-
PDF
- Split View
-
Views
-
Cite
Cite
Hannah Gora, Tasnim Hasan, Simon Smith, Ian Wilson, Mark Mayo, Celeste Woerle, Jessica R Webb, Bart J Currie, Josh Hanson, Ella M Meumann, Melioidosis of the Central Nervous System: Impact of the bimABm Allele on Patient Presentation and Outcome, Clinical Infectious Diseases, Volume 78, Issue 4, 15 April 2024, Pages 968–975, https://doi.org/10.1093/cid/ciac111
Close - Share Icon Share
Abstract
The autotransporter protein Burkholderia intracellular motility A (BimA) facilitates the entry of Burkholderia pseudomallei into the central nervous system (CNS) in mouse models of melioidosis. Its role in the pathogenesis of human cases of CNS melioidosis is incompletely defined.
Consecutive culture-confirmed cases of melioidosis at 2 sites in tropical Australia after 1989 were reviewed. Demographic, clinical, and radiological data of the patients with CNS melioidosis were recorded. The bimA allele (bimABm or bimABp) of the B. pseudomallei isolated from each patient was determined.
Of the 1587 cases diagnosed at the 2 sites during the study period, 52 (3.3%) had confirmed CNS melioidosis: 20 (38.5%) had a brain abscess, 18 (34.6%) had encephalomyelitis, 4 (7.7%) had isolated meningitis, and 10 (19.2%) had extra-meningeal disease. Among the 52 patients, there were 8 (15.4%) deaths; 17/44 (38.6%) survivors had residual disability. The bimA allele was characterized in 47/52; 17/47 (36.2%) had the bimABm allele and 30 (63.8%) had the bimABp allele. Patients with a bimABm variant were more likely to have a predominantly neurological presentation (odds ratio [OR]: 5.60; 95% confidence interval: 1.52–20.61; P = .01), to have brainstem involvement (OR: 7.33; 1.92–27.95; P = .004), and to have encephalomyelitis (OR: 4.69; 1.30–16.95; P = .02). Patients with a bimABm variant were more likely to die or have residual disability (OR: 4.88; 1.28–18.57; P = .01).
The bimA allele of B. pseudomallei has a significant impact on the clinical presentation and outcome of patients with CNS melioidosis.
Melioidosis is caused by Burkholderia pseudomallei, a gram-negative bacterium that lives in the soil and surface water in tropical regions [1, 2]. The vast majority of B. pseudomallei infections are subclinical; however, some individuals develop life-threatening disease, particularly those with specific comorbidities that include diabetes mellitus, hazardous alcohol use, chronic kidney disease, and chronic lung disease [3]. Melioidosis can affect almost any organ, but central nervous system (CNS) involvement—which occurs in approximately 4% of cases—is one of the most feared manifestations [4, 5]. Even in well-resourced settings the case-fatality rate can rise to 50%, with permanent disability common in survivors [4, 6, 7].
Central nervous system melioidosis can present as encephalomyelitis, brain abscess, meningitis, or extra-meningeal disease. Encephalomyelitis is common in northern Australia, but it is rare in other countries where macroscopic brain abscesses are seen more frequently [4, 5]. Indeed, 63% of all cases of encephalomyelitis have been reported from Australia, while case series from Malaysia and Singapore describe brain abscesses as the sole manifestation of CNS melioidosis [4, 8, 9]. It has been postulated that these geographical differences in the clinical presentation of CNS melioidosis are related, at least in part, to regional B. pseudomallei genotypic variation [10].
Burkholderia pseudomallei has a large genome comprising 2 chromosomes 4.07 and 3.17 mega-base pairs in size, high rates of homologous recombination, and an open pangenome [11]. Long-range dissemination of B. pseudomallei is rare, and B. pseudomallei populations in Australia, Asia, Africa, and the Americas remain largely distinct [12]. All B. pseudomallei isolates carry bimA, a virulence gene that encodes an autotransporter protein (BimA) that mediates actin-based motility [13]. Most isolates carry the bimABp allele; however, a small number carry the Burkholderia mallei–like bimABm allele. There is significant geographical variation in the prevalence of the bimABm allele: to date, it has only been reported from Australia (~12% of isolates), India (4.5% of isolates), and Sri Lanka (18.5% of isolates) [10, 12, 14–17].
Humans infected with the bimABm variant are 14 times more likely to present with neurological involvement than those with bimABp [10]. In mouse models, B. pseudomallei carrying bimABm is more virulent and has been shown to directly invade the CNS after intranasal inoculation via nerve route translocation along cranial nerves [18, 19]. Human cases where encephalomyelitis has developed after B. pseudomallei culture–positive facial skin infections support the hypothesis that the organism can invade the CNS via nerve root translocation along the facial nerve, with BimA again thought to play an important role [20, 21].
This study was performed to provide an overview of the clinical presentation of CNS melioidosis in tropical Australia, and to gain a greater insight into its pathophysiology by examining the association between different bimA alleles and the clinical features of the cases. Recent advances in medical imaging, critical care, and disease-specific management have seen the case-fatality rate of melioidosis fall significantly in Australia [5, 22]. This study was performed to determine if this was also the case in patients with CNS disease.
METHODS
The study was performed at Royal Darwin Hospital (RDH) and Cairns Hospital in tropical Australia. Royal Darwin Hospital, a 350-bed tertiary-referral hospital in the Northern Territory, serves a population of approximately 160 000 people from the Top End of Australia, an area of nearly 500 000 km2. Cairns Hospital, a 531-bed tertiary-referral hospital in Far North Queensland, serves a population of approximately 280 000 people dispersed across an area of over 380 000 km2 [23]. Australian First Nations populations—the Aboriginal and Torres Strait Islander peoples—comprise a significant proportion of both regions’ populations. All patients with culture-confirmed CNS melioidosis between 1 October 1989 and 30 June 2021 at RDH, and between 1 January 1998 and 30 June 2021 at Cairns Hospital, were eligible for inclusion. The clinical details of each case were recorded as described previously [5, 24]. A patient was defined as having a risk factor for melioidosis if they had documented diabetes mellitus, hazardous alcohol use, chronic kidney disease, chronic lung disease, immunosuppression, malignancy, kava consumption, rheumatic heart disease, or cardiac failure. Patients and/or families were asked about potential inoculation events prior to presentation [5, 24]
The diagnosis of CNS melioidosis required radiological changes consistent with active CNS infection and a positive B. pseudomallei culture. Central nervous system involvement was further categorized as encephalomyelitis, a brain abscess, meningitis, or extra-meningeal involvement [4]. Primary CNS melioidosis was defined as cases where neurological symptoms were the main presenting manifestation. Patients were said to have secondary CNS melioidosis if their presenting symptoms originated from a nonneurological primary focus of infection—melioidosis pneumonia, for instance—with CNS involvement identified subsequently.
For CNS melioidosis cases, the bimA allele of the infecting B. pseudomallei isolate was determined either by polymerase chain reaction (PCR) or in silico analysis of whole-genome sequence data. PCR primers were as previously described [10]. In silico bimA typing was done by aligning short read sequence data to the bimABm allele (BURPS668_A2118 in B. pseudomallei MSHR668; GenBank accession CP000571.1) and to the bimABp allele (BPSS1492 in B. pseudomallei K96243; GenBank accession BX571966.1) using SRST2 version 0.2.0 [25]. In all available isolates either the bimABm allele or the bimABp allele was detected.
Data were entered into an electronic database (Microsoft Excel; Microsoft Corporation) and analyzed using statistical software (Stata version 14.2; StataCorp LP). Groups were compared using the Kruskal-Wallis, chi-square, Fisher’s exact test and logistic regression, as appropriate. The Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research (HREC 02/38) and the Far North Queensland Human Research Ethics Committee (HREC/2020/QCH/59103-1428, HREC/15/QCH/46-977) provided ethical approval for the study.
RESULTS
Of 1587 melioidosis cases diagnosed at the 2 sites during the study periods, 52 (3.3%) had confirmed CNS melioidosis (Supplementary Tables 1 and 2). Children were more likely to have CNS involvement than were adults (6/76 [7.9%] vs 46/1511 [3.0%]; P = .04). All 6 children (aged 3–14 years) with CNS involvement had no risk factor for melioidosis, while only 5 of 46 (10.9%) adults with CNS involvement had no risk factor (P < .0001). The only other significant difference between patients who did, and did not, have CNS involvement was that CNS involvement was more common in those with a remote residential address (Table 1). Of the 52 patients with CNS involvement, 20 (38.5%) had brain abscesses, 18 (34.6%) had encephalomyelitis, 4 (7.7%) had isolated meningitis, and 10 (19.2%) had extra-meningeal disease (Supplementary Table 1, Figure 1). Primary CNS melioidosis was present in 24 of 52 patients (46.2%); 28 of 52 patients (53.9%) had secondary CNS melioidosis. Patients with a primary CNS melioidosis presentation were more likely to have encephalomyelitis than those with secondary CNS melioidosis (13/24 [54.2%] vs 5/28 [17.9%]; odds ratio [OR]: 5.43; 95% confidence interval [CI]: 1.55–19.11; P = .008) and less likely to be bacteremic (6/24 [25%] vs 25/28 [89.3%]; OR: .04; 95% CI: .01–.18; P < .0001). There was no difference in the demographic characteristics or comorbidities of the patients with primary and secondary CNS melioidosis (Table 2).
Comparison of the Demographic, Epidemiological, and Clinical Characteristics of the Patients With Laboratory-Confirmed Melioidosis in the Study Who Did—and Did Not—Have Central Nervous System Involvement
| . | CNS Involvement (n = 52) . | No CNS Involvement (n = 1535) . | P . |
|---|---|---|---|
| Age, years | 46 (33–59) | 51 (40–61) | .03 |
| Child <18 years | 6/52 (11.5%) | 70/1535 (4.6%) | .02 |
| Male gender | 34/52 (65.4%) | 991/1535 (64.6%) | .90 |
| First Nations Australian | 30/52 (57.7%) | 789/1535 (51.4%) | .37 |
| Remote residential address | 33/52 (63.5%) | 707/1535 (46.1%) | .01 |
| Presentation during wet season | 34/52 (65.4%) | 1050/1535 (68.4%) | .65 |
| Diabetes mellitus | 21/52 (40.4%) | 724/1524 (47.5%) | .31 |
| Hazardous alcohol consumption | 20/52 (38.5%) | 612/1511 (40.5%) | .77 |
| Chronic kidney disease | 4/52 (7.7%) | 194/1521 (12.8%) | .28 |
| Chronic lung disease | 10/52 (19.2%) | 383/1515 (25.3%) | .32 |
| No documented risk factor | 11/52 (21.2%) | 232/1535 (15.1%) | .23 |
| Bacteremia | 31/52 (59.6%) | 903/1535 (58.8%) | .91 |
| ICU admission | 31/52 (59.6%) | 355/1521 (23.3%) | <.001 |
| Died before hospital discharge | 8/52 (15.4%) | 164/1535 (10.7%) | .28 |
| . | CNS Involvement (n = 52) . | No CNS Involvement (n = 1535) . | P . |
|---|---|---|---|
| Age, years | 46 (33–59) | 51 (40–61) | .03 |
| Child <18 years | 6/52 (11.5%) | 70/1535 (4.6%) | .02 |
| Male gender | 34/52 (65.4%) | 991/1535 (64.6%) | .90 |
| First Nations Australian | 30/52 (57.7%) | 789/1535 (51.4%) | .37 |
| Remote residential address | 33/52 (63.5%) | 707/1535 (46.1%) | .01 |
| Presentation during wet season | 34/52 (65.4%) | 1050/1535 (68.4%) | .65 |
| Diabetes mellitus | 21/52 (40.4%) | 724/1524 (47.5%) | .31 |
| Hazardous alcohol consumption | 20/52 (38.5%) | 612/1511 (40.5%) | .77 |
| Chronic kidney disease | 4/52 (7.7%) | 194/1521 (12.8%) | .28 |
| Chronic lung disease | 10/52 (19.2%) | 383/1515 (25.3%) | .32 |
| No documented risk factor | 11/52 (21.2%) | 232/1535 (15.1%) | .23 |
| Bacteremia | 31/52 (59.6%) | 903/1535 (58.8%) | .91 |
| ICU admission | 31/52 (59.6%) | 355/1521 (23.3%) | <.001 |
| Died before hospital discharge | 8/52 (15.4%) | 164/1535 (10.7%) | .28 |
All numbers are presented as n/N (%) or median (interquartile range) as appropriate. Abbreviations: CNS, central nervous system; ICU, intensive care unit.
Comparison of the Demographic, Epidemiological, and Clinical Characteristics of the Patients With Laboratory-Confirmed Melioidosis in the Study Who Did—and Did Not—Have Central Nervous System Involvement
| . | CNS Involvement (n = 52) . | No CNS Involvement (n = 1535) . | P . |
|---|---|---|---|
| Age, years | 46 (33–59) | 51 (40–61) | .03 |
| Child <18 years | 6/52 (11.5%) | 70/1535 (4.6%) | .02 |
| Male gender | 34/52 (65.4%) | 991/1535 (64.6%) | .90 |
| First Nations Australian | 30/52 (57.7%) | 789/1535 (51.4%) | .37 |
| Remote residential address | 33/52 (63.5%) | 707/1535 (46.1%) | .01 |
| Presentation during wet season | 34/52 (65.4%) | 1050/1535 (68.4%) | .65 |
| Diabetes mellitus | 21/52 (40.4%) | 724/1524 (47.5%) | .31 |
| Hazardous alcohol consumption | 20/52 (38.5%) | 612/1511 (40.5%) | .77 |
| Chronic kidney disease | 4/52 (7.7%) | 194/1521 (12.8%) | .28 |
| Chronic lung disease | 10/52 (19.2%) | 383/1515 (25.3%) | .32 |
| No documented risk factor | 11/52 (21.2%) | 232/1535 (15.1%) | .23 |
| Bacteremia | 31/52 (59.6%) | 903/1535 (58.8%) | .91 |
| ICU admission | 31/52 (59.6%) | 355/1521 (23.3%) | <.001 |
| Died before hospital discharge | 8/52 (15.4%) | 164/1535 (10.7%) | .28 |
| . | CNS Involvement (n = 52) . | No CNS Involvement (n = 1535) . | P . |
|---|---|---|---|
| Age, years | 46 (33–59) | 51 (40–61) | .03 |
| Child <18 years | 6/52 (11.5%) | 70/1535 (4.6%) | .02 |
| Male gender | 34/52 (65.4%) | 991/1535 (64.6%) | .90 |
| First Nations Australian | 30/52 (57.7%) | 789/1535 (51.4%) | .37 |
| Remote residential address | 33/52 (63.5%) | 707/1535 (46.1%) | .01 |
| Presentation during wet season | 34/52 (65.4%) | 1050/1535 (68.4%) | .65 |
| Diabetes mellitus | 21/52 (40.4%) | 724/1524 (47.5%) | .31 |
| Hazardous alcohol consumption | 20/52 (38.5%) | 612/1511 (40.5%) | .77 |
| Chronic kidney disease | 4/52 (7.7%) | 194/1521 (12.8%) | .28 |
| Chronic lung disease | 10/52 (19.2%) | 383/1515 (25.3%) | .32 |
| No documented risk factor | 11/52 (21.2%) | 232/1535 (15.1%) | .23 |
| Bacteremia | 31/52 (59.6%) | 903/1535 (58.8%) | .91 |
| ICU admission | 31/52 (59.6%) | 355/1521 (23.3%) | <.001 |
| Died before hospital discharge | 8/52 (15.4%) | 164/1535 (10.7%) | .28 |
All numbers are presented as n/N (%) or median (interquartile range) as appropriate. Abbreviations: CNS, central nervous system; ICU, intensive care unit.
Demographics, Comorbidities, Clinical Phenotype, and Clinical Course of the Patients With Primary and Secondary Central Nervous System Melioidosis
| . | Primary CNS Melioidosis (n = 24) . | Secondary CNS Melioidosis (n = 28) . | P . |
|---|---|---|---|
| Age, years | 39 (25–56) | 48 (38–63) | .10 |
| Child <18 years | 4 (16.7%) | 2 (7.1%) | .40 |
| Male gender | 17 (70.8%) | 17 (60.7%) | .56 |
| First Nations Australian | 9 (37.5%) | 13 (46.4%) | .58 |
| Diabetes mellitus | 7 (29.2%) | 13 (46.4%) | .26 |
| Hazardous alcohol use | 8 (33.3%) | 13 (46.4%) | .40 |
| Chronic kidney disease | 0 | 3 (10.7%) | .24 |
| Chronic lung disease | 3 (12.5%) | 8 (28.6%) | .19 |
| No documented risk factor | 7 (29.2%) | 4 (14.3%) | .31 |
| Encephalomyelitis | 13 (54.2%) | 5 (17.9%) | .009 |
| Brain abscess | 8 (33.3%) | 12 (42.9%) | .57 |
| Extra-meningeal disease | 2 (8.3%) | 8 (28.6%) | .09 |
| Meningitis | 1 (4.2%) | 3 (10.7%) | .62 |
| Brainstem involvement | 13 (54.2%) | 4 (14.3%) | .003 |
| Bacteremic | 6 (25%) | 25 (89.3%) | <.0001 |
| Burkholderia pseudomallei cultured outside CNS | 12 (50%) | 28 (100%) | <.0001 |
| Documented exposure event | 11 (45.8%) | 7 (25.0%) | .15 |
| Immersion in water or face/scalp lesion | 5 (20.8%) | 2 (7.1%) | .23 |
| bimABm varianta | 12/21 (57.1%) | 5/26 (19.2%) | .01 |
| Had septic shock during hospitalization | 2 (8.3%) | 13 (46.4%) | .005 |
| ICU admission | 12 (50%) | 20 (71.4%) | .16 |
| Neurosurgery | 8 (33.3%) | 8 (28.6%) | .77 |
| Died before discharge | 5 (20.8%) | 3 (10.7%) | .45 |
| Death or residual disability | 17 (70.8%) | 8 (28.6%) | .005 |
| . | Primary CNS Melioidosis (n = 24) . | Secondary CNS Melioidosis (n = 28) . | P . |
|---|---|---|---|
| Age, years | 39 (25–56) | 48 (38–63) | .10 |
| Child <18 years | 4 (16.7%) | 2 (7.1%) | .40 |
| Male gender | 17 (70.8%) | 17 (60.7%) | .56 |
| First Nations Australian | 9 (37.5%) | 13 (46.4%) | .58 |
| Diabetes mellitus | 7 (29.2%) | 13 (46.4%) | .26 |
| Hazardous alcohol use | 8 (33.3%) | 13 (46.4%) | .40 |
| Chronic kidney disease | 0 | 3 (10.7%) | .24 |
| Chronic lung disease | 3 (12.5%) | 8 (28.6%) | .19 |
| No documented risk factor | 7 (29.2%) | 4 (14.3%) | .31 |
| Encephalomyelitis | 13 (54.2%) | 5 (17.9%) | .009 |
| Brain abscess | 8 (33.3%) | 12 (42.9%) | .57 |
| Extra-meningeal disease | 2 (8.3%) | 8 (28.6%) | .09 |
| Meningitis | 1 (4.2%) | 3 (10.7%) | .62 |
| Brainstem involvement | 13 (54.2%) | 4 (14.3%) | .003 |
| Bacteremic | 6 (25%) | 25 (89.3%) | <.0001 |
| Burkholderia pseudomallei cultured outside CNS | 12 (50%) | 28 (100%) | <.0001 |
| Documented exposure event | 11 (45.8%) | 7 (25.0%) | .15 |
| Immersion in water or face/scalp lesion | 5 (20.8%) | 2 (7.1%) | .23 |
| bimABm varianta | 12/21 (57.1%) | 5/26 (19.2%) | .01 |
| Had septic shock during hospitalization | 2 (8.3%) | 13 (46.4%) | .005 |
| ICU admission | 12 (50%) | 20 (71.4%) | .16 |
| Neurosurgery | 8 (33.3%) | 8 (28.6%) | .77 |
| Died before discharge | 5 (20.8%) | 3 (10.7%) | .45 |
| Death or residual disability | 17 (70.8%) | 8 (28.6%) | .005 |
All numbers are presented as n (%) or median (interquartile range) as appropriate. Abbreviations: CNS, central nervous system; ICU, intensive care unit.
It was only possible to determine the bimA allele in 47 of the 52 patients.
Demographics, Comorbidities, Clinical Phenotype, and Clinical Course of the Patients With Primary and Secondary Central Nervous System Melioidosis
| . | Primary CNS Melioidosis (n = 24) . | Secondary CNS Melioidosis (n = 28) . | P . |
|---|---|---|---|
| Age, years | 39 (25–56) | 48 (38–63) | .10 |
| Child <18 years | 4 (16.7%) | 2 (7.1%) | .40 |
| Male gender | 17 (70.8%) | 17 (60.7%) | .56 |
| First Nations Australian | 9 (37.5%) | 13 (46.4%) | .58 |
| Diabetes mellitus | 7 (29.2%) | 13 (46.4%) | .26 |
| Hazardous alcohol use | 8 (33.3%) | 13 (46.4%) | .40 |
| Chronic kidney disease | 0 | 3 (10.7%) | .24 |
| Chronic lung disease | 3 (12.5%) | 8 (28.6%) | .19 |
| No documented risk factor | 7 (29.2%) | 4 (14.3%) | .31 |
| Encephalomyelitis | 13 (54.2%) | 5 (17.9%) | .009 |
| Brain abscess | 8 (33.3%) | 12 (42.9%) | .57 |
| Extra-meningeal disease | 2 (8.3%) | 8 (28.6%) | .09 |
| Meningitis | 1 (4.2%) | 3 (10.7%) | .62 |
| Brainstem involvement | 13 (54.2%) | 4 (14.3%) | .003 |
| Bacteremic | 6 (25%) | 25 (89.3%) | <.0001 |
| Burkholderia pseudomallei cultured outside CNS | 12 (50%) | 28 (100%) | <.0001 |
| Documented exposure event | 11 (45.8%) | 7 (25.0%) | .15 |
| Immersion in water or face/scalp lesion | 5 (20.8%) | 2 (7.1%) | .23 |
| bimABm varianta | 12/21 (57.1%) | 5/26 (19.2%) | .01 |
| Had septic shock during hospitalization | 2 (8.3%) | 13 (46.4%) | .005 |
| ICU admission | 12 (50%) | 20 (71.4%) | .16 |
| Neurosurgery | 8 (33.3%) | 8 (28.6%) | .77 |
| Died before discharge | 5 (20.8%) | 3 (10.7%) | .45 |
| Death or residual disability | 17 (70.8%) | 8 (28.6%) | .005 |
| . | Primary CNS Melioidosis (n = 24) . | Secondary CNS Melioidosis (n = 28) . | P . |
|---|---|---|---|
| Age, years | 39 (25–56) | 48 (38–63) | .10 |
| Child <18 years | 4 (16.7%) | 2 (7.1%) | .40 |
| Male gender | 17 (70.8%) | 17 (60.7%) | .56 |
| First Nations Australian | 9 (37.5%) | 13 (46.4%) | .58 |
| Diabetes mellitus | 7 (29.2%) | 13 (46.4%) | .26 |
| Hazardous alcohol use | 8 (33.3%) | 13 (46.4%) | .40 |
| Chronic kidney disease | 0 | 3 (10.7%) | .24 |
| Chronic lung disease | 3 (12.5%) | 8 (28.6%) | .19 |
| No documented risk factor | 7 (29.2%) | 4 (14.3%) | .31 |
| Encephalomyelitis | 13 (54.2%) | 5 (17.9%) | .009 |
| Brain abscess | 8 (33.3%) | 12 (42.9%) | .57 |
| Extra-meningeal disease | 2 (8.3%) | 8 (28.6%) | .09 |
| Meningitis | 1 (4.2%) | 3 (10.7%) | .62 |
| Brainstem involvement | 13 (54.2%) | 4 (14.3%) | .003 |
| Bacteremic | 6 (25%) | 25 (89.3%) | <.0001 |
| Burkholderia pseudomallei cultured outside CNS | 12 (50%) | 28 (100%) | <.0001 |
| Documented exposure event | 11 (45.8%) | 7 (25.0%) | .15 |
| Immersion in water or face/scalp lesion | 5 (20.8%) | 2 (7.1%) | .23 |
| bimABm varianta | 12/21 (57.1%) | 5/26 (19.2%) | .01 |
| Had septic shock during hospitalization | 2 (8.3%) | 13 (46.4%) | .005 |
| ICU admission | 12 (50%) | 20 (71.4%) | .16 |
| Neurosurgery | 8 (33.3%) | 8 (28.6%) | .77 |
| Died before discharge | 5 (20.8%) | 3 (10.7%) | .45 |
| Death or residual disability | 17 (70.8%) | 8 (28.6%) | .005 |
All numbers are presented as n (%) or median (interquartile range) as appropriate. Abbreviations: CNS, central nervous system; ICU, intensive care unit.
It was only possible to determine the bimA allele in 47 of the 52 patients.
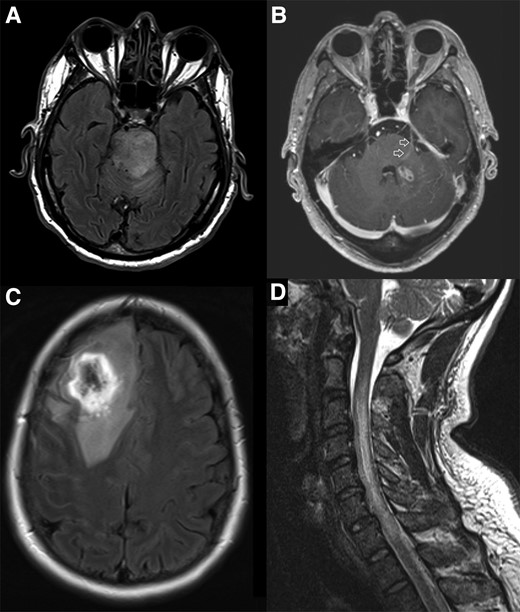
Magnetic resonance imaging from cases in this cohort highlighting selected features of CNS melioidosis. A, MRI FLAIR sequence demonstrating hyperintense expansile lesion within the brainstem extending into the left middle cerebellar peduncle and cerebral peduncles bilaterally. The patient presented with pneumonia and bacteremia, with neurological symptoms developing subsequently; the Burkholderia pseudomallei isolate carried the bimABm allele. B, MRI T1 post-contrast study demonstrating small, ovoid lesion with rim enhancement in the left cerebellar peduncle and enhancement of the adjacent trigeminal nerve and tract as it traverses the pons (arrows). The patient had a neurological presentation; the B. pseudomallei isolate carried the bimABm allele. C, MRI FLAIR sequence demonstrating a very hyperintense ring lesion with extensive increased signal in adjacent white matter consistent with vasogenic edema. The patient presented with pneumonia and seizures; it was not possible to determine the bimA allele in this case. D, MRI T2 sequence demonstrating cord expansion with high signal from the C2/3 to C7/T1 level. The patient had a neurological presentation; the B. pseudomallei isolate carried the bimABm allele. Abbreviations: CNS, central nervous system; FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging.
All patients had brain imaging performed. Computed tomography (CT) was performed in 26 of 52 patients (50%), with 8 of 26 scans (30.8%) reported as normal. Magnetic resonance imaging (MRI) was performed in 37 of 52 patients (71.2%), and all 37 were reported as abnormal. Inflammatory changes with a predilection for white matter tracts were commonly demonstrated (Figure 1). A lumbar puncture was performed in 32 of 52 patients (61.5%); lymphocytes were predominant (≥50% of cerebrospinal fluid [CSF] leukocytes) in 19 of 31 patients (61.3%) who had a CSF leukocyte count performed. Burkholderia pseudomallei was cultured from CSF in only 9 of 32 patients (28.1%); this included 4 of 15 (26.7%) cases of encephalomyelitis and 4 of 10 (40%) cases of brain abscess in which a lumbar puncture was performed (Supplementary Table 3).
The intensive-phase antibiotic therapy was recorded in 51 of 52 patients; 23 (45.1%) received meropenem, 12 (23.5%) received ceftazidime, and 16 (31.4%) received both (sequentially) (Supplementary Table 4). There were 32 of 52 patients (61.5%) admitted to the intensive care unit (ICU), while 16 of 52 (30.8%) received neurosurgical intervention, including abscess drainage in 11, laminectomy in 2, ventricular drain insertion in 2, and hemicraniectomy in 1.
There were 8 of 52 patients (15.4%) who died before hospital discharge at a median (IQR) of 14 (8–44) days after diagnosis. The median (IQR) duration of intravenous therapy in survivors was 8 (6–8) weeks; the median (IQR) duration of subsequent oral eradication therapy in survivors was 6 (3.2–7.6) months. Trimethoprim-sulfamethoxazole (TMP-SMX) was prescribed as oral eradication therapy in 37 of 44 (84.1%) survivors, 5 (13.5%) of whom had an adverse drug reaction to this medication (Supplementary Table 4). Among the 44 survivors, 17 (38.6%) had residual neurological deficits (Supplementary Table 1). There was a single relapse of culture-confirmed CNS melioidosis: a patient with brain abscess who did not receive neurosurgery and who received only 4 weeks of intravenous intensive therapy and 3 months of oral doxycycline eradication therapy.
The B. pseudomallei bimA allele was characterized in 47 of 52 patients (in 5 cases the isolate was not available); 17 of 47 (36.2%) had the bimABm allele and 30 (63.8%) had the bimABp allele. Patients with infection with the bimABm variant were more likely to have a primary CNS melioidosis presentation than a secondary CNS melioidosis presentation (12/17 [70.6%] vs 9/30 [30.0%]; OR: 5.60; 95% CI: 1.52–20.61; P = .01), more likely to have brainstem involvement (11/17 [64.7%] vs 6/30 [20%]; OR: 7.33; 9% CI: 1.92–27.95; P = .004), and more likely to have encephalomyelitis (10/17 [58.8%] vs 7/30 [23.3%]; OR: 4.69; 95% CI: 1.30–16.95; P = .02). Patients with a bimABm variant were also more likely to die or have residual disability (13/17 [76.5%] vs 12/30 [40.0%]; OR: 4.88; 95% CI: 1.28–18.57; P = .01) (Table 3).
Demographics, Comorbidities, Clinical Phenotype, and Clinical Course of the Patients With the bimABm and bimABp Alleles
| . | bimABm Allele (n = 17)a . | bimABp Allele (n = 30)a . | P . |
|---|---|---|---|
| Age, years | 48 (29–62) | 43 (37–58) | .89 |
| Child <18 years | 2 (11.8%) | 3 (10%) | 1.0 |
| Male gender | 13 (76.5%) | 20 (66.7%) | .53 |
| First Nations Australian | 6 (35.3%) | 13 (43.3%) | .76 |
| Diabetes mellitus | 3 (17.7%) | 16 (53.3%) | .03 |
| Hazardous alcohol use | 5 (29.4%) | 13 (43.3%) | .53 |
| Chronic kidney disease | 1 (5.9%) | 1 (3.3%) | 1.0 |
| Chronic lung disease | 4 (23.5%) | 5 (16.7%) | .70 |
| No risk factor | 4 (23.5%) | 6 (20%) | 1.0 |
| Primary CNS presentation | 12 (70.6%) | 9 (30%) | .01 |
| Parenchymal disease | 17 (100%) | 16 (53.3%) | .001 |
| Encephalomyelitis | 10 (58.8%) | 7 (23.3%) | .03 |
| Brain abscess | 7 (41.2%) | 9 (30%) | .53 |
| Extra-meningeal CNS disease | 0 | 10 (30%) | .008 |
| Meningitis | 0 | 4 (13.3%) | .28 |
| Brainstem involvement | 11 (64.7%) | 6 (20%) | .004 |
| Bacteremic | 8 (47.1%) | 22 (73.3%) | .11 |
| Burkholderia pseudomallei cultured outside CNS | 11 (64.7%) | 26 (86.7%) | .14 |
| Exposure event | 8 (47.1%) | 9 (30%) | .35 |
| Immersion or face/scalp lesion | 4 (23.5%) | 3 (10%) | .24 |
| Septic shock during admission | 4 (23.5%) | 9 (30%) | .74 |
| ICU admission | 13 (76.5%) | 15 (50%) | .12 |
| Neurosurgery | 3 (17.7%) | 10 (33.3%) | .32 |
| Died before discharge | 4 (23.5%) | 4 (13.3%) | .44 |
| Residual disabilityb | 9/13 (69.2%) | 8/26 (30.8%) | .04 |
| Death or residual disability | 13 (76.5%) | 12 (40.0%) | .03 |
| . | bimABm Allele (n = 17)a . | bimABp Allele (n = 30)a . | P . |
|---|---|---|---|
| Age, years | 48 (29–62) | 43 (37–58) | .89 |
| Child <18 years | 2 (11.8%) | 3 (10%) | 1.0 |
| Male gender | 13 (76.5%) | 20 (66.7%) | .53 |
| First Nations Australian | 6 (35.3%) | 13 (43.3%) | .76 |
| Diabetes mellitus | 3 (17.7%) | 16 (53.3%) | .03 |
| Hazardous alcohol use | 5 (29.4%) | 13 (43.3%) | .53 |
| Chronic kidney disease | 1 (5.9%) | 1 (3.3%) | 1.0 |
| Chronic lung disease | 4 (23.5%) | 5 (16.7%) | .70 |
| No risk factor | 4 (23.5%) | 6 (20%) | 1.0 |
| Primary CNS presentation | 12 (70.6%) | 9 (30%) | .01 |
| Parenchymal disease | 17 (100%) | 16 (53.3%) | .001 |
| Encephalomyelitis | 10 (58.8%) | 7 (23.3%) | .03 |
| Brain abscess | 7 (41.2%) | 9 (30%) | .53 |
| Extra-meningeal CNS disease | 0 | 10 (30%) | .008 |
| Meningitis | 0 | 4 (13.3%) | .28 |
| Brainstem involvement | 11 (64.7%) | 6 (20%) | .004 |
| Bacteremic | 8 (47.1%) | 22 (73.3%) | .11 |
| Burkholderia pseudomallei cultured outside CNS | 11 (64.7%) | 26 (86.7%) | .14 |
| Exposure event | 8 (47.1%) | 9 (30%) | .35 |
| Immersion or face/scalp lesion | 4 (23.5%) | 3 (10%) | .24 |
| Septic shock during admission | 4 (23.5%) | 9 (30%) | .74 |
| ICU admission | 13 (76.5%) | 15 (50%) | .12 |
| Neurosurgery | 3 (17.7%) | 10 (33.3%) | .32 |
| Died before discharge | 4 (23.5%) | 4 (13.3%) | .44 |
| Residual disabilityb | 9/13 (69.2%) | 8/26 (30.8%) | .04 |
| Death or residual disability | 13 (76.5%) | 12 (40.0%) | .03 |
Abbreviations: CNS, central nervous system; ICU, intensive care unit.
It was only possible to determine the bimA allele in 47 of the 52 patients.
It was only possible to determine the bimA allele in 39 of the 44 survivors.
Demographics, Comorbidities, Clinical Phenotype, and Clinical Course of the Patients With the bimABm and bimABp Alleles
| . | bimABm Allele (n = 17)a . | bimABp Allele (n = 30)a . | P . |
|---|---|---|---|
| Age, years | 48 (29–62) | 43 (37–58) | .89 |
| Child <18 years | 2 (11.8%) | 3 (10%) | 1.0 |
| Male gender | 13 (76.5%) | 20 (66.7%) | .53 |
| First Nations Australian | 6 (35.3%) | 13 (43.3%) | .76 |
| Diabetes mellitus | 3 (17.7%) | 16 (53.3%) | .03 |
| Hazardous alcohol use | 5 (29.4%) | 13 (43.3%) | .53 |
| Chronic kidney disease | 1 (5.9%) | 1 (3.3%) | 1.0 |
| Chronic lung disease | 4 (23.5%) | 5 (16.7%) | .70 |
| No risk factor | 4 (23.5%) | 6 (20%) | 1.0 |
| Primary CNS presentation | 12 (70.6%) | 9 (30%) | .01 |
| Parenchymal disease | 17 (100%) | 16 (53.3%) | .001 |
| Encephalomyelitis | 10 (58.8%) | 7 (23.3%) | .03 |
| Brain abscess | 7 (41.2%) | 9 (30%) | .53 |
| Extra-meningeal CNS disease | 0 | 10 (30%) | .008 |
| Meningitis | 0 | 4 (13.3%) | .28 |
| Brainstem involvement | 11 (64.7%) | 6 (20%) | .004 |
| Bacteremic | 8 (47.1%) | 22 (73.3%) | .11 |
| Burkholderia pseudomallei cultured outside CNS | 11 (64.7%) | 26 (86.7%) | .14 |
| Exposure event | 8 (47.1%) | 9 (30%) | .35 |
| Immersion or face/scalp lesion | 4 (23.5%) | 3 (10%) | .24 |
| Septic shock during admission | 4 (23.5%) | 9 (30%) | .74 |
| ICU admission | 13 (76.5%) | 15 (50%) | .12 |
| Neurosurgery | 3 (17.7%) | 10 (33.3%) | .32 |
| Died before discharge | 4 (23.5%) | 4 (13.3%) | .44 |
| Residual disabilityb | 9/13 (69.2%) | 8/26 (30.8%) | .04 |
| Death or residual disability | 13 (76.5%) | 12 (40.0%) | .03 |
| . | bimABm Allele (n = 17)a . | bimABp Allele (n = 30)a . | P . |
|---|---|---|---|
| Age, years | 48 (29–62) | 43 (37–58) | .89 |
| Child <18 years | 2 (11.8%) | 3 (10%) | 1.0 |
| Male gender | 13 (76.5%) | 20 (66.7%) | .53 |
| First Nations Australian | 6 (35.3%) | 13 (43.3%) | .76 |
| Diabetes mellitus | 3 (17.7%) | 16 (53.3%) | .03 |
| Hazardous alcohol use | 5 (29.4%) | 13 (43.3%) | .53 |
| Chronic kidney disease | 1 (5.9%) | 1 (3.3%) | 1.0 |
| Chronic lung disease | 4 (23.5%) | 5 (16.7%) | .70 |
| No risk factor | 4 (23.5%) | 6 (20%) | 1.0 |
| Primary CNS presentation | 12 (70.6%) | 9 (30%) | .01 |
| Parenchymal disease | 17 (100%) | 16 (53.3%) | .001 |
| Encephalomyelitis | 10 (58.8%) | 7 (23.3%) | .03 |
| Brain abscess | 7 (41.2%) | 9 (30%) | .53 |
| Extra-meningeal CNS disease | 0 | 10 (30%) | .008 |
| Meningitis | 0 | 4 (13.3%) | .28 |
| Brainstem involvement | 11 (64.7%) | 6 (20%) | .004 |
| Bacteremic | 8 (47.1%) | 22 (73.3%) | .11 |
| Burkholderia pseudomallei cultured outside CNS | 11 (64.7%) | 26 (86.7%) | .14 |
| Exposure event | 8 (47.1%) | 9 (30%) | .35 |
| Immersion or face/scalp lesion | 4 (23.5%) | 3 (10%) | .24 |
| Septic shock during admission | 4 (23.5%) | 9 (30%) | .74 |
| ICU admission | 13 (76.5%) | 15 (50%) | .12 |
| Neurosurgery | 3 (17.7%) | 10 (33.3%) | .32 |
| Died before discharge | 4 (23.5%) | 4 (13.3%) | .44 |
| Residual disabilityb | 9/13 (69.2%) | 8/26 (30.8%) | .04 |
| Death or residual disability | 13 (76.5%) | 12 (40.0%) | .03 |
Abbreviations: CNS, central nervous system; ICU, intensive care unit.
It was only possible to determine the bimA allele in 47 of the 52 patients.
It was only possible to determine the bimA allele in 39 of the 44 survivors.
A possible inoculation event was reported in 18 of 52 patients (34.6%). In 7 patients this was immersion or a preceding skin lesion (Supplementary Table 5); 4 (57.1%) of these 7 cases had no predisposing risk factor for melioidosis compared with 7 of 45 (15.6%) of the remaining cases (P = .03). However, only 1 of these 4 cases without a risk factor had the bimABm variant, a 3-year-old child who presented with brainstem encephalomyelitis after having a boil on his scalp for over 1 week. A 10-year-old child from Papua New Guinea without predisposing risk factors for melioidosis developed a subdural empyema and an intracerebral abscess after plant products were scraped across a scalp wound; he had the BimABp variant and his case has been previously reported [26].
DISCUSSION
The study highlights the diversity of presentations of CNS melioidosis, but it also demonstrates that if patients are recognized promptly, receive care in a well-resourced setting, and have access to sophisticated imaging and ICU support, many are able to survive without permanent sequelae. It also suggests that the different alleles of the B. pseudomallei bimA gene have a critical role in the pathogenesis of CNS melioidosis, with a major impact on clinical presentation and prognosis.
Clinicians have long recognized that patients with CNS melioidosis can present as primary or secondary disease, with clinical and experimental data supporting the hypothesis that the clinical phenotype is influenced by the mechanism by which B. pseudomallei enters the CNS. In secondary CNS disease, patients are usually bacteremic, multiorgan involvement is often apparent, and the clinical presentation is dominated by nonneurological manifestations [27]. In these cases, B. pseudomallei is likely to enter the CNS via a hematogenous route, either directly or within infected leukocytes [9, 28]. In contrast, patients with primary CNS disease have a predominantly neurological presentation and are more likely to have encephalomyelitis and less likely to be bacteremic or have B. pseudomallei cultured outside the CNS. In primary disease, it is hypothesized that, in many cases, the organism enters the brainstem or spinal cord directly, travelling along peripheral and cranial nerves to gain access to the CNS [20, 21].
This study suggests that the bimA allele of the infecting B. pseudomallei strain plays a critical role in determining whether primary or secondary CNS melioidosis is more likely. Patients with the bimABm, instead of the bimABp, variant were more than 5 times more likely to have a predominantly neurological presentation, over 7 times more likely to have brainstem involvement, and over 4 times more likely to have encephalomyelitis. All patients in the cohort with the bimABm variant had parenchymal CNS disease compared to just over half of those with bimABp allele. The bimA allele of the infecting strain—likely through its association with brainstem involvement—also appears to have prognostic value, with patients infected with the bimABm variant being almost 5 times more likely to die or have residual disability.
Precisely how different bimA alleles might lead to different clinical phenotypes is unclear. In mouse models, the bimABm variant is more virulent—disseminating rapidly—and persists for longer in phagocytic cells [18]. The bimA gene mediates actin-based motility and it is hypothesized that the bimABm allele facilitates bacterial invasion of the olfactory mucosa to gain access to olfactory and trigeminal nerve endings [29]. Alternatively, BimABm might influence bacterial uptake by Schwann cells, facilitating entry to the nerve fascicle, although BimA is thought unlikely to be directly involved in movement within the nerve fascicles themselves [29]. It has been postulated that B. pseudomallei carrying bimABm might enter the CNS via nerve route translocation across the nasal or oral mucosa after water immersion, or as a complication of B. pseudomallei elsewhere in the body, particularly infection of the face and scalp [20]. However, while it was notable that CNS disease was more common in individuals without traditional risk factors for melioidosis than in those with these risk factors, it was not possible to demonstrate a link between the bimABm allele and an inoculation event in this small cohort.
None of the 6 children (who comprised >10% of the cohort) had traditional risk factors for melioidosis. Although 5 of the 6 children had a putative exposure history, in only 2 was this felt to be strong. In another series from Queensland, 4 of 12 cases of CNS melioidosis occurred in children, although the presence, or absence, of predisposing factors was not reported [7]. Melioidosis is well recognized to occur in individuals without apparent risk factors, especially in children [1, 20]. That children were more likely to have CNS involvement than adults reflects, at least in part, that they have lower rates of disseminated disease than adults [20], but it may also reflect a higher predisposition to inoculating events such as water immersion.
The case-fatality rate (15.4%) in this cohort compares with case-fatality rates of 25% and 45% in Australian studies published in 2000 and 2013, respectively [6, 7]. This cohort’s lower case-fatality rate is likely explained, predominantly, by continuing improvements in critical care; almost all the patients in this study were able to receive prompt, multimodal ICU support [30]. The study period also coincided with improved access to MRI imaging at both sites, which, in several cases, expedited diagnosis. There has been a significant recent rise in the incidence of melioidosis in Far North Queensland, increasing local clinicians’ index of suspicion for the disease [31]. Prompt administration of antibiotics with activity against B. pseudomallei is also likely to have contributed. This has been facilitated by the electronic promulgation of national guidelines for the management of melioidosis, and which also recommend empirical meropenem for cases of severe sepsis in tropical Australia when melioidosis is a possible diagnosis [32]. The low relapse rate can be explained by evolving neurosurgical support for both sites over the course of the study and evolution of the recommended duration of antibiotic therapy, particularly prolongation of the duration of the intravenous induction period [33]. The combination of intravenous meropenem and adjunctive oral TMP-SMX is now recommended in Australia for the 8-week intensive phase of management of CNS melioidosis. Eradication therapy with oral TMP-SMX is then continued for at least 6 months [1, 34].
One of the explanations for the poor outcome described with CNS melioidosis in many series is a failure to consider the diagnosis or—even if the diagnosis is considered—a difficulty in establishing the diagnosis, both of which can delay therapy [35]. This is partly due to its highly variable presentation, which can mimic other infectious and noninfectious neurological conditions. It is also due to challenges in establishing a microbiological diagnosis, particularly in low- and middle-income countries where access to high-quality laboratory services may be limited [36]. However, even with access to microbiological services, CSF culture is commonly negative—less than one-third of CSF cultures were positive in this cohort—and CSF microscopy findings are highly variable, although it was notable that, unusually for a bacterial infection, lymphocytes were predominant in the CSF of many cases (Supplementary Table 3).
The study again demonstrates the limited sensitivity of CT imaging in the early diagnosis of disease. While all MRI scans demonstrated abnormalities, there is limited access to MRI in many parts of the world where B. pseudomallei is endemic. Although the MRI findings in CNS melioidosis are variable, the presence of rim-enhancing abscesses with contrast enhancement of white matter tracts and cranial nerves supports the diagnosis [37]. Cranial neuropathies—especially of the trigeminal nerve—are characteristic clinical findings [4, 6, 37]. Enhancement of the trigeminal nerve with associated brainstem micro-abscesses in 1 case suggests that B. pseudomallei entered the CNS via this path (Figure 1B).
This study has several limitations. Almost 40% of the Queensland cases had data collected retrospectively. The relatively small cohort—despite a study period of greater than 20 years—makes type 2 errors inevitable. However, the findings provide support for current hypotheses—derived from animal models—about the pathogenesis of CNS melioidosis in humans. Future research might determine the BimA protein’s potential as a target for vaccines or adjuvant therapy and the utility of adjunctive TMP-SMX in the initial therapy for CNS melioidosis [21, 38, 39].
Conclusions
In the well-resourced Australian healthcare system, the case-fatality rate from melioidosis is declining, but CNS involvement remains a feared complication. Infection with the bimABm variant increases the likelihood of primary CNS disease, particularly brainstem encephalomyelitis, and is associated with poorer outcomes. A greater understanding of the clinical associations of the organism’s virulence factors—like BimA—provides an insight into the pathophysiology of B. pseudomallei infection and has the potential to inform future therapeutic strategies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge and thank Rob Baird and the laboratory scientists at the Royal Darwin Hospital for their expertise in retrieval and identification of B. pseudomallei and Linda Ward at Menzies School of Health Research for maintaining the Darwin Prospective Melioidosis Study database and providing statistical support.
Financial support. The work was supported by the Australian National Health and Medical Research Council (grant numbers 1046812, 1098337, and 1131932) (the HOT NORTH initiative).
References
Author notes
J. H. and E. M. M. contributed equally.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




