-
PDF
- Split View
-
Views
-
Cite
Cite
Nadia Sabet, Tanvier Omar, Minja Milovanovic, Tebogo Magajane, Modiehi Mosala, Tumelo Moloantoa, Nalukenge Kato-Kalule, Lenise Varela Semedo, Floris Swanepoel, Carole Wallis, Pattamukkil Abraham, Limakatso Lebina, Ebrahim Variava, Neil Martinson, Undiagnosed Pulmonary Tuberculosis (TB) and Coronavirus Disease 2019 (COVID-19) in Adults Dying at Home in a High-TB-Burden Setting, Before and During Pandemic COVID-19: An Autopsy Study, Clinical Infectious Diseases, Volume 77, Issue 3, 1 August 2023, Pages 453–459, https://doi.org/10.1093/cid/ciad212
Close - Share Icon Share
Abstract
Missing or undiagnosed patients with tuberculosis (TB) or coronavirus disease 2019 (COVID-19) are of concern. Identifying both infections in patients with no diagnosis prior to death contributes to understanding the burden of disease. To confirm reports of global reduction in TB incidence, a 2012 autopsy study of adults dying at home of natural causes in a high-TB-burden setting was repeated, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) assessments after the first COVID-19 surge in South Africa.
Adult decedents who died at home with insufficient information to determine cause of death, no recent hospitalization, and no current antemortem TB or COVID-19 diagnosis were identified between March 2019 and October 2020 with a 4-month halt during lockdown. A standardized verbal autopsy followed by minimally invasive needle autopsy (MIA) was performed. Biopsies were taken for histopathology from liver, bilateral brain and lung; bronchoalveolar lavage fluid was collected for Xpert (MTB/RIF) and mycobacterial culture, and blood for human immunodeficiency virus (HIV) polymerase chain reaction (PCR) testing. After the start of the COVID-19 pandemic, a nasopharyngeal swab and lung tissue were subjected to SARS-CoV-2 PCR testing.
Sixty-six MIAs were completed in 25 men and 41 women (median age, 60 years); 68.2% had antemortem respiratory symptoms and 30.3% were people with HIV. Overall, TB was diagnosed in 11 of 66 (16.7%) decedents, and 14 of 41 (34.1%) in the COVID-19 pandemic were SARS-CoV-2 positive.
Undiagnosed TB in adults dying at home has decreased but remains unacceptably high. Forty percent of decedents had undiagnosed COVID-19, suggesting that estimates of excess deaths may underestimate the impact of SARS-CoV-2 on mortality.
Globally, the majority of deaths occur out of hospital, either at home or in the community, with a higher proportion in low- and middle-income countries [1–3]. In the latter setting, largely due to unreliable or nonrepresentative death registration statistics, cause-specific mortality is poorly described [4, 5]. In 2020, tuberculosis (TB) caused the death of 1.5 million people globally [6] and remains the leading cause of death in adults in South Africa [1]. The majority of these decedents are people with human immunodeficiency virus (HIV) despite widespread availability of antiretroviral therapy (ART) [7]. Deaths of patients receiving TB treatment are likely to be reported both on TB and death registers. However, global concern about mismatch between estimates of annual incident TB patients and counts of patients diagnosed with TB suggest that there is a substantial group of people with undiagnosed and untreated TB and, consequently, underreported mortality [8, 9].
A 2012 autopsy study of deaths at home by our group reported that 31.8% of adults dying at home, due to natural causes and without an immediately apparent cause of death, had undiagnosed TB [10]. Since then, social and health system improvements have been made, including coverage of social grants, housing quality, TB diagnostics and therapeutics and TB preventive therapy [11–13], and widespread HIV testing with immediate ART initiation [14]. These advances, however, were impacted by the coronavirus disease 2019 (COVID-19) pandemic, which, at the time of writing, has caused >6 million deaths [15] and substantially disrupted TB control [16]. Restricted healthcare access and decreased testing have contributed to the pandemic's excess deaths [17]. Identifying COVID-19 deaths from other corollary causes may assist in understanding the impact of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic on TB control.
The 2012 autopsy study in Matlosana, South Africa, was therefore repeated to assess the proportion of adults dying at home with pulmonary TB 7 years later. During recruitment of the current study, the first patient in South Africa was diagnosed with COVID-19 on 5 March 2020; recruitment was continued after a brief hiatus, additionally assessing the proportion dying at home with undiagnosed COVID-19.
MATERIALS AND METHODS
For temporal comparisons, we adhered to similar study procedures as the prior autopsy study [10]. The study recruited from March 2019 to October 2020, with a temporary recruitment pause for COVID-19 lockdown restrictions from March to May 2020. At the time of this study, annual TB incidence in the district was 690 per 100 000 [18] (a decrease of 25% since 2012). The reported count of COVID-19 cases in the North West province at the end of the study in October 2020 was 13 783, contributing 4.5% of the cumulative national number [19].
Fifteen private subdistrict undertakers and physicians in clinics of the Matlosana health subdistrict were approached and requested to refer family members of decedents who died at home. Study staff contacted decedents’ families to obtain informed consent and confirm eligibility. A brief screening verbal autopsy administered to next of kin assessed antemortem health status, health service utilization, and risk factors for and symptoms of TB and, more latterly, COVID-19. Where available, medical records were reviewed. The study included adults ≥18 years of age who died at home and whose death was not related to trauma, suicide, a surgical procedure, or pregnancy and in whom no likely cause was apparent. Those with a recent hospital admission, a TB diagnosis in the last 6 months, or who were receiving TB treatment at the time of death were excluded. We focused on pulmonary TB as the leading form of TB globally and, from a public health perspective, the most pressing to combat as untreated pulmonary TB is responsible for TB transmission.
Autopsy Procedures
Minimally invasive autopsies (MIAs) directed at the lung and its airways were performed by a trained nurse or doctor at private undertakers’ premises where almost all decedents (excluding pauper and unclaimed bodies) in South Africa are taken after death. Appropriate personal protective equipment was worn. Core needle biopsies of bilateral lungs and the liver were taken with 14G × 11 cm Multicore automatic tissue biopsy needles (Sterylab, Milan, Italy) [20], bilateral hemispheres of the brain were biopsied with 14G × 15 cm Trucut manual tissue biopsy needles (Carefusion, Vernon Hills, Illinois) [21], and modified bronchoalveolar lavage was performed through a midline tracheotomy incision by instillation of normal saline through an 8Fr feeding tube and suctioned a few minutes later following chest percussion. If HIV status was unknown or negative, a postmortem blood sample was taken for HIV polymerase chain reaction (PCR) testing. Where HIV PCR was reported as indeterminate or invalid and the medical record or family report was not available, the participant's HIV status was recorded as indeterminate.
All tissue biopsies were examined histopathologically after staining with hematoxylin and eosin (H&E). Ziehl-Neelsen and Grocott staining for acid-fast bacilli and fungi, respectively, were performed on all lung, liver, and brain samples. Other stains were guided by findings on H&E. Nonspecific interstitial pneumonia (NSIP) was diagnosed as a histological reaction pattern when lung biopsy material showed interstitial inflammation with or without fibrosis, with no disruption of alveolar architecture, but the biopsy lacked specific features and could not be classified further in the absence of clinical, radiological, or serological supporting evidence.
Liquid mycobacterial culture was performed on all lung, brain, and lavage specimens using automated Mycobacterial Growth Indicator Tube (MGIT) system (Becton Dickinson Microbiology Systems, Franklin Lakes, New Jersey) culture. Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, California) was used to test lung and lavage specimens. Testing of brain tissue was discontinued halfway (except if testing SARS-CoV-2 positive) as almost all were histologically unremarkable. Postmortem nasopharyngeal (NP) swabs were collected for real-time reverse-transcription PCR (rtPCR) testing following government mandate [22]. Additionally, rtPCR was performed on paraffin-embedded lung tissue specimens of patients testing COVID-19 positive on NP swab, those with histopathological findings raising the possibility of COVID pneumonia, and in 9 randomly selected participants predating the pandemic. Approval was obtained from the University of the Witwatersrand Human Research Ethics Committee (Reference: 180511), the North West Department of Health, and local hospital research committees.
Data Analysis
We defined a deceased person with TB disease using a more conservative definition of at least 2 different testing modalities suggestive or diagnostic of TB, and also a less strict definition of at least 1 diagnostic assay positive. This was done to recognize that histology based on limited sampling alone is likely an inaccurate method of TB diagnosis and that a positive Xpert may reflect a prior episode of TB [23, 24]. Participant demographics and laboratory results are reported with median and interquartile range (IQR) and proportions as percentages with 95% confidence intervals. The χ2 test was performed to compare binary data and Cohen kappa (κ) to assess concordance. Findings are reported as overall and stratified by pre–COVID-19 and COVID-19 pandemic eras using 1 January 2020 as the separator.
RESULTS
From March 2019 to October 2020, 138 adults who died at home were approached; 32 families refused participation. After staff review of medical records and the verbal autopsy, 36 of 106 (34.0%) decedents were ineligible, of whom half (n = 18) were reportedly receiving TB treatment at the time of their death (Figure 1). Study procedures were performed on 66 enrolled participants: 25 (37.9%) in the pre–COVID-19 era and the remaining 41 (62.1%) during the COVID-19 pandemic. Overall, 25 of 66 (37.9%) were men and the median age of all decedents was 60 years (IQR, 50–72 years) (Table 1).
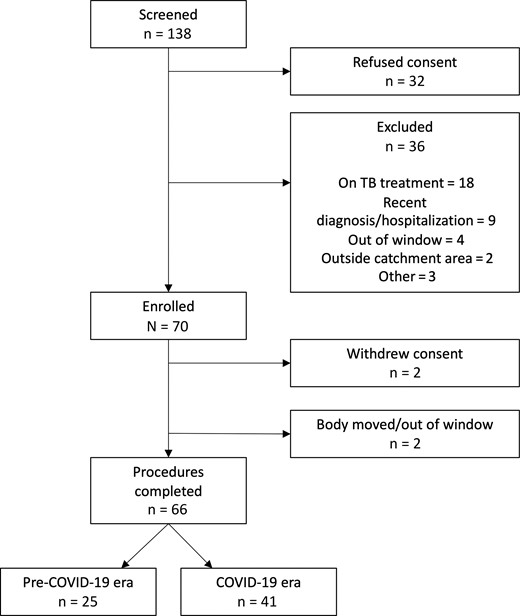
Flow diagram of study recruitment. Pre–coronavirus disease 2019 (COVID-19) and COVID-19 pandemic eras were defined using the separator of 1 January 2020. Abbreviations: COVID-19, coronavirus disease 2019; TB, tuberculosis.
| Characteristic . | Pre–COVID-19 Era (n = 25) . | COVID-19 Era (n = 41) . | Overall (N = 66) . |
|---|---|---|---|
| Age at death, y, median (IQR) | 62 (41–73) | 59 (53–68) | 60 (50–72) |
| Male sex | 12 (48.0) | 13 (31.7) | 25 (37.9) |
| HIV status, as reported | |||
| Positive | 7 (28.0) | 10 (24.4) | 17 (25.8) |
| Negative | 14 (56.0) | 25 (61.0) | 39 (59.1) |
| Unknown | 4 (16.0) | 6 (14.6) | 10 (15.2) |
| HIV positive, reported and tested | 7 (28.0) | 13 (31.7) | 20 (30.3) |
| Chronic illness | |||
| Cardiac | 3 (12.0) | 1 (2.4) | 4 (6.1) |
| Renal | 7 (28.0) | 5 (12.2) | 12 (18.2) |
| Hypertension | … | 21 (51.2) | … |
| Neurological | 1 (4.0) | 1 (2.4) | 2 (3.0) |
| Respiratory | … | 5 (12.2) | … |
| Malnutrition | … | … | … |
| Cancer | 1 (4.0) | 1 (2.4) | 2 (3.0) |
| Other | 10 (40.0) | 18 (43.9) | 28 (42.4) |
| Reported symptoms | |||
| Cough | 13 (52.0) | 18 (43.9) | 31 (47.0) |
| Loss of weight | 9 (36.0) | 19 (46.3) | 28 (42.4) |
| Chest pain | 9 (36.0) | 12 (29.2) | 21 (31.8) |
| Shortness of breath | 13 (52.0) | 17 (41.5) | 30 (45.5) |
| Other | 14 (56.0) | 33 (80.5) | 47 (71.2) |
| Duration of symptoms, d, median (IQR) | |||
| Cough | 30 (7–30) | 7 (4.5–14) | 7 (5–15) |
| Loss of weight | 48.5 (7–90) | 14 (7–25.5) | 14 (7–30) |
| Chest pain | 30 (1–90) | 7.5 (5–14) | 8 (5–30) |
| Shortness of breath | 30 (3–90) | 12 (5–75.5) | 14 (5–90) |
| Other | 5 (3–7) | 11 (4–30) | 7 (3–30) |
| Occupation | |||
| Employed | 4 (16.0) | 7 (17.1) | 11 (16.7) |
| Unemployed | 4 (16.0) | 14 (34.1) | 18 (27.3) |
| Pensioner | 17 (68.0) | 21 (51.2) | 38 (57.6) |
| Underground work | 3 (12.0) | 10 (24.4) | 13 (19.7) |
| Median duration, y | 8 | 12 | 9.5 |
| Smokes cigarettes | 14 (56.0) | 21 (51.2) | 35 (53.0) |
| Mean cigarettes per d | 9.2 | 9.2 | 9.2 |
| Accessed healthcare in year preceding death, % | |||
| Outpatient/casualty | 12.0 | 29.3 | 22.7 |
| Clinic | 32.0 | 9.8 | 18.2 |
| Private GP | 4.0 | 7.3 | 6.1 |
| Traditional healer | … | 2.4 | 1.5 |
| Characteristic . | Pre–COVID-19 Era (n = 25) . | COVID-19 Era (n = 41) . | Overall (N = 66) . |
|---|---|---|---|
| Age at death, y, median (IQR) | 62 (41–73) | 59 (53–68) | 60 (50–72) |
| Male sex | 12 (48.0) | 13 (31.7) | 25 (37.9) |
| HIV status, as reported | |||
| Positive | 7 (28.0) | 10 (24.4) | 17 (25.8) |
| Negative | 14 (56.0) | 25 (61.0) | 39 (59.1) |
| Unknown | 4 (16.0) | 6 (14.6) | 10 (15.2) |
| HIV positive, reported and tested | 7 (28.0) | 13 (31.7) | 20 (30.3) |
| Chronic illness | |||
| Cardiac | 3 (12.0) | 1 (2.4) | 4 (6.1) |
| Renal | 7 (28.0) | 5 (12.2) | 12 (18.2) |
| Hypertension | … | 21 (51.2) | … |
| Neurological | 1 (4.0) | 1 (2.4) | 2 (3.0) |
| Respiratory | … | 5 (12.2) | … |
| Malnutrition | … | … | … |
| Cancer | 1 (4.0) | 1 (2.4) | 2 (3.0) |
| Other | 10 (40.0) | 18 (43.9) | 28 (42.4) |
| Reported symptoms | |||
| Cough | 13 (52.0) | 18 (43.9) | 31 (47.0) |
| Loss of weight | 9 (36.0) | 19 (46.3) | 28 (42.4) |
| Chest pain | 9 (36.0) | 12 (29.2) | 21 (31.8) |
| Shortness of breath | 13 (52.0) | 17 (41.5) | 30 (45.5) |
| Other | 14 (56.0) | 33 (80.5) | 47 (71.2) |
| Duration of symptoms, d, median (IQR) | |||
| Cough | 30 (7–30) | 7 (4.5–14) | 7 (5–15) |
| Loss of weight | 48.5 (7–90) | 14 (7–25.5) | 14 (7–30) |
| Chest pain | 30 (1–90) | 7.5 (5–14) | 8 (5–30) |
| Shortness of breath | 30 (3–90) | 12 (5–75.5) | 14 (5–90) |
| Other | 5 (3–7) | 11 (4–30) | 7 (3–30) |
| Occupation | |||
| Employed | 4 (16.0) | 7 (17.1) | 11 (16.7) |
| Unemployed | 4 (16.0) | 14 (34.1) | 18 (27.3) |
| Pensioner | 17 (68.0) | 21 (51.2) | 38 (57.6) |
| Underground work | 3 (12.0) | 10 (24.4) | 13 (19.7) |
| Median duration, y | 8 | 12 | 9.5 |
| Smokes cigarettes | 14 (56.0) | 21 (51.2) | 35 (53.0) |
| Mean cigarettes per d | 9.2 | 9.2 | 9.2 |
| Accessed healthcare in year preceding death, % | |||
| Outpatient/casualty | 12.0 | 29.3 | 22.7 |
| Clinic | 32.0 | 9.8 | 18.2 |
| Private GP | 4.0 | 7.3 | 6.1 |
| Traditional healer | … | 2.4 | 1.5 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: COVID-19, coronavirus disease 2019; GP, general practitioner; HIV, human immunodeficiency virus; IQR, interquartile range.
| Characteristic . | Pre–COVID-19 Era (n = 25) . | COVID-19 Era (n = 41) . | Overall (N = 66) . |
|---|---|---|---|
| Age at death, y, median (IQR) | 62 (41–73) | 59 (53–68) | 60 (50–72) |
| Male sex | 12 (48.0) | 13 (31.7) | 25 (37.9) |
| HIV status, as reported | |||
| Positive | 7 (28.0) | 10 (24.4) | 17 (25.8) |
| Negative | 14 (56.0) | 25 (61.0) | 39 (59.1) |
| Unknown | 4 (16.0) | 6 (14.6) | 10 (15.2) |
| HIV positive, reported and tested | 7 (28.0) | 13 (31.7) | 20 (30.3) |
| Chronic illness | |||
| Cardiac | 3 (12.0) | 1 (2.4) | 4 (6.1) |
| Renal | 7 (28.0) | 5 (12.2) | 12 (18.2) |
| Hypertension | … | 21 (51.2) | … |
| Neurological | 1 (4.0) | 1 (2.4) | 2 (3.0) |
| Respiratory | … | 5 (12.2) | … |
| Malnutrition | … | … | … |
| Cancer | 1 (4.0) | 1 (2.4) | 2 (3.0) |
| Other | 10 (40.0) | 18 (43.9) | 28 (42.4) |
| Reported symptoms | |||
| Cough | 13 (52.0) | 18 (43.9) | 31 (47.0) |
| Loss of weight | 9 (36.0) | 19 (46.3) | 28 (42.4) |
| Chest pain | 9 (36.0) | 12 (29.2) | 21 (31.8) |
| Shortness of breath | 13 (52.0) | 17 (41.5) | 30 (45.5) |
| Other | 14 (56.0) | 33 (80.5) | 47 (71.2) |
| Duration of symptoms, d, median (IQR) | |||
| Cough | 30 (7–30) | 7 (4.5–14) | 7 (5–15) |
| Loss of weight | 48.5 (7–90) | 14 (7–25.5) | 14 (7–30) |
| Chest pain | 30 (1–90) | 7.5 (5–14) | 8 (5–30) |
| Shortness of breath | 30 (3–90) | 12 (5–75.5) | 14 (5–90) |
| Other | 5 (3–7) | 11 (4–30) | 7 (3–30) |
| Occupation | |||
| Employed | 4 (16.0) | 7 (17.1) | 11 (16.7) |
| Unemployed | 4 (16.0) | 14 (34.1) | 18 (27.3) |
| Pensioner | 17 (68.0) | 21 (51.2) | 38 (57.6) |
| Underground work | 3 (12.0) | 10 (24.4) | 13 (19.7) |
| Median duration, y | 8 | 12 | 9.5 |
| Smokes cigarettes | 14 (56.0) | 21 (51.2) | 35 (53.0) |
| Mean cigarettes per d | 9.2 | 9.2 | 9.2 |
| Accessed healthcare in year preceding death, % | |||
| Outpatient/casualty | 12.0 | 29.3 | 22.7 |
| Clinic | 32.0 | 9.8 | 18.2 |
| Private GP | 4.0 | 7.3 | 6.1 |
| Traditional healer | … | 2.4 | 1.5 |
| Characteristic . | Pre–COVID-19 Era (n = 25) . | COVID-19 Era (n = 41) . | Overall (N = 66) . |
|---|---|---|---|
| Age at death, y, median (IQR) | 62 (41–73) | 59 (53–68) | 60 (50–72) |
| Male sex | 12 (48.0) | 13 (31.7) | 25 (37.9) |
| HIV status, as reported | |||
| Positive | 7 (28.0) | 10 (24.4) | 17 (25.8) |
| Negative | 14 (56.0) | 25 (61.0) | 39 (59.1) |
| Unknown | 4 (16.0) | 6 (14.6) | 10 (15.2) |
| HIV positive, reported and tested | 7 (28.0) | 13 (31.7) | 20 (30.3) |
| Chronic illness | |||
| Cardiac | 3 (12.0) | 1 (2.4) | 4 (6.1) |
| Renal | 7 (28.0) | 5 (12.2) | 12 (18.2) |
| Hypertension | … | 21 (51.2) | … |
| Neurological | 1 (4.0) | 1 (2.4) | 2 (3.0) |
| Respiratory | … | 5 (12.2) | … |
| Malnutrition | … | … | … |
| Cancer | 1 (4.0) | 1 (2.4) | 2 (3.0) |
| Other | 10 (40.0) | 18 (43.9) | 28 (42.4) |
| Reported symptoms | |||
| Cough | 13 (52.0) | 18 (43.9) | 31 (47.0) |
| Loss of weight | 9 (36.0) | 19 (46.3) | 28 (42.4) |
| Chest pain | 9 (36.0) | 12 (29.2) | 21 (31.8) |
| Shortness of breath | 13 (52.0) | 17 (41.5) | 30 (45.5) |
| Other | 14 (56.0) | 33 (80.5) | 47 (71.2) |
| Duration of symptoms, d, median (IQR) | |||
| Cough | 30 (7–30) | 7 (4.5–14) | 7 (5–15) |
| Loss of weight | 48.5 (7–90) | 14 (7–25.5) | 14 (7–30) |
| Chest pain | 30 (1–90) | 7.5 (5–14) | 8 (5–30) |
| Shortness of breath | 30 (3–90) | 12 (5–75.5) | 14 (5–90) |
| Other | 5 (3–7) | 11 (4–30) | 7 (3–30) |
| Occupation | |||
| Employed | 4 (16.0) | 7 (17.1) | 11 (16.7) |
| Unemployed | 4 (16.0) | 14 (34.1) | 18 (27.3) |
| Pensioner | 17 (68.0) | 21 (51.2) | 38 (57.6) |
| Underground work | 3 (12.0) | 10 (24.4) | 13 (19.7) |
| Median duration, y | 8 | 12 | 9.5 |
| Smokes cigarettes | 14 (56.0) | 21 (51.2) | 35 (53.0) |
| Mean cigarettes per d | 9.2 | 9.2 | 9.2 |
| Accessed healthcare in year preceding death, % | |||
| Outpatient/casualty | 12.0 | 29.3 | 22.7 |
| Clinic | 32.0 | 9.8 | 18.2 |
| Private GP | 4.0 | 7.3 | 6.1 |
| Traditional healer | … | 2.4 | 1.5 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: COVID-19, coronavirus disease 2019; GP, general practitioner; HIV, human immunodeficiency virus; IQR, interquartile range.
Verbal Next of Kin Report
According to next of kin reports, 45 of 66 (68.2%) of decedents had antemortem respiratory symptoms. Cough was reported for 31 of 66 (47.0%) decedents for a median of 7 days (IQR, 5–15 days). Only 5 of 66 (7.6%) were reportedly asymptomatic prior to death. Median time from death to autopsy was 3 days (IQR, 2–4 days).
Tuberculosis
Overall, 11 of 66 (16.7%) decedents had laboratory-confirmed TB, diagnosed on at least 1 laboratory assay (Table 2). Of these, 4 of 25 (16.0%) and 7 of 41 (17.1%) were sampled in the pre–COVID-19 and COVID-19 eras, respectively (P = .91). Using stricter definitions of >1 testing modality to improve specificity and suggest with greater accuracy those with TB disease, only 4 of 66 (6.1%) decedents had TB at death. Decedents with TB who were people with HIV, those without HIV, and those whose HIV status was indeterminate were 5 of 20 (25.0%), 2 of 26 (7.7%), and 4 of 20 (20.0%), respectively. Prevalence of TB diagnosed at autopsy by at least 1 modality in men and women was 4 of 11 (36.4%) and 7 of 11 (63.6%), respectively.
Tuberculosis, Coronavirus Disease 2019, and Other Diagnoses in Decedents by Diagnostic Modality
| Diagnosis . | No. (%) . |
|---|---|
| Tuberculosis (n = 66) | |
| TB diagnosed on at least 1 laboratory modality | 11 (16.7) |
| TB diagnosed on ≥2 modalities | 4 (6.1) |
| BAL specimens with TB (n = 7) | |
| Xpert MTB/RIF Ultra | 6 (9.1) |
| MGIT positive | 3 (4.5) |
| Lung biopsy specimens with TB (n = 5)a | |
| Xpert MTB/RIF Ultra | 5 (8.1) |
| MGIT positive | 3 (4.8) |
| Histology suggestive of TBb | 4 (6.5) |
| Brain tissue biopsy specimens with TB (n = 1) | … |
| MGIT positive | 1 (2.0) |
| Histology suggestive of TB | 0 (0.0) |
| Liver tissue biopsy specimens with TB (n = 1) | … |
| Histology suggestive of TB | 1 (4.8) |
| COVID-19 (n = 41) | |
| COVID-19 diagnosed on at least 1 laboratory modality | 13 (31.7) |
| COVID-19 diagnosed on ≥2 modalities | 4 (9.8) |
| NP swab specimens SARS-CoV-2 positive (n = 30) | 9 (30.0) |
| Lung biopsy specimens SARS-CoV-2 positive | |
| SARS-CoV-2 rtPCR (n = 30) | 10 (33.3) |
| Histology suggestive of COVID-19c | 12 (29.3) |
| Histopathological diagnoses (n = 63)d | |
| Other bacterial pneumonia | 13 (20.6) |
| Nonspecific interstitial pneumonia | 13 (20.6) |
| Incomplete septal fibrosis | 31 (49.2) |
| Other (adenocarcinoma, evidence of left ventricular failure) | 4 (6.3) |
| Diagnosis . | No. (%) . |
|---|---|
| Tuberculosis (n = 66) | |
| TB diagnosed on at least 1 laboratory modality | 11 (16.7) |
| TB diagnosed on ≥2 modalities | 4 (6.1) |
| BAL specimens with TB (n = 7) | |
| Xpert MTB/RIF Ultra | 6 (9.1) |
| MGIT positive | 3 (4.5) |
| Lung biopsy specimens with TB (n = 5)a | |
| Xpert MTB/RIF Ultra | 5 (8.1) |
| MGIT positive | 3 (4.8) |
| Histology suggestive of TBb | 4 (6.5) |
| Brain tissue biopsy specimens with TB (n = 1) | … |
| MGIT positive | 1 (2.0) |
| Histology suggestive of TB | 0 (0.0) |
| Liver tissue biopsy specimens with TB (n = 1) | … |
| Histology suggestive of TB | 1 (4.8) |
| COVID-19 (n = 41) | |
| COVID-19 diagnosed on at least 1 laboratory modality | 13 (31.7) |
| COVID-19 diagnosed on ≥2 modalities | 4 (9.8) |
| NP swab specimens SARS-CoV-2 positive (n = 30) | 9 (30.0) |
| Lung biopsy specimens SARS-CoV-2 positive | |
| SARS-CoV-2 rtPCR (n = 30) | 10 (33.3) |
| Histology suggestive of COVID-19c | 12 (29.3) |
| Histopathological diagnoses (n = 63)d | |
| Other bacterial pneumonia | 13 (20.6) |
| Nonspecific interstitial pneumonia | 13 (20.6) |
| Incomplete septal fibrosis | 31 (49.2) |
| Other (adenocarcinoma, evidence of left ventricular failure) | 4 (6.3) |
Abbreviations: BAL, bronchoalveolar lavage; COVID-19, coronavirus disease 2019; MGIT, Mycobacteria Growth Indicator Tube; MTB, Mycobacterium tuberculosis; NP, nasopharyngeal; RIF, rifampin; rtPCR, real-time reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TB, tuberculosis; Xpert, Xpert MTB/RIF Ultra.
Inadequate samples (not containing specified tissue) on histopathological evaluation excluded.
One case was positive on Ziehl-Neelsen staining of tissue biopsy.
Viral pneumonitis with diffuse alveolar damage and/or pulmonary thrombi.
Cases may have had >1 histological diagnosis.
Tuberculosis, Coronavirus Disease 2019, and Other Diagnoses in Decedents by Diagnostic Modality
| Diagnosis . | No. (%) . |
|---|---|
| Tuberculosis (n = 66) | |
| TB diagnosed on at least 1 laboratory modality | 11 (16.7) |
| TB diagnosed on ≥2 modalities | 4 (6.1) |
| BAL specimens with TB (n = 7) | |
| Xpert MTB/RIF Ultra | 6 (9.1) |
| MGIT positive | 3 (4.5) |
| Lung biopsy specimens with TB (n = 5)a | |
| Xpert MTB/RIF Ultra | 5 (8.1) |
| MGIT positive | 3 (4.8) |
| Histology suggestive of TBb | 4 (6.5) |
| Brain tissue biopsy specimens with TB (n = 1) | … |
| MGIT positive | 1 (2.0) |
| Histology suggestive of TB | 0 (0.0) |
| Liver tissue biopsy specimens with TB (n = 1) | … |
| Histology suggestive of TB | 1 (4.8) |
| COVID-19 (n = 41) | |
| COVID-19 diagnosed on at least 1 laboratory modality | 13 (31.7) |
| COVID-19 diagnosed on ≥2 modalities | 4 (9.8) |
| NP swab specimens SARS-CoV-2 positive (n = 30) | 9 (30.0) |
| Lung biopsy specimens SARS-CoV-2 positive | |
| SARS-CoV-2 rtPCR (n = 30) | 10 (33.3) |
| Histology suggestive of COVID-19c | 12 (29.3) |
| Histopathological diagnoses (n = 63)d | |
| Other bacterial pneumonia | 13 (20.6) |
| Nonspecific interstitial pneumonia | 13 (20.6) |
| Incomplete septal fibrosis | 31 (49.2) |
| Other (adenocarcinoma, evidence of left ventricular failure) | 4 (6.3) |
| Diagnosis . | No. (%) . |
|---|---|
| Tuberculosis (n = 66) | |
| TB diagnosed on at least 1 laboratory modality | 11 (16.7) |
| TB diagnosed on ≥2 modalities | 4 (6.1) |
| BAL specimens with TB (n = 7) | |
| Xpert MTB/RIF Ultra | 6 (9.1) |
| MGIT positive | 3 (4.5) |
| Lung biopsy specimens with TB (n = 5)a | |
| Xpert MTB/RIF Ultra | 5 (8.1) |
| MGIT positive | 3 (4.8) |
| Histology suggestive of TBb | 4 (6.5) |
| Brain tissue biopsy specimens with TB (n = 1) | … |
| MGIT positive | 1 (2.0) |
| Histology suggestive of TB | 0 (0.0) |
| Liver tissue biopsy specimens with TB (n = 1) | … |
| Histology suggestive of TB | 1 (4.8) |
| COVID-19 (n = 41) | |
| COVID-19 diagnosed on at least 1 laboratory modality | 13 (31.7) |
| COVID-19 diagnosed on ≥2 modalities | 4 (9.8) |
| NP swab specimens SARS-CoV-2 positive (n = 30) | 9 (30.0) |
| Lung biopsy specimens SARS-CoV-2 positive | |
| SARS-CoV-2 rtPCR (n = 30) | 10 (33.3) |
| Histology suggestive of COVID-19c | 12 (29.3) |
| Histopathological diagnoses (n = 63)d | |
| Other bacterial pneumonia | 13 (20.6) |
| Nonspecific interstitial pneumonia | 13 (20.6) |
| Incomplete septal fibrosis | 31 (49.2) |
| Other (adenocarcinoma, evidence of left ventricular failure) | 4 (6.3) |
Abbreviations: BAL, bronchoalveolar lavage; COVID-19, coronavirus disease 2019; MGIT, Mycobacteria Growth Indicator Tube; MTB, Mycobacterium tuberculosis; NP, nasopharyngeal; RIF, rifampin; rtPCR, real-time reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TB, tuberculosis; Xpert, Xpert MTB/RIF Ultra.
Inadequate samples (not containing specified tissue) on histopathological evaluation excluded.
One case was positive on Ziehl-Neelsen staining of tissue biopsy.
Viral pneumonitis with diffuse alveolar damage and/or pulmonary thrombi.
Cases may have had >1 histological diagnosis.
COVID-19
Overall, 12 of 30 (40.0%) decedents tested positive on either/both NP swab or tissue rtPCR for SARS-CoV-2; an additional 2 had histological evidence strongly suggestive of COVID-19 pneumonia, with overall COVID-19 and/or SARS-CoV-2 infection prevalence of 14 of 41 (34.1%) in the COVID era. Prevalence of COVID-19 and or SARS-CoV-2 infection diagnosed at autopsy in men or women was equal. All those with COVID-19 or SARS-CoV-2 infection had antemortem symptoms. Of those with a positive NP swab, 7 of 9 (77.8%) were positive for SARS-CoV-2 on lung rtPCR testing. Three patients in the COVID era with negative NP swab results were positive for SARS-CoV-2 on lung rtPCR, and 2 of these had histopathological changes in keeping with NSIP. All lung rtPCR results for SARS-CoV-2 on pre–COVID-19–era decedents were negative. Diagnostic concordance between NP and tissue rtPCR was 68.8% (Cohen κ = 0.355) in 30 patients with NP swabs taken.
Histopathology
Of 66 MIAs performed, 3 (4.5%) had inadequate lung biopsy specimens for histological assessment and another 27 (42.9%) showed no specific pathological changes (Table 2). Four of 63 (6.0%) decedents had histological evidence of a mycobacterial infection (Supplementary Figure 1) on at least 1 tissue biopsy sample, of which 1 was positive on Ziehl-Neelsen staining. Among all COVID-19–era patients, 29.3% (12/41) had histopathological findings consistent with COVID-19 pneumonia (Supplementary Figure 2A and 2B). All showed features of diffuse alveolar damage (DAD), and 3 (25.0%) had superimposed bacterial pneumonia.
Thirteen of 63 (20.6%) lung biopsies had features of NSIP (Supplementary Figure 3A and 3B). In 4 decedents, the presence of superimposed DAD, in decedents sampled in the COVID-19 era, raised the possibility of COVID-19 pneumonia. Two of these 4 were positive for SARS-CoV-2 on tissue rtPCR, confirming COVID-19 pneumonia.
Acute bacterial pneumonia (Supplementary Figure 4), either in isolation or coexisting with COVID-19 pneumonia, NSIP, or TB, was seen in 12 of 63 samples (19.0%). No lung specimens had features of both TB and COVID-19 coinfection and no specimens had evidence of DAD prior to January 2020. In 4 of 63 (6.4%) biopsies, established septal fibrosis indicated prior lung injury. Additionally, microscopic foci of delicate interstitial collagen deposition was noted in 15 of 63 (23.8%) cases across all diagnostic categories including normal lung biopsies, in both people with HIV and participants without HIV. In 1 decedent an undiagnosed adenocarcinoma (likely bronchogenic) was identified, but not coexistent with TB.
Fifty-one participants had brain sampling. One was inadequate, 2 of 66 (3.0%) showed gliosis, and the rest were unremarkable. A single patient with disseminated TB and positive microbiological confirmation in brain tissue had no histopathological evidence of TB.
DISCUSSION
This follow-up autopsy study suggests that in those dying at home, the prevalence of undiagnosed, pulmonary and potentially infectious TB was approximately half (16.7%) the prevalence of a similar study completed 7 years previously (31.8%) [10]. Moreover, a substantial proportion of decedents in the COVID-19 era had a PCR test positive for SARS-CoV-2, the majority of whom also had lung changes suggestive of COVID-19, confirming that SARS-CoV-2 at least partially contributed to their death.
Our results show a clear decreased proportion of undiagnosed TB among those dying at home in Matlosana, likely due to a combination of new global and national TB diagnostic, therapeutic, and preventive measures [11], widespread access to HIV diagnosis with immediate ART initiation, and improvements in social conditions. The first increase in global deaths due to TB in 30 years, since COVID-19 has impacted these measures, was reported in 2020 [25], though these were not measured in our data.
As in our prior study, molecular diagnostics and culture identified more specimens with TB than histology, possibly due to sampling bias as core biopsies may miss localized pathology. The postmortem examination was largely restricted to respiratory pathology, which likely reduced the proportion of undiagnosed extrapulmonary TB reported as not all organs were reviewed and only core biopsies were taken. In contrast to 2012 findings, no cases of Mycobacterium other than TB were identified in the present sample [10], perhaps due to sampling bias or because all people with HIV were on ART [26].
Similar to potential biases in mortality statistics [27], TB prevalence may have been underestimated when calculated using the inflated denominator of the additional deaths due to COVID-19 during the pandemic. However, if decedents with proven COVID-19 are excluded from the prevalence estimation, the proportion who died with TB is close to 21%, still a large reduction from our prior 2012 study. The high number of decedents excluded because they died while receiving TB treatment or had a recent TB diagnosis is a cause of concern. Indeed the minimal estimate of TB prevalence in people dying at home among all those we screened was 27.3%, compared to 41.8% in our prior study. While lower than estimates in 2012, clearly more needs to be done to reduce mortality to reach the End TB targets of a 90% reduction in mortality by 2030 [11]. Whereas men experience a higher burden of TB, our study recruited a higher proportion of women, which may have led to an underestimation of TB mortality [28]; this may be due to a higher acceptability by families of autopsies of female decedents and to shifting mortality patterns in South Africa during the pandemic [17, 29]. Since most autopsy studies are performed in hospitalized patients with in-built selection bias [30, 31], a strength of this study was restricting inclusion to people dying at home without a clear premortem cause of death. Furthermore, while complete diagnostic autopsy remains the gold standard, in our experience next of kin are reluctant to consent for a full autopsy on their loved one [10, 32].
The high proportion of decedents with microscopic foci of interstitial collagen deposition has previously been noted by 1 of the authors [32], particularly in the setting of HIV but more recently also in biopsies with COVID-19 pneumonia. This collagenization does not distort lung microanatomy and to our knowledge is not well described. Its distribution along alveolar septae suggests prior healed interstitial insults; possible causes considered include interstitial pneumonia, DAD, and smoking.
Concordance between NP swab and lung tissue rtPCR was fair [33]. Pre–COVID-19–era SARS-CoV-2 tissue rtPCR results suggest that, at least at this site, earlier, undetected COVID-19 patients prior to January 2020—as reported in Italy [34]—are unlikely. The high proportion of decedents with undiagnosed COVID-19 supports hypotheses based on excess death data that COVID-19 mortality, particularly in South Africa, may be underreported [17]. A substantial proportion had not attended health facilities prior to death despite respiratory symptoms, consistent with previous report of poor health-seeking behavior or restrictions to access during the pandemic [35]. The high concordance between SARS-CoV-2 PCR positivity and COVID-19 features on lung histology suggests that MIA may be a useful tool in this setting for disease surveillance and characterizing excess deaths [36].
Similar to most autopsy studies requiring consent from next of kin, this sample is likely not generalizable to all deaths nor to other settings, a limitation exacerbated by small sample size and recruiting at a single site. Next of kin reports are likely subject to respondent bias, especially among grieving relatives [37]. Proportions of decedents with hypertension were increased and prevalence of cigarette smoking was marginally reduced compared to the prior study, in keeping with larger studies and trends in this population [38–40]. This shift in risk of death from noncommunicable disease and risk of death due to COVID-19 may provide an alternate explanation for the reduction in undiagnosed deaths due to TB, irrespective of a reduction in TB prevalence. Unfortunately, data on additional noncommunicable diseases associated with mortality and both TB and COVID-19, such as diabetes mellitus, were not collected. Prior episodes of TB, particularly important in this high-prevalence setting, were also not documented. Last, while HIV testing was performed, HIV status was indeterminate for a large proportion of participants.
Our findings suggest that although prevalence of undiagnosed TB in adults who die at home is dramatically reduced, those dying with undiagnosed TB remains too high to meet current global targets [11] and these individuals, in addition to those with undiagnosed COVID-19, appear to experience obstacles to accessing care, which likely contribute to transmission, morbidity, and mortality. Response strategies to continuing resurgences of COVID-19 should accurately document deaths from both TB and COVID-19. Additionally, barriers to diagnosis of TB, especially those due to COVID-19 restrictions, must be overcome as a matter of urgency.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank ExHALE Study team members: Nthabiseng Koloane, Ashley Ringane, and Nxosana Xabanisa; and the families of the deceased, who gave so generously of their time while grieving.
Financial support. This work was supported by the University of the Witwatersrand Faculty of Research Committee's Multi/interdisciplinary Collaborative Research Award (grant number 0012838438201512110500000000000000004550).
References
Sterylab.
Author notes
N. S. and T. O. contributed equally to this work.
Potential conflicts of interest. C. W. reports consulting fees from International Partnership for Microbicides. L. L. reports grants or contracts from Gilead Sciences (paid to institution). N. M. reports grants or contracts from Pfizer for an unrelated observational study of patients with pneumonia (paid to institution); reports participation on a data and safety monitoring board or advisory board for Strat TB for a clinical trial assessing treatment for TB meningitis; and was a board member of the Setshaba Research Center in Soshanguve, South Africa. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




