or “against” a particular management strategy, the panel decided to make a “no recommendation.”
According to the GRADE approach, strong recommendations in the setting of lower-quality evidence were only assigned when the panelists believed they conformed to one or several paradigmatic conditions. As per GRADE guidance [13] on discordant recommendations, there are 5 paradigmatic situations that can be conceptualized as ones in which there are clear benefits in the setting of a life-threatening situation, clear catastrophic harms, or equivalence between 2 interventions with clear harms for 1 of the alternatives.
Although there is ongoing need for research on virtually all of the topics considered in this guideline, “research needs” were noted for recommendations in which the need was believed by the panelists to be particularly relevant.
Review Process
The draft guideline underwent a rapid review for approval by the IDSA Board of Directors Executive Committee external to the guideline development panel. The guideline was reviewed and endorsed by SHEA, PIDS, and the American Society of Microbiology. The IDSA Board of Directors Executive Committee reviewed and approved the guideline prior to dissemination.
Updating Process
Regular, frequent screening of the literature will take place to determine the need for revisions based on the likelihood that any new data will have an impact on the recommendations. If necessary, the entire expert panel will be reconvened to discuss potential changes.
Definitions
Surgical Masks
Masks with or without plastic shields are used as a physical barrier to protect the user from hazards, such as splashes of large droplets of blood or body fluids. Surgical masks also protect other people against infection from the person wearing the surgical mask. Such masks trap large particles of body fluids that may contain bacteria or viruses expelled by the wearer [14]. Surgical masks and medical masks are used interchangeably in this document.
Respirator
Devices used to protect HCP from airborne particles that can lead to infection. This includes N95 filtering facepiece respirators and higher-level “mask-like” respirators (eg, N99, N100) and powered air-purifying respirators (PAPRs) and controlled air-purifying respirators.
Donning and Doffing Procedures
Donning refers to the practice of putting on PPE. Doffing refers to the practice of taking off PPE.
Crisis Standards of Care
Conventional capacity: Usual supplies available and used [1].
Contingency capacity: Conservation, adaptation, and substitution of supplies with occasional reuse of select supplies.
Crisis capacity: Critical supplies lacking.
PPE extended use: The use of PPE for greater than a single patient encounter and without removing the PPE, with or without the use of additional devices (eg, a face shield over a surgical mask). Recommended for use only in contingency or crisis capacity settings [3].
PPE reuse: The use of PPE that is doffed after each patient encounter and redonned after a period of time and/or a processing step. Recommended for use only in contingency or crisis capacity settings [3].
RESULTS
For all recommendations listed below, the panel emphasizes the importance of “appropriate PPE,” which includes gowns, gloves, and eye protection and adherence to standards for donning and doffing to minimize transmission.
In Conventional Settings
Recommendation 1: The IDSA guideline panel recommends that HCP caring for patients with suspected or known COVID-19 use either a surgical mask or N95 (or N99 or PAPR) respirator compared with no mask as part of appropriate PPE.* Strong recommendation, moderate certainty of evidence.
In Contingency or Crisis Capacity Settings
Recommendation 2: While in contingency or crisis capacity settings (respirator shortages), the IDSA guideline panel recommends that HCP caring for patients with suspected or known COVID-19 use a surgical mask or reprocessed respirator instead of no mask as part of appropriate PPE.* Strong recommendation, moderate certainty of evidence.
*Appropriate PPE includes, in addition to a mask or respirator, eye protection, gown and gloves.
Summary of the Evidence
Direct evidence from the early stages of the COVID-19 pandemic provides information about the risk of infection among HCP and the effectiveness of N95 respirators and surgical masks. According to these studies, approximately 30% of unprotected HCP (wearing no masks) exposed to COVID-19 patients developed infection [15]. In a retrospective cohort study that compared HCP wearing N95 respirators (N = 278) caring for high-risk COVID-19 patients with unmasked HCP (N = 213) caring for low-risk patients, 10/213 unmasked HCP became infected compared with 0/278 who wore N95 respirators [16]. Overall, rates of infections in HCP were 3 times higher compared with the general population, likely due to inadequate PPE practices, although the most frequent failure mechanism (lack of proper masks, face shield, or contact precautions such as hand washing) remains unclear [17].
Indirect evidence from the SARS epidemic was used to inform the question about the effectiveness of masks. Based on an existing systematic review of 5 observational studies in HCP, wearing any mask (surgical mask or N95 respirator) demonstrated a large reduction in the risk of developing infection (surgical masks: odds ratio [OR], 0.13; 95% confidence interval [CI], .03–.62 or N95 respirator: OR, 0.12; 95% CI, .06–.26) [18] (Table 1). Studies that compared N95 respirators with surgical masks on rates of SARS infection failed to show or exclude a beneficial effect (OR, 0.86; 95% CI, .22–3.33) on rates of SARS infections. Four studies compared N95 respirators with surgical masks for prevention of viral respiratory infections (VRIs) also failed to show or exclude a beneficial effect (OR, 0.94; 95% CI, .80–1.11) [19] (see Table 2 and Supplementary Figure 2).
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile: N95/Surgical Mask Compared With No Personal Protective Equipment (No Mask) or Infrequent PPE (Inconsistent Use of Mask)
| Certainty Assessment . | . | . | . | . | . | . | No. of Patients . | . | Effect . | . | Certainty . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies . | Study Design . | Risk of Bias . | Inconsistency . | Indirectness . | Imprecision . | Other Considerations . | N95 . | No Personal Protective Equipment . | Relative (95% CI) . | Absolute (95% CI) . | . |
| Severe acute respiratory syndrome infection | |||||||||||
| 5 [20–24] | Observational | Not serious | Not serious | Not serious a | Not serious | Strong association b | 9/163 (5.5%) | 86/234 (36.8%) | Odds ratio 0.12 (.06 –.26) | 302 fewer per 1000 (from 334 fewer to 236 fewer) | ⨁⨁⨁◯ Moderate |
| Certainty Assessment . | . | . | . | . | . | . | No. of Patients . | . | Effect . | . | Certainty . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies . | Study Design . | Risk of Bias . | Inconsistency . | Indirectness . | Imprecision . | Other Considerations . | N95 . | No Personal Protective Equipment . | Relative (95% CI) . | Absolute (95% CI) . | . |
| Severe acute respiratory syndrome infection | |||||||||||
| 5 [20–24] | Observational | Not serious | Not serious | Not serious a | Not serious | Strong association b | 9/163 (5.5%) | 86/234 (36.8%) | Odds ratio 0.12 (.06 –.26) | 302 fewer per 1000 (from 334 fewer to 236 fewer) | ⨁⨁⨁◯ Moderate |
Abbreviation: CI, confidence interval.
aAlthough the studies reported on the severe acute respiratory syndrome outbreak, given the similarities between severe acute respiratory syndrome coronavirus 1 and severe acute respiratory syndrome coronavirus 2, we did not rate down for indirectness.
bThe evidence was rated up for large magnitude of effect.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile: N95/Surgical Mask Compared With No Personal Protective Equipment (No Mask) or Infrequent PPE (Inconsistent Use of Mask)
| Certainty Assessment . | . | . | . | . | . | . | No. of Patients . | . | Effect . | . | Certainty . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies . | Study Design . | Risk of Bias . | Inconsistency . | Indirectness . | Imprecision . | Other Considerations . | N95 . | No Personal Protective Equipment . | Relative (95% CI) . | Absolute (95% CI) . | . |
| Severe acute respiratory syndrome infection | |||||||||||
| 5 [20–24] | Observational | Not serious | Not serious | Not serious a | Not serious | Strong association b | 9/163 (5.5%) | 86/234 (36.8%) | Odds ratio 0.12 (.06 –.26) | 302 fewer per 1000 (from 334 fewer to 236 fewer) | ⨁⨁⨁◯ Moderate |
| Certainty Assessment . | . | . | . | . | . | . | No. of Patients . | . | Effect . | . | Certainty . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies . | Study Design . | Risk of Bias . | Inconsistency . | Indirectness . | Imprecision . | Other Considerations . | N95 . | No Personal Protective Equipment . | Relative (95% CI) . | Absolute (95% CI) . | . |
| Severe acute respiratory syndrome infection | |||||||||||
| 5 [20–24] | Observational | Not serious | Not serious | Not serious a | Not serious | Strong association b | 9/163 (5.5%) | 86/234 (36.8%) | Odds ratio 0.12 (.06 –.26) | 302 fewer per 1000 (from 334 fewer to 236 fewer) | ⨁⨁⨁◯ Moderate |
Abbreviation: CI, confidence interval.
aAlthough the studies reported on the severe acute respiratory syndrome outbreak, given the similarities between severe acute respiratory syndrome coronavirus 1 and severe acute respiratory syndrome coronavirus 2, we did not rate down for indirectness.
bThe evidence was rated up for large magnitude of effect.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile: N95 Respirator Compared With Surgical Mask
| Certainty Assessment . | . | . | . | . | . | . | No. of patients . | . | Effect . | . | Certainty . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies . | Study Design . | Risk of Bias . | Inconsistency . | Indirectness . | Imprecision . | Other Considerations . | N95 . | Surgical Mask . | Relative (95% CI) . | Absolute (95% CI) . | . |
| Severe acute respiratory syndrome infection | |||||||||||
| 3 [20, 22, 25] | Observational | Serious a | Not serious | Not serious b | Serious c | None | 4/141 (2.8%) | 24/452 (5.3%) | OR 0.86 (.22–3.33) | 7 fewer per 1000 (from 41 fewer to 104 more) | ⨁◯◯◯ Very low |
| Viral respiratory illness | |||||||||||
| 4 [26–29] | Randomized | Not serious d | Not serious | Serious e | Serious c | None | 393/2464 (15.9%) | 416/1989 (20.9%) | OR 0.96 (.85–1.08) | 7 fewer per 1000 (from 26 fewer to 13 more) | ⨁⨁◯◯ Low |
| Certainty Assessment . | . | . | . | . | . | . | No. of patients . | . | Effect . | . | Certainty . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies . | Study Design . | Risk of Bias . | Inconsistency . | Indirectness . | Imprecision . | Other Considerations . | N95 . | Surgical Mask . | Relative (95% CI) . | Absolute (95% CI) . | . |
| Severe acute respiratory syndrome infection | |||||||||||
| 3 [20, 22, 25] | Observational | Serious a | Not serious | Not serious b | Serious c | None | 4/141 (2.8%) | 24/452 (5.3%) | OR 0.86 (.22–3.33) | 7 fewer per 1000 (from 41 fewer to 104 more) | ⨁◯◯◯ Very low |
| Viral respiratory illness | |||||||||||
| 4 [26–29] | Randomized | Not serious d | Not serious | Serious e | Serious c | None | 393/2464 (15.9%) | 416/1989 (20.9%) | OR 0.96 (.85–1.08) | 7 fewer per 1000 (from 26 fewer to 13 more) | ⨁⨁◯◯ Low |
Abbreviation: CI, confidence interval.
aThere were concerns about recall bias.
bAlthough the studies reported on the severe acute respiratory syndrome outbreak, given the similarities between severe acute respiratory syndrome coronavirus 1 and severe acute respiratory syndrome coronavirus 2, we did not rate down for indirectness.
cThere were concerns about imprecision with a low event rate and that the boundaries of the CI cross the clinical threshold.
dAlthough compliance to the assigned mask type was self-reported and it is not clear if there was a performance bias, study staff conducted regular checks on the study participants to control for performance bias, thus, we did not rate down for risk of bias.
eThere were concerns about indirectness since upper respiratory infection viruses in addition to coronavirus were included in this outcome.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile: N95 Respirator Compared With Surgical Mask
| Certainty Assessment . | . | . | . | . | . | . | No. of patients . | . | Effect . | . | Certainty . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies . | Study Design . | Risk of Bias . | Inconsistency . | Indirectness . | Imprecision . | Other Considerations . | N95 . | Surgical Mask . | Relative (95% CI) . | Absolute (95% CI) . | . |
| Severe acute respiratory syndrome infection | |||||||||||
| 3 [20, 22, 25] | Observational | Serious a | Not serious | Not serious b | Serious c | None | 4/141 (2.8%) | 24/452 (5.3%) | OR 0.86 (.22–3.33) | 7 fewer per 1000 (from 41 fewer to 104 more) | ⨁◯◯◯ Very low |
| Viral respiratory illness | |||||||||||
| 4 [26–29] | Randomized | Not serious d | Not serious | Serious e | Serious c | None | 393/2464 (15.9%) | 416/1989 (20.9%) | OR 0.96 (.85–1.08) | 7 fewer per 1000 (from 26 fewer to 13 more) | ⨁⨁◯◯ Low |
| Certainty Assessment . | . | . | . | . | . | . | No. of patients . | . | Effect . | . | Certainty . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies . | Study Design . | Risk of Bias . | Inconsistency . | Indirectness . | Imprecision . | Other Considerations . | N95 . | Surgical Mask . | Relative (95% CI) . | Absolute (95% CI) . | . |
| Severe acute respiratory syndrome infection | |||||||||||
| 3 [20, 22, 25] | Observational | Serious a | Not serious | Not serious b | Serious c | None | 4/141 (2.8%) | 24/452 (5.3%) | OR 0.86 (.22–3.33) | 7 fewer per 1000 (from 41 fewer to 104 more) | ⨁◯◯◯ Very low |
| Viral respiratory illness | |||||||||||
| 4 [26–29] | Randomized | Not serious d | Not serious | Serious e | Serious c | None | 393/2464 (15.9%) | 416/1989 (20.9%) | OR 0.96 (.85–1.08) | 7 fewer per 1000 (from 26 fewer to 13 more) | ⨁⨁◯◯ Low |
Abbreviation: CI, confidence interval.
aThere were concerns about recall bias.
bAlthough the studies reported on the severe acute respiratory syndrome outbreak, given the similarities between severe acute respiratory syndrome coronavirus 1 and severe acute respiratory syndrome coronavirus 2, we did not rate down for indirectness.
cThere were concerns about imprecision with a low event rate and that the boundaries of the CI cross the clinical threshold.
dAlthough compliance to the assigned mask type was self-reported and it is not clear if there was a performance bias, study staff conducted regular checks on the study participants to control for performance bias, thus, we did not rate down for risk of bias.
eThere were concerns about indirectness since upper respiratory infection viruses in addition to coronavirus were included in this outcome.
Other Considerations
Evidence to support the use of N95 respirators or surgical masks (compared with no masks) was based on observational studies that showed a very large reduction in the risk of infection during the SARS outbreak. The overall certainty of evidence was moderate. The quality of data on the use of N95 respirators compared with surgical masks for SARS or other VRIs was low or very low. If N95 respirators are used and the supply is in a contingency state, access may be mitigated by extending use (covering the respirator with a face shield or mask) over >1 patient encounter. The limitations of the evidence included small numbers of events, recall bias, and data on all viral infections (not limited to coronavirus).
Conclusions and Research Needs
The guideline panel recommends that in conventional settings, HCP caring for confirmed or suspected COVID-19 patients use a surgical mask or an N95 or higher-grade respirator (such as an N99 or PAPR). Use of masks or respirators must be in conjunction with other recommended PPE and appropriate hand hygiene. Because of the risk of serious harm, the panel recommends that HCP not be exposed to suspected or confirmed COVID-19 patients without a mask or respirator.
Additional well-designed RCTs or prospective cohort studies with appropriate comparison groups and integration with prospective outcome registries are needed to address the potential superiority of N95 or higher-grade respirators compared with surgical masks in HCP caring for COVID-19 patients.
In Conventional, Contingency, or Crisis Capacity Settings
Recommendation 3: The IDSA guideline panel makes no recommendation for the use of double gloves vs single gloves for HCP caring for patients with suspected or known COVID-19 as part of appropriate PPE.* Knowledge gap.
*Appropriate PPE includes, in addition to a mask or respirator, eye protection, gown, and gloves.
Summary of the Evidence
There were no comparative studies that compared double vs single gloves to decrease infection rates. However, there is a theoretical risk of organism transfer from contaminated PPE to hands after removal of the contaminated gloves or clothing that may contribute to infection. In 1 study, swabs from 30 HCP PPE were collected after personnel exited COVID-19 patient rooms. There were no positive samples out of 90 collected swabs [30]. Furthermore, in a laboratory experiment that simulated droplet contamination [31], 2 groups of participants were contaminated with bacteriophage MS2 after both groups donned a full set of PPE as per CDC guidance [32]. One group wore 1 pair of gloves over the gown sleeve. The second group donned 2 pairs of latex gloves. The first (inner) pair of gloves was applied under the gown sleeve and the second (outer) pair was placed over the first pair and positioned over the gown sleeve. During the doffing phase, the inner pair of gloves was removed last. The double-glove strategy was associated with less contamination than the single-glove strategy [31]. However, there was no report of hand hygiene or use of hand sanitizer between doffing sequences in the single-glove group as per CDC recommendations, which may have decreased the contamination in the single-glove group [32].
Other Considerations
The panel determined that there was insufficient evidence to make a recommendation on the use of double gloves.
Conclusions and Research Needs
There is conflicting indirect and experimental evidence on the importance of double-gloving as a component of appropriate PPE when caring for a patient with suspected or known COVID-19. Further studies are needed to compare different glove doffing strategies to prevent infection in HCP providing usual care for COVID-19 patients.
In Conventional, Contingency, or Crisis Capacity Settings
Recommendation 4: The IDSA guideline panel makes no recommendation for the use of shoe covers vs no shoe covers for HCP caring for patients with suspected or known COVID-19 as part of appropriate PPE.* Knowledge gap.
*Appropriate PPE includes, in addition to a mask or respirator, eye protection, gown, and gloves.
Summary of the Evidence
There were no studies that evaluated shoe covers as part of routine PPE and COVID-19 transmission. In the study by Ong et al [19], HCP PPE was swabbed (approximately 90 swabs obtained) after personnel exited COVID-19 patient rooms. Only 1 PPE swab obtained from the surface of a shoe front was found positive [30].
Other Considerations
The panel determined that there was insufficient evidence to make a recommendation on the use of shoe covers.
Conclusions and Research Needs
Current guidance on PPE endorses the use of shoe covers when there is concern for splash risk from fluids that may contain pathogens. Further studies are needed to determine if shoe covers are needed to protect HCP from contamination in the context of COVID-19.
AEROSOL-GENERATING PROCEDURES
Background
Person-to-person transmission of SARS-CoV-2 occurs primarily via exposure to an infectious person’s respiratory droplets. Respiratory protection (ie, surgical mask) prevents droplets from contacting the mucous membranes of a person’s nose and mouth. Some medical procedures are more likely to generate higher concentrations of infectious small-particle (<0.5 µm) respiratory aerosols. These procedures, referred to as aerosol-generating procedures (AGPs), could potentially increase HCP exposure risk to SARS-CoV-2 (see Table 3 for the lists of AGPs from various organizations). Thus, a higher level of respiratory protection is likely needed to protect HCP from inhaling smaller aerosolized particles. N95 and higher-level respirators, such as disposable filtering facepiece respirators, PAPR, and elastomeric respirators, provide additional protection due to their filtering capabilities. As with droplet transmission, eye protection in the form of goggles or face shield is required.
| Organization . | Aerosol-generating Procedures . | . | . | . |
|---|---|---|---|---|
| CDC: COVID-19 Guidance [36] | Open suctioning of airways, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, noninvasive ventilation (eg, bilevel positive airway pressure, continuous positive airway pressure), bronchoscopy, manual ventilation | |||
| CDC: Seasonal Influenza Guidance [37] | Bronchoscopy, sputum induction, elective intubation and extubation, autopsies, cardiopulmonary resuscitation, emergent intubation, open suctioning of airways | |||
| WHO: COVID-19 Guidance [38] | Tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, bronchoscopy | |||
| WHO: Epidemic- and Pandemic-Prone Acute Respiratory Diseases [39] | Aspiration of respiratory tract, intubation, resuscitation, bronchoscopy, autopsy | |||
| Organization | CDC (COVID-19 Guidance)[36] | CDC (Seasonal Influenza Guidance)[37] | WHO (COVID-19 Guidance)[38] | WHO (Epidemic and Pandemic -Prone Acute Respiratory Diseases)[39] |
| Procedures listed | Open suctioning of airways, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, noninvasive ventilation (eg, bilevel positive airway pressure, continuous positive airway pressure), bronchoscopy, manual ventilation | Bronchoscopy, sputum induction, elective intubation and extubation, autopsies, cardiopulmonary resuscitation, emergent intubation and open suctioning of airways | Tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, and bronchoscopy | Aspiration of respiratory tract, intubation, resuscitation, bronchoscopy, autopsy |
| Organization . | Aerosol-generating Procedures . | . | . | . |
|---|---|---|---|---|
| CDC: COVID-19 Guidance [36] | Open suctioning of airways, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, noninvasive ventilation (eg, bilevel positive airway pressure, continuous positive airway pressure), bronchoscopy, manual ventilation | |||
| CDC: Seasonal Influenza Guidance [37] | Bronchoscopy, sputum induction, elective intubation and extubation, autopsies, cardiopulmonary resuscitation, emergent intubation, open suctioning of airways | |||
| WHO: COVID-19 Guidance [38] | Tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, bronchoscopy | |||
| WHO: Epidemic- and Pandemic-Prone Acute Respiratory Diseases [39] | Aspiration of respiratory tract, intubation, resuscitation, bronchoscopy, autopsy | |||
| Organization | CDC (COVID-19 Guidance)[36] | CDC (Seasonal Influenza Guidance)[37] | WHO (COVID-19 Guidance)[38] | WHO (Epidemic and Pandemic -Prone Acute Respiratory Diseases)[39] |
| Procedures listed | Open suctioning of airways, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, noninvasive ventilation (eg, bilevel positive airway pressure, continuous positive airway pressure), bronchoscopy, manual ventilation | Bronchoscopy, sputum induction, elective intubation and extubation, autopsies, cardiopulmonary resuscitation, emergent intubation and open suctioning of airways | Tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, and bronchoscopy | Aspiration of respiratory tract, intubation, resuscitation, bronchoscopy, autopsy |
Accessed 16 April 2020.
Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; WHO, World Health Organization.
| Organization . | Aerosol-generating Procedures . | . | . | . |
|---|---|---|---|---|
| CDC: COVID-19 Guidance [36] | Open suctioning of airways, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, noninvasive ventilation (eg, bilevel positive airway pressure, continuous positive airway pressure), bronchoscopy, manual ventilation | |||
| CDC: Seasonal Influenza Guidance [37] | Bronchoscopy, sputum induction, elective intubation and extubation, autopsies, cardiopulmonary resuscitation, emergent intubation, open suctioning of airways | |||
| WHO: COVID-19 Guidance [38] | Tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, bronchoscopy | |||
| WHO: Epidemic- and Pandemic-Prone Acute Respiratory Diseases [39] | Aspiration of respiratory tract, intubation, resuscitation, bronchoscopy, autopsy | |||
| Organization | CDC (COVID-19 Guidance)[36] | CDC (Seasonal Influenza Guidance)[37] | WHO (COVID-19 Guidance)[38] | WHO (Epidemic and Pandemic -Prone Acute Respiratory Diseases)[39] |
| Procedures listed | Open suctioning of airways, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, noninvasive ventilation (eg, bilevel positive airway pressure, continuous positive airway pressure), bronchoscopy, manual ventilation | Bronchoscopy, sputum induction, elective intubation and extubation, autopsies, cardiopulmonary resuscitation, emergent intubation and open suctioning of airways | Tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, and bronchoscopy | Aspiration of respiratory tract, intubation, resuscitation, bronchoscopy, autopsy |
| Organization . | Aerosol-generating Procedures . | . | . | . |
|---|---|---|---|---|
| CDC: COVID-19 Guidance [36] | Open suctioning of airways, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, noninvasive ventilation (eg, bilevel positive airway pressure, continuous positive airway pressure), bronchoscopy, manual ventilation | |||
| CDC: Seasonal Influenza Guidance [37] | Bronchoscopy, sputum induction, elective intubation and extubation, autopsies, cardiopulmonary resuscitation, emergent intubation, open suctioning of airways | |||
| WHO: COVID-19 Guidance [38] | Tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, bronchoscopy | |||
| WHO: Epidemic- and Pandemic-Prone Acute Respiratory Diseases [39] | Aspiration of respiratory tract, intubation, resuscitation, bronchoscopy, autopsy | |||
| Organization | CDC (COVID-19 Guidance)[36] | CDC (Seasonal Influenza Guidance)[37] | WHO (COVID-19 Guidance)[38] | WHO (Epidemic and Pandemic -Prone Acute Respiratory Diseases)[39] |
| Procedures listed | Open suctioning of airways, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, noninvasive ventilation (eg, bilevel positive airway pressure, continuous positive airway pressure), bronchoscopy, manual ventilation | Bronchoscopy, sputum induction, elective intubation and extubation, autopsies, cardiopulmonary resuscitation, emergent intubation and open suctioning of airways | Tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, and bronchoscopy | Aspiration of respiratory tract, intubation, resuscitation, bronchoscopy, autopsy |
Accessed 16 April 2020.
Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; WHO, World Health Organization.
In Conventional Settings
Recommendation 5: The IDSA guideline panel recommends that HCP involved with AGPs on suspected or known COVID-19 patients use an N95 (or N99 or PAPR) respirator instead of a surgical mask as part of appropriate PPE.* Strong recommendation, very low certainty of evidence.
Comment: Despite the very low-quality and indirect evidence supporting this recommendation, the IDSA guideline panel placed a high value on avoiding serious harms to exposed HCP.
*Appropriate PPE includes, in addition to a mask or respirator, eye protection, gown, and gloves.
Summary of the Evidence
There was no direct evidence on AGPs and rates of COVID-19 infection among HCP. Indirect evidence from the SARS epidemic was used to inform this recommendation. Based on observational data, among infected HCP with SARS, exposure to an AGP such as tracheal intubation was associated with a higher risk of infection (see Table 4) [33]. Evidence from laboratory simulation data also provided indirect evidence on the viability of aerosolized SARS-CoV-2 [34]. Additionally, data on environmental contamination was obtained by sampling various surfaces and air samples from confirmed COVID-19 patient rooms: 87% (13/15) of room sites (including air exhaust outlet fans) returned positive SARS-CoV-2 on reverse-transcription polymerase chain reaction (RT-PCR) results and 60% (3/5) of toilet sites (including toilet bowl, sink, and door handle) returned positive SARS-CoV-2 on RT-PCR results. Air samples were negative despite the extent of environmental contamination [35].
Risk of Severe Acute Respiratory Syndrome Transmission to Healthcare Workers Exposed and Not Exposed to Aerosol-generating Procedure, and Aerosol-generating Procedures as Risk Factors for SARS Transmission
| Type of Aerosol-generating Procedure . | Odds Ratio . | 95% Confidence Interval . |
|---|---|---|
| Tracheal intubation | 6.6 | 2.3–18.9 |
| Manipulation of oxygen mask | 4.6 | .6–32.5 |
| Tracheotomy | 4.2 | 1.5–11.5 |
| Manipulation of bilevel positive airway pressure mask | 4.2 | .6–27.4 |
| Suction before intubation | 3.5 | .5– 24.6 |
| Noninvasive ventilation | 3.1 | 1.4–7.2 |
| Manual ventilation before intubation | 2.8 | 1.3–6.4 |
| Collection of sputum sample | 2.7 | .9–8.2 |
| Defibrillation | 2.5 | .1–43.9 |
| Bronchoscopy | 1.9 | .2–14.2 |
| Chest compressions | 1.4 | .2–11.2 |
| Insertion of nasogastric tube | 1.2 | .4–4.0 |
| Type of Aerosol-generating Procedure . | Odds Ratio . | 95% Confidence Interval . |
|---|---|---|
| Tracheal intubation | 6.6 | 2.3–18.9 |
| Manipulation of oxygen mask | 4.6 | .6–32.5 |
| Tracheotomy | 4.2 | 1.5–11.5 |
| Manipulation of bilevel positive airway pressure mask | 4.2 | .6–27.4 |
| Suction before intubation | 3.5 | .5– 24.6 |
| Noninvasive ventilation | 3.1 | 1.4–7.2 |
| Manual ventilation before intubation | 2.8 | 1.3–6.4 |
| Collection of sputum sample | 2.7 | .9–8.2 |
| Defibrillation | 2.5 | .1–43.9 |
| Bronchoscopy | 1.9 | .2–14.2 |
| Chest compressions | 1.4 | .2–11.2 |
| Insertion of nasogastric tube | 1.2 | .4–4.0 |
Adapted from [32].
Abbreviation: SARS, severe acute respiratory syndrome.
Risk of Severe Acute Respiratory Syndrome Transmission to Healthcare Workers Exposed and Not Exposed to Aerosol-generating Procedure, and Aerosol-generating Procedures as Risk Factors for SARS Transmission
| Type of Aerosol-generating Procedure . | Odds Ratio . | 95% Confidence Interval . |
|---|---|---|
| Tracheal intubation | 6.6 | 2.3–18.9 |
| Manipulation of oxygen mask | 4.6 | .6–32.5 |
| Tracheotomy | 4.2 | 1.5–11.5 |
| Manipulation of bilevel positive airway pressure mask | 4.2 | .6–27.4 |
| Suction before intubation | 3.5 | .5– 24.6 |
| Noninvasive ventilation | 3.1 | 1.4–7.2 |
| Manual ventilation before intubation | 2.8 | 1.3–6.4 |
| Collection of sputum sample | 2.7 | .9–8.2 |
| Defibrillation | 2.5 | .1–43.9 |
| Bronchoscopy | 1.9 | .2–14.2 |
| Chest compressions | 1.4 | .2–11.2 |
| Insertion of nasogastric tube | 1.2 | .4–4.0 |
| Type of Aerosol-generating Procedure . | Odds Ratio . | 95% Confidence Interval . |
|---|---|---|
| Tracheal intubation | 6.6 | 2.3–18.9 |
| Manipulation of oxygen mask | 4.6 | .6–32.5 |
| Tracheotomy | 4.2 | 1.5–11.5 |
| Manipulation of bilevel positive airway pressure mask | 4.2 | .6–27.4 |
| Suction before intubation | 3.5 | .5– 24.6 |
| Noninvasive ventilation | 3.1 | 1.4–7.2 |
| Manual ventilation before intubation | 2.8 | 1.3–6.4 |
| Collection of sputum sample | 2.7 | .9–8.2 |
| Defibrillation | 2.5 | .1–43.9 |
| Bronchoscopy | 1.9 | .2–14.2 |
| Chest compressions | 1.4 | .2–11.2 |
| Insertion of nasogastric tube | 1.2 | .4–4.0 |
Adapted from [32].
Abbreviation: SARS, severe acute respiratory syndrome.
Other Considerations
Evidence to support the use of N95 or higher-level respirators instead of surgical masks for HCP involved in AGPs was based on observational studies and experimental laboratory data. The overall certainty of evidence was very low due to limitations in the retrospective observational data and recall bias. However, the IDSA guideline panel made a strong recommendation for N95 or higher-level respirators, placing a high value on preventing infection among HCP.
Conclusions and Research Needs
The guideline panel recommends that when an AGP is being performed on a patient with suspected or known COVID-19, all involved HCP should wear an N95 or higher-level respirator, in addition to a gown, gloves, and eye protection. Additional clinical studies are needed to inform our understanding of SARS-CoV-2 respiratory transmission in the healthcare setting. Studies are especially needed to clarify which medical procedures require a higher level of respiratory protection.
In Contingency or Crisis Capacity Settings
Recommendation 6: While in contingency or crisis capacity settings (N95 respirator shortages), the IDSA guideline panel suggests that HCP involved with AGPs on suspected or known COVID-19 patients use a reprocessed N95 respirator for reuse as part of appropriate PPE.* Conditional recommendation, very low certainty evidence.
*Appropriate PPE includes, in addition to a mask or respirator, eye protection, gown, and gloves.
Summary of the Evidence
No direct evidence was found on infection rates among HCP using reprocessed and reused N95 respirators. Furthermore, indirect evidence from other pandemic outbreaks also did not reveal empiric data on infection rates. Indirect evidence on reprocessing strategies that use ultraviolet (UV) radiation, heat, 70% ethanol, or vaporized hydrogen peroxide (VHP) was used to inform this recommendation. These data were taken from experiments under laboratory conditions or anecdotal reports on reprocessing and reuse of N95 respirators with COVID-19 patients from different medical centers in the United States.
Three studies conducted in a laboratory setting using VHP showed effective decontamination of N95 respirators with no observable physical changes and no degradation to the filtration media after up to 30–50 cycles of exposure to VHP. However, after 20 cycles, the elastic straps became stiffer and there were concerns about respirator fit and comfort [40–42].
UV germicidal irradiation (UVGI) to decontaminate and reuse N95 respirators showed similar results in up to 20 decontamination cycles with no effect on filtration efficacy in various laboratory studies [40, 43–45]. However, there was discrepancy in fit testing after 10–20 cycles of UVGI, depending on the model of N95 respirator tested [44]. Furthermore, anecdotal reports from hospitals that used UVGI for N95 decontamination showed that up to 50 cycles was acceptable before significant degradation in filtration efficiency was noted but that the average number of times masks were reused before fit testing failures was 3 [46].
Dry heat as a decontamination method was used in 4 studies reporting that heat administered at temperatures of 70°C–80°C had no effect on the filtration efficiency or degradation of the N95 respirator [45, 47, 48]. In 1 study, N95 respirator fit was impaired; therefore, only 2 reuses after heat decontamination are recommended [49].
Other Considerations
No studies that evaluated the effectiveness of reprocessed masks on prevention of COVID-19 infection among HCP were found. The available evidence to inform this recommendation included anecdotal reports and experiments under laboratory conditions to assess mask integrity, filtration efficiency (filter aerosol penetration, airflow resistance), and fit performance of various reprocessing strategies. The overall certainty of evidence was very low due to the following limitations: no comparison of reprocessed N95 respirators with new or unprocessed N95 respirators and no direct evidence on infection rates using reprocessed masks.
Conclusions and Research Needs
The guideline panel recommends that during a contingency or crisis situation with shortages of N95 respirators, reprocessed N95 respirators be reused instead of surgical masks as part of appropriate PPE when HCP are involved in AGPs on patients with suspected or known COVID-19. Additional experimental and clinical studies are needed to inform research for the risk of dispersal and acquisition of SARS-CoV-2 during AGPs in clinics, acute care and critical care wards, and airborne infection isolation facilities. Further investigations are needed to inform research for the optimal methods of reprocessing N95 respirators to meet the safety requirement for HCP.
Recommendation 7: While in contingency or crisis settings (respirator shortages), the IDSA guideline panel recommends that HCP involved with AGPs on suspected or known COVID-19 patients add a face shield or surgical mask as a cover for the N95 respirator to allow for extended use as part of appropriate PPE.* Strong recommendation, very low certainty evidence. Comment: This recommendation assumes correct doffing sequence and hand hygiene are performed before and after removing the face shield or surgical mask covering the respirator.
Recommendation 8: While in contingency or crisis settings (respirator shortages), the IDSA guideline panel suggests that HCP involved with AGPs on suspected or known COVID-19 patients add a face shield or surgical mask as a cover for the N95 respirator to allow for reuse as part of appropriate PPE.* Conditional recommendation, very low certainty evidence. Comment: This recommendation assumes correct doffing sequence and hand hygiene are performed before and after removing the face shield or surgical mask covering the respirator.
*Appropriate PPE includes, in addition to a mask or respirator, eye protection, gown, and gloves.
Summary of the Evidence
Extended use [50] is defined as wearing the same N95 respirator for multiple different and consecutive patient encounters without removal between encounters. The CDC recommends a maximum extended use period of 8–12 hours [50]. Reuse is defined as wearing the same N95 respirator for multiple different patient encounters but doffing between encounters. Unless the manufacturer specifies otherwise, the CDC suggests limiting N95 respirator reuse to no more than 5 times per device [50]. In contingency or crisis capacity settings (shortage of N95 respirators), no direct evidence on extended use or reuse of N95 respirator was identified. Additionally, no indirect comparative evidence on infection rates among HCP was identified (Table 5).
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile: Extended Use/Reuse of the Same N95 Respirator vs Surgical Masks for Coronavirus 2019 Prevention
| Certainty Assessment . | . | . | Impact . |
|---|---|---|---|
| No. of Studies . | Study Design . | Certainty . | . |
| Infection with COVID-19 | |||
| 9 [50–55, 57–59] | Anecdotal reports, experiments under laboratory conditions | ⨁◯◯◯ Very lowa,b | There was no direct evidence found on infection rates with extended use of N95 respirators during the COVID-19 pandemic. Furthermore, indirect evidence from other pandemic outbreaks did not reveal empiric data on infection rates. However, there were reports of anecdotal experience on extended use, laboratory experiments, and mathematical models. Experiments on tolerability of the N95 respirator with prolonged use showed that HCP were able to tolerate the N95 respirator for 89 of 215 (41%) total shifts of 8 hours. In the remaining shifts, N95 respirators were discarded before 8 hours because of contamination or due to intolerance [57]. Anecdotal reports also showed that more than 40% of HCP reported extended use or reuse of their N95 respirator during the H1N1 pandemic [50, 51]. A mathematical model to calculate the potential influenza contamination of office masks from aerosol sources in various exposure scenarios showed that surgical mask contamination levels from a single cough (approximately equal to 19 viruses) were much less than likely levels from aerosols (4473 viruses on filtering facepiece respirator and 3476 viruses on surgical masks) [58]. Laboratory tests have reported that 5 consecutive donnings can be performed before fit factors consistently drop to unsafe levels [54]. Extended use of N95 respirators during the COVID-19 pandemic has been associated with skin irritation. In a survey study, 97% of first-line HCP reported (mostly mild) skin damage [55]. Anecdotal reports of the use of surgical masks over N95 respirators as a barrier to pathogens (so as to extend the life of the N95 respirator) have been published [53]. This strategy was sparingly utilized during the SARS outbreak, but the effect on HCP infections was not reported. Narrative reports, news conference reports, including the Centers for Disease Control and Prevention recommendation [52] during the H1N1 pandemic advised use of a cleanable face shield or surgical mask to reduce N95 respirator contamination [59]. |
| Certainty Assessment . | . | . | Impact . |
|---|---|---|---|
| No. of Studies . | Study Design . | Certainty . | . |
| Infection with COVID-19 | |||
| 9 [50–55, 57–59] | Anecdotal reports, experiments under laboratory conditions | ⨁◯◯◯ Very lowa,b | There was no direct evidence found on infection rates with extended use of N95 respirators during the COVID-19 pandemic. Furthermore, indirect evidence from other pandemic outbreaks did not reveal empiric data on infection rates. However, there were reports of anecdotal experience on extended use, laboratory experiments, and mathematical models. Experiments on tolerability of the N95 respirator with prolonged use showed that HCP were able to tolerate the N95 respirator for 89 of 215 (41%) total shifts of 8 hours. In the remaining shifts, N95 respirators were discarded before 8 hours because of contamination or due to intolerance [57]. Anecdotal reports also showed that more than 40% of HCP reported extended use or reuse of their N95 respirator during the H1N1 pandemic [50, 51]. A mathematical model to calculate the potential influenza contamination of office masks from aerosol sources in various exposure scenarios showed that surgical mask contamination levels from a single cough (approximately equal to 19 viruses) were much less than likely levels from aerosols (4473 viruses on filtering facepiece respirator and 3476 viruses on surgical masks) [58]. Laboratory tests have reported that 5 consecutive donnings can be performed before fit factors consistently drop to unsafe levels [54]. Extended use of N95 respirators during the COVID-19 pandemic has been associated with skin irritation. In a survey study, 97% of first-line HCP reported (mostly mild) skin damage [55]. Anecdotal reports of the use of surgical masks over N95 respirators as a barrier to pathogens (so as to extend the life of the N95 respirator) have been published [53]. This strategy was sparingly utilized during the SARS outbreak, but the effect on HCP infections was not reported. Narrative reports, news conference reports, including the Centers for Disease Control and Prevention recommendation [52] during the H1N1 pandemic advised use of a cleanable face shield or surgical mask to reduce N95 respirator contamination [59]. |
Abbreviations: COVID-19, coronavirus disease 2019; HCP, healthcare personnel; SARS, severe acute respiratory syndrome.
aExperimental data and observational evidence with no comparator to understand the risk of the acceptable protection from COVID-19.
bThere are multiple layers of indirectness, including different populations (some studies reported on influenza virus or simulation studies on healthy volunteers), and indirect outcomes (tolerability of the mask or laboratory evidence of contamination) instead of infection rates among HCP.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile: Extended Use/Reuse of the Same N95 Respirator vs Surgical Masks for Coronavirus 2019 Prevention
| Certainty Assessment . | . | . | Impact . |
|---|---|---|---|
| No. of Studies . | Study Design . | Certainty . | . |
| Infection with COVID-19 | |||
| 9 [50–55, 57–59] | Anecdotal reports, experiments under laboratory conditions | ⨁◯◯◯ Very lowa,b | There was no direct evidence found on infection rates with extended use of N95 respirators during the COVID-19 pandemic. Furthermore, indirect evidence from other pandemic outbreaks did not reveal empiric data on infection rates. However, there were reports of anecdotal experience on extended use, laboratory experiments, and mathematical models. Experiments on tolerability of the N95 respirator with prolonged use showed that HCP were able to tolerate the N95 respirator for 89 of 215 (41%) total shifts of 8 hours. In the remaining shifts, N95 respirators were discarded before 8 hours because of contamination or due to intolerance [57]. Anecdotal reports also showed that more than 40% of HCP reported extended use or reuse of their N95 respirator during the H1N1 pandemic [50, 51]. A mathematical model to calculate the potential influenza contamination of office masks from aerosol sources in various exposure scenarios showed that surgical mask contamination levels from a single cough (approximately equal to 19 viruses) were much less than likely levels from aerosols (4473 viruses on filtering facepiece respirator and 3476 viruses on surgical masks) [58]. Laboratory tests have reported that 5 consecutive donnings can be performed before fit factors consistently drop to unsafe levels [54]. Extended use of N95 respirators during the COVID-19 pandemic has been associated with skin irritation. In a survey study, 97% of first-line HCP reported (mostly mild) skin damage [55]. Anecdotal reports of the use of surgical masks over N95 respirators as a barrier to pathogens (so as to extend the life of the N95 respirator) have been published [53]. This strategy was sparingly utilized during the SARS outbreak, but the effect on HCP infections was not reported. Narrative reports, news conference reports, including the Centers for Disease Control and Prevention recommendation [52] during the H1N1 pandemic advised use of a cleanable face shield or surgical mask to reduce N95 respirator contamination [59]. |
| Certainty Assessment . | . | . | Impact . |
|---|---|---|---|
| No. of Studies . | Study Design . | Certainty . | . |
| Infection with COVID-19 | |||
| 9 [50–55, 57–59] | Anecdotal reports, experiments under laboratory conditions | ⨁◯◯◯ Very lowa,b | There was no direct evidence found on infection rates with extended use of N95 respirators during the COVID-19 pandemic. Furthermore, indirect evidence from other pandemic outbreaks did not reveal empiric data on infection rates. However, there were reports of anecdotal experience on extended use, laboratory experiments, and mathematical models. Experiments on tolerability of the N95 respirator with prolonged use showed that HCP were able to tolerate the N95 respirator for 89 of 215 (41%) total shifts of 8 hours. In the remaining shifts, N95 respirators were discarded before 8 hours because of contamination or due to intolerance [57]. Anecdotal reports also showed that more than 40% of HCP reported extended use or reuse of their N95 respirator during the H1N1 pandemic [50, 51]. A mathematical model to calculate the potential influenza contamination of office masks from aerosol sources in various exposure scenarios showed that surgical mask contamination levels from a single cough (approximately equal to 19 viruses) were much less than likely levels from aerosols (4473 viruses on filtering facepiece respirator and 3476 viruses on surgical masks) [58]. Laboratory tests have reported that 5 consecutive donnings can be performed before fit factors consistently drop to unsafe levels [54]. Extended use of N95 respirators during the COVID-19 pandemic has been associated with skin irritation. In a survey study, 97% of first-line HCP reported (mostly mild) skin damage [55]. Anecdotal reports of the use of surgical masks over N95 respirators as a barrier to pathogens (so as to extend the life of the N95 respirator) have been published [53]. This strategy was sparingly utilized during the SARS outbreak, but the effect on HCP infections was not reported. Narrative reports, news conference reports, including the Centers for Disease Control and Prevention recommendation [52] during the H1N1 pandemic advised use of a cleanable face shield or surgical mask to reduce N95 respirator contamination [59]. |
Abbreviations: COVID-19, coronavirus disease 2019; HCP, healthcare personnel; SARS, severe acute respiratory syndrome.
aExperimental data and observational evidence with no comparator to understand the risk of the acceptable protection from COVID-19.
bThere are multiple layers of indirectness, including different populations (some studies reported on influenza virus or simulation studies on healthy volunteers), and indirect outcomes (tolerability of the mask or laboratory evidence of contamination) instead of infection rates among HCP.
During the H1N1 pandemic, more than 40% of HCP reported extended use or reuse of an N95 respirator [51, 52]. During an influenza pandemic or other widespread respiratory pathogen outbreak, the CDC recommends the addition of a cleanable face shield on top of an N95 respirator to reduce respirator contamination [53]. Surgical masks being worn over N95 respirators were anecdotally reported during the SARS outbreak. The face shield or surgical mask is felt to serve as a barrier to surface contamination, thereby extending the life of the N95 respirator. However, the effect of extended use of this combination on infection rates among HCP has not been reported [54].
Based on laboratory evidence, in vitro testing on durability and endurance of N95 respirators suggests that 3–5 consecutive donnings can be performed before fit factors consistently drop, predicting an unsafe fit [55]. In a survey of front-line HCP, 97% reported predominantly mild skin damage with extended use of an N95 respirator during the current COVID-19 pandemic [56].
Other Considerations
The available evidence to inform this recommendation included anecdotal reports, experimental laboratory data, and mathematical models. Strategies of using a face shield or surgical mask to cover an N95 respirator and extend the life of the respirator were used in other pandemics. Additionally, in vitro testing was performed on durability and fit endurance of N95 respirators. The overall certainty of the evidence was low due to concerns about the observational data and lack of evidence on infection rates in HCP using N95 respirators for extended periods or reusing respirators.
Conclusions and Research Needs
The guideline panel recommends that should extended use or reuse of an N95 respirator become necessary in a contingency or crisis setting (ie, N95 respirator shortage), HCP should place some type of barrier (face shield or surgical mask) over the N95 respirator while performing AGPs to reduce contamination of the N95 respirator. Either extended use or reuse strategies are preferred to a surgical mask alone when performing AGPs. These recommendations are based on indirect evidence that suggests that masks/respirators are frequently contaminated during AGPs and direct evidence that suggests that HCP routinely touch masks/respirators while wearing them. As a result, the guideline panel believes these recommendations lead to increased safety for HCP and decreased risk of self-inoculation from a contaminated N95 respirator when worn for an extended period or reused on separate occasions.
RCTs and prospective outcome registries are needed to inform strategies to prevent infection of HCP while in contingency and crisis settings in which recommendations for use of PPE in conventional settings cannot be adhered to. Additional studies are also needed to characterize the true impact of extended use and reuse on N95 respirator fit and filtration, including identifying simple thresholds above which these strategies would no longer be recommended. Techniques for safely storing an N95 respirator between uses (eg, in a clean, breathable container) and for preventing HCP contamination during donning and doffing require evaluation. Combining extended use or reuse with other conservation strategies, such as alternating between different N95 respirators at a set interval or performing N95 disinfection, may further improve safety and merits investigation.
NARRATIVE SUMMARIES
In addition to the clinical questions addressed above, the panel identified several infection prevention topics for which additional data are needed to formulate recommendations. Narrative summaries are provided below.
Does the Use of N95 Respirators Require Fit-testing Beyond Ensuring a Good Seal?
An N95 filtering facepiece respirator should be fit-tested in order to demonstrate that a tight seal is maintained during routine activity. This will ensure maximal protection when HCP are involved in performing an AGP on COVID-19 patients [60]. Many different respirators are manufactured, and appropriate testing should occur to ensure appropriate fit. The Occupational Safety Health Administration requires an initial fit test to identify the appropriate model, style, and size of the respirator [61]. Several methods for fit-testing can be used to establish appropriate fit, including inward leakage [62], qualitative fit test [63], quantitative 8-step fit test [64], fast 5-step test [65], and, for certain respirators, even a panel passing rate [66]. In a crisis situation, however, a respirator that has the best facial fit without actual fit-testing can be used.
What Is the Role of PAPRs in Contingency or Crisis Capacity Settings?
Access to PAPRs may be even more limited due to cost and need for routine maintenance; they can be considered if a hospital has an established PAPR program that can help service, disinfect, and turn around these devices for the next user. Programs that incorporate PAPRs need to include programs for battery supply and maintenance. HCP also need formal training on how to use PAPRs appropriately, as well as how to safely doff the PAPR hood and avoid self-contamination in the process. Removal of PAPR hoods is more complicated than removal of an N95 or similar respirator and may increase the risk of self-contamination.
Should Universal Masking Be Used to Prevent COVID-19 Transmission in Healthcare Settings?
In addition to personal protection, the major purpose of universal masking is to limit transmission of viral particles from individuals wearing a mask (who may be asymptomatic or minimally symptomatic) to other individuals and the environment (ie, source control). Although no studies exist regarding the effectiveness of universal masking for the prevention of transmission of COVID-19 in healthcare settings, we identified 4 studies of universal masking in a tertiary care hospital and certain high-risk settings. During the influenza A H1N1 pandemic in 2009, surgical mask use by HCP and visitors was promoted in the hospital. With these measures, only 4 (0.48%) of 836 persons who were exposed to laboratory-confirmed patients and staff with A/2009/H1N1 infection were confirmed to have A/2009/H1N1 infection. Not wearing a surgical mask, either by exposed persons during contact with index cases (4/4 vs 264/832) or vice versa (4/4 vs 300/832), was found to be a significant risk factor for the nosocomial acquisition of A/2009/H1N1 infection [67]. One prospective single-center study examined the impact of universal masking of all staff and visitors, regardless of symptoms or season, when in direct contact with hemopoietic stem cell transplant (HSCT) patients. Using a time series approach adjusted for season and year, the authors demonstrated a significant decrease in VRIs from 10.3% (95/920 patients) in the premask period (2003–2009) to 4.4% (40/911) in the postmask period (2010–2014), regardless of transplant type [68]. Another single-center quality improvement study assessed the impact of universal masking of all staff and visitors on the incidence of RVIs in a HSCT unit in a pre–post comparison without adjustment. There were 14 RVIs over 15 001 patient-days in the premask period vs 2 RVIs over 15 608 patient-days in the postmask period [69]. A similar pre–post study of universal masking was performed in a neonatal intensive care unit (NICU) and special care nursery in Sweden. The incidence of RVIs during the premasking period (January 2014–September 2015) was compared to the incidence in the postmasking period (October 2015–September 2016). A nonsignificant decline in the RVI rate was observed in the special care nursery (5 per 10 000 patient-days to 2 per 10 000), but declines were not observed in the NICU (1 per 10 000 vs 2 per 10 000) [70].
These clinical studies likely demonstrated declines that are also attributable to concomitant adherence to hand hygiene and other appropriate precautions in high-risk settings, as masks alone are not likely to mitigate spread. Nonetheless, there may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCP and visitors.
Does the Addition of a Negative-pressure Room/Airborne Infection Isolation Room Provide Increased Safety for HCP Working With Suspected or Known COVID-19 Patients?
The role of an airborne infection isolation room (AIIR), or negative-pressure room, as an intervention to increase safety for HCP caring for suspected or known COVID-19 patients is unclear, with the exception of those involved in AGPs (eg, intubation). Negative-pressure rooms are routinely used to prevent transmission of pathogens spread via airborne nuclei, such as measles, tuberculosis, or varicella. To date, there are no data to suggest that SARS-CoV-2 is routinely spread via long-distance airborne nuclei during routine care or following AGPs. In fact, important transmission metrics such as the Ro (the number of new infections estimated to stem from a single case) and secondary household attack rate of SARS-CoV-2 are significantly lower than pathogen spread via airborne nuclei[71, 72]. While laboratory-based experiments demonstrate that SARS-CoV-2 can remain viable in experimentally generated aerosols in a constantly rotating drum designed to minimize particle settle for up to 3 hours, available data demonstrate environmental contamination consistent with spread via droplets and aerosols [34, 35]. Additional studies are required to better characterize transmission dynamics of SARS-CoV-2, including the role of the “turbulent gas cloud” [73], and to tailor effective infection prevention strategies to protect HCP. In facilities with limited AIIR access, these rooms should be reserved for patients undergoing AGPs.
DISCUSSION
Many infection prevention and control (IPC) recommendations are based on minimal or no research studies or RCTs, instead relying on observational data, quality improvement projects, and indirect evidence. This is despite the large impact of healthcare-associated infections (HAIs), estimated to lead to 1.7 million infections and nearly 100 000 deaths per year in the United States [74]. Most IPC programs focus on a variety of high-risk HAIs, such as central line–associated bloodstream infections and Clostridioides difficile infection, that can be monitored and are amenable to interventions. Intervening to prevent these infections also may prevent HAIs that are not part of surveillance programs by improving overall hand hygiene, environmental cleaning, and other behaviors [75]. These programs are also responsible for recommendations for PPE to protect HCP and to respond to infectious disease outbreaks. However, the lack of research-based recommendations can be a significant challenge when responding to outbreaks that put HCP at risk. This challenge is magnified when we are faced with a novel pathogen, such as SARS-CoV-2 that has spread rapidly across the globe and is associated with a very broad spectrum of symptoms. With rapidly moving and large epidemics, the supply of PPE is put at risk, further complicating how recommendations for PPE usage are developed and implemented.
The SARS-CoV-2 pandemic was first described as an outbreak of pneumonia at the end of December 2019 [76]. The rapid identification of the virus and its relation to human and zoonotic coronaviruses led to early recommendations and expectations for modes of transmission [77, 78]. Over the following weeks, hospital- and clinic-based IPC recommendations were proposed that evolved in response to reports from the field and laboratory. A significant challenge has been the reliance on clinical observations; molecular assays as proxies for transmission; extrapolations from SARS-CoV, MERS-CoV, human coronaviruses, and influenza; and other indirect evidence. This is problematic as we learn more about SARS-CoV-2 and its pathogenesis, such as the rapid decrease in viral burden following the onset of symptoms, which is, for example, different than the kinetics of SARS-CoV [79].
The WHO published guidelines for the use of PPE that has been mirrored by the national public health authorities in Canada [80], Australia [81], and England [82]. The CDC adopted more conservative recommendations early in the pandemic and has maintained these recommendations, including the use of an N95 respirator for the routine care of patients with known or suspected COVID-19, while allowing the use of a procedure mask with eye protection if there is a shortage of N95 respirators. These conflicting guidelines have put IPC leaders and teams in challenging positions, especially when balancing ongoing supply chain challenges, an unclear forecast for the trajectory of the epidemic, and the number of future cases. One agreed-on objective is to maintain HCP safety in all patient care scenarios, but this can ultimately lead to increasing the risk to patients. When supply chains are exhausted, the only option is to retreat to maximal PPE coverage of the HCP and to not remove PPE between patient encounters. This, combined with breakdowns in administrative and engineering controls, can lead to transmission of pathogens within the healthcare setting.
In an effort to establish a baseline for IPC recommendations and to highlight opportunities for research, the IDSA guideline panel used the best available evidence to provide recommendations on the use of PPE and potential risks within the healthcare environment. We recognize there are many more questions that desperately need answers, and we hope to be able to pursue additional questions in the near future. The panel recognizes the rapidly evolving nature of the pandemic, the incredible pace of discovery, and the growing access to clinical and laboratory data. Where possible, recommendations were made based on the evidence. In the absence of even indirect data, recommendations were made that prioritized the safety of HCP. The panel hopes that highlighting the distinct lack of data to guide many recommendations will increase the attention of the research community and funders to address these gaps. Answers will likely require observational and nonexperimental studies, but the panel would like to emphasize that RCTs are possible and should be encouraged.
HCP rely on PPE for their safety, and the data to support specific pieces or combinations of equipment are critical. Studies must include the other elements of PPE and practices, including the use of hand hygiene, since no PPE is used in isolation. The practice of hand hygiene when using PPE is critical to prevent contamination and transmission [83, 84], but adherence to this practice in studies of PPE use is not routinely reported. The role of education, HCP comfort and familiarity with equipment, and PPE use in routine clinical scenarios (eg, resuscitation, psychiatric emergency department, or by public safety officers) also need attention. Other tools that can increase adherence and safety, such as the use of a trained observer for donning and doffing, are being used heterogeneously and without guidance in most regions.
Unlike areas of clinical research that include treatment and diagnostics, the use of PPE and HCP practices are embedded in human behavior and human factors. The participation of researchers with expertise in human behavior, ergonomics, psychology, and anthropology is needed to evaluate how and why PPE is used by HCP, perceptions of risk, and adherence to local guidelines. While not always feasible, the panel recommends that outcome measures be used in research studies of PPE and, if not possible, rigorous definitions of process measures that incorporate other measures of HCP behavior should be used to ensure comparability of studies (eg, hand hygiene). Also, more data are needed on the types of higher-risk procedures that are considered AGPs as well as considerations for PPE and AIIR or negative-pressure rooms for HCP involved with HCP.
Healthcare systems and smaller clinical settings, such as clinics attached to shelters and in other congregate settings, are currently being called on to protect their healthcare workforce in the face of a pandemic. This situation has an impact on HCP directly in their role as essential personnel who leave their homes to work and as members of communities. The risk of SARS-CoV-2 acquisition exists in both scenarios, which means that HCP will continue to become infected even with appropriate use of PPE within the patient care environment. Regardless, it remains the responsibility of these organizations to protect the healthcare workforce now and into the future. Finally, while determining how to address the research gaps for PPE and protocols for SARS-CoV-2, the panel also recommends attention to how PPE use is perceived by patients and what tools are needed to mediate communication between HCP and specific patient communities, such as people who use lip reading or depend on facial recognition. These questions and many more remain in critical need of attention.
This is a living guideline that will be frequently updated as new data emerge. Updates and changes to the guideline will be posted to the IDSA website.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Panel members: John B. Lynch (lead), Deverick J. Anderson, Adarsh Bhimraj, Vincent Chi-Chung Cheng, Judith Guzman-Cottrill (representative, Society for Healthcare Epidemiology of America), Jasmine Dhindsa, Abhijit Duggal, Mamta K. Jain, Grace M. Lee (representative, Pediatric Infectious Diseases Society), Stephen Y. Liang, and Allison McGeer.
Methodologists: Shahnaz Sultan (lead), Perica Davitkov, Valery Lavergne, M. Hassan Murad, Reem A. Mustafa, Rebecca L. Morgan, and Yngve Falck-Ytter.
Acknowledgments. The expert panel thanks the Infectious Diseases Society of America (IDSA) for supporting guideline development, specifically Cynthia Sears, Dana Wollins, Genet Demisashi, and Rebecca Goldwater for their continued support throughout the guideline process.
Financial support. This project was funded by the IDSA.
References
Author notes
Potential conflicts of interest. The following list displays what has been reported to the IDSA. To provide thorough transparency, the IDSA requires full disclosure of all relationships, regardless of relevancy to the guideline topic. Evaluation of such relationships as potential conflicts of interest (COI) is determined by a review process that includes assessment by the Board of Directors liaison to the Standards and Practice Guideline Committee and, if necessary, the COI and Ethics Committee. The assessment of disclosed relationships for possible COI is based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The reader of these guidelines should be mindful of this when the list of disclosures is reviewed. P. D. receives research funding from the University Hospitals Seidman Cancer Center. D. J. A. receives research funding from the Centers for Disease Control and Prevention (CDC), the Agency for Health Care Research and Quality (AHRQ), and the National Institutes of Health (NIH) and receives Duke University Educational Program funding from the National Football League. V. C.-C. C. receives research funding from the Health and Medical Research Fund. J. G. C. serves as an advisor in pediatric infectious diseases (ID) and infection for Children’s Health Alliance and as an advisor in ID and infection control for the Oregon Health Authority and receives research funding from the CDC. A. D. receives research funding from the National Heart, Lung, and Blood Institute, NIH. A-Lung, and Sunnybrook Health Sciences Centre. M. K. J. serves as a consultant for Gilead Sciences, Inc and receives research funding from Gilead Sciences, Inc, Regeneron Pharmaceuticals, Inc, Janssen Pharmaceutica, Merck, GlaxoSmithKline, ViiV Healthcare, and VasoGene. G. M. L. serves as vice chair for the Advisory Committee on Immunization Practices, CDC. S. Y. L. receives research funding from the CDC, Barnes-Jewish Hospital Foundation, and ContraFect Corporation. A. M. receives research funding from Pfizer, Inc, Merck, Sanofi Pasteur, Seqirus, the Canadian Institutes of Health Research, the Joint Programming Initiative on Antimicrobial Resistance, the Canadian Immunization Research Network, the Ontario Ministry of Health, and the Natural Sciences and Engineering Research Council; receives Build in Canada Innovation Program funding from Canada; serves as an advisory board member for GlaxoSmithKline, Merck, Cidara, and Seqirus; and receives honoraria for lectures on influenza for Cidara. M. H. M. receives research funding from AHRQ, the Endocrine Society, the Society for Vascular Surgery, and the American Society of Hematology and is a board member for the Evidence Foundation. Y. F.-Y. receives honoraria for evidence reviews and teaching from the Evidence Foundation, honoraria for evidence reviews for the American Gastroenterological Association, and serves as a director for the Evidence Foundation and for the US GRADE Network. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




