-
PDF
- Split View
-
Views
-
Cite
Cite
Jarl Emanuel Strange, Lauge Østergaard, Lars Køber, Henning Bundgaard, Kasper Iversen, Marianne Voldstedlund, Gunnar Hilmar Gislason, Jonas Bjerring Olesen, Emil Loldrup Fosbøl, Patient Characteristics, Microbiology, and Mortality of Infective Endocarditis After Transcatheter Aortic Valve Implantation, Clinical Infectious Diseases, Volume 77, Issue 12, 15 December 2023, Pages 1617–1625, https://doi.org/10.1093/cid/ciad431
Close - Share Icon Share
Abstract
Infective endocarditis (IE) after transcatheter aortic valve implantation (TAVI) is associated with high mortality and surgery is rarely performed. Thus, to inform on preventive measures and treatment strategies, we investigated patient characteristics and microbiology of IE after TAVI.
Using Danish nationwide registries, we identified patients with IE after TAVI, IE after non-TAVI prosthetic valve (nTPV), and native valve IE. Patient characteristics; overall, early (≤12 m), and late IE (>12 m) microbiology; and unadjusted and adjusted mortality were compared.
We identified 273, 1022, and 5376 cases of IE after TAVI, IE after nTPV, and native valve IE. Age and frailty were highest among TAVI IE (4.8%; median age: 82 y; 61.9% frail). Enterococcus spp. were common for IE after TAVI (27.1%) and IE after nTPV (21.2%) compared with native valve IE (11.4%). Blood culture–negative IE was rare in IE after TAVI (5.5%) compared with IE after nTPV (15.2%) and native valve IE (13.5%). The unadjusted 90-day mortality was comparable, but the 5-year mortality was highest for IE after TAVI (75.2% vs 57.2% vs 53.6%). In Cox models adjusted for patient characteristics and bacterial etiology for 1–90 days and 91–365 days, there was no significant difference in mortality rates.
Patients with IE after TAVI are older and frailer, enterococci and streptococci are often the etiologic agents, and are rarely blood culture negative compared with other IE patients. Future studies regarding antibiotic prophylaxis strategies covering enterococci should be considered in this setting.
Transcatheter aortic valve implantation (TAVI) is a well-established treatment option in selected patients with severe, symptomatic aortic stenosis; however, TAVI and aortic stenosis also come with an increased risk of infective endocarditis (IE) [1–4]. With the rapid increase in procedures performed, this patient group may likely grow in the future [5]. Patients who undergo TAVI are often above 80 years of age and with significant comorbidities, which also adds to the disposition to IE [6]. Although the comorbidity burden has declined, rates of IE after TAVI remain stable [4, 7, 8].
Yet, large-scale data on IE after TAVI remain sparse [9–16]. Previous larger studies on IE after TAVI have been based on data from voluntary centers with a high risk of selection/referral bias or in selected study populations [9, 10, 16]. With regard to outcomes, estimates of in-hospital mortality differ markedly in previous studies, from 11.1% to 63.6% [3, 4]. Notably, surgical treatment in patients with IE after TAVI is rarely performed despite the majority of patients meeting the criteria for an indication for surgery [3, 4].
Altogether, knowledge on patient characteristics, microbiology, and associated mortality in patients with IE after TAVI is limited, yet needed, to give an understanding of how IE after TAVI is differentiated from native valve IE. A more detailed description of the microbiological etiology may help guide empirical and prophylactic antibiotic strategies. Consequently, we sought to investigate patient characteristics, microbiological etiology, and the prognosis of IE after TAVI compared with IE after non-TAVI prosthetic valve and native valve IE.
METHODS
Data Sources
In Denmark, all permanent residents are provided a unique personal identification number. This number is used to crosslink information from the following registers: the Danish Civil Registration System [17], the Danish National Patient Registry [18], and the Danish National Prescription Registry were used [19]. These registries have previously been described [8]. All registries have been extensively used for research purposes and, in general, the positive-predictive value of cardiac diagnoses, procedures, and surgeries is high [20, 21].
Finally, the Danish Microbiology Database was used [22]. It comprises copies of reports from all 11 Departments of Clinical Microbiology in Denmark, which analyzes samples from general practitioners, outpatient clinics, and public and private hospitals. Data on all blood cultures were extracted from January 2010 to December 2021 [22, 23].
Study Design and Population
All patients diagnosed with a primary or secondary diagnosis code of first-time IE from January 2010 to December 2021 with a minimum hospital length of stay of 14 days were included as well as patients who died during admission. When determining hospital length of stay, we included transfers between departments within 24 hours. This was done in accordance with a previous study validating the definition of IE with a positive-predictive value of 90% [24]. Finally, patients had to have a minimum of 1 blood culture drawn within a period of 30 days prior to IE admission and IE discharge [24]. The cohort was stratified into 3 groups: (1) IE after TAVI, (2) IE after non-TAVI prosthetic valve, and (3) native valve IE. Baseline was defined as the date of admission for IE.
If a patient had multiple samples of bacteremia with different bacterial species, the bacterial etiology was defined as “polymicrobial.” Other groups were defined as “Staphylococcus aureus” IE, “Enterococcus spp.” IE, “Streptococcus spp.” IE, “coagulase-negative staphylococci (CoNS)” IE, “other” IE, and “blood culture–negative” IE.
Outcomes and Follow-up
The primary outcome of the study was of a descriptive nature, examining the microbiological distribution of IE after TAVI compared with IE after non-TAVI prosthetic valve and native valve IE. For IE after TAVI, the microbiological distribution was further examined according to time from valve implantation to IE; early IE after TAVI was defined as IE 12 months or less after valve implantation and late IE after TAVI was defined as IE more than 12 months after valve implantation. Secondary outcomes of interest were 90-day mortality rates and the 90-day mortality according to microbiological etiology within each group. Moreover, we investigated the 5-year mortality rates and the proportion of patients who underwent in-hospital cardiac surgery. Patients were followed from date of admission for IE until date of death, 5 years of follow-up, emigration, or end of the study period.
Definition of Comorbidities and Co-medication
Frailty was defined using the Hospital Frailty Risk Score [25]. All hospital contacts up to 10 years prior to IE were identified and the Hospital Frailty Risk Score was calculated (see Supplementary Table 1 for codes and points). Frailty was then categorized as nonfrail (0–4 points) versus frail (≥5 points) or hierarchically as low (0–4 points), intermediate (5–15 points), and high (>15 points).
Other individual comorbidities were identified within the same period. For co-medication, we identified filled prescriptions less than 180 days before date of IE (see Supplementary Table 2 for codes). As done previously, diabetes was defined as a filled prescription of a glucose-lowering drug [26]. Moreover, hypertension was defined as 2 or more prescriptions filled for blood pressure–lowering drugs [27].
Statistical Methodology
Baseline characteristics are presented stratified on IE groups. Continuous variables are reported with medians and interquartile ranges (IQRs) and categorical variables as counts and percentages. The bacterial etiology is presented with proportions stratified on IE groups. Differences in microbiological etiology were tested with the chi-square test. The use of in-hospital cardiac surgery was estimated with the Aalen-Johansen estimator with the competing risk of death [28].
The 90-day and 5-year rates of death were estimated with the reverse Kaplan-Meier estimator. As the proportional hazards assumption was violated in the Cox model for 5-year mortality rates, we used 2 Cox models: a 0–90-days Cox model and a 91-days–5-years Cox model. All models were adjusted for bacterial etiology, age groups (≤65, 66–75, 76–85, ≥86 y), sex, calendar year of IE, frailty, heart failure, liver disease, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, and cancer. Analysis of variance (ANOVA) was used to test for an interaction between bacterial etiology and the exposure group on the outcome of 90-day mortality rates. The level of statistical significance was set at 5%. Data management, analyses, figures, and tables were all done using the statistical software R version 4.0.3 [29].
Sensitivity Analysis
Because differences in patient characteristics between IE groups may influence the risk of endocarditis and the microbiology, we matched patients with IE after TAVI to patients with IE after non-TAVI prosthetic valve and native valve IE in a 1:2:3 ratio. Matching criteria were sex, age, and calendar year of endocarditis. Exact matching was used for sex, and the nearest-neighbor method for age and calendar year of IE estimated with logistic regression (ie, the propensity score). One control could only be matched to 1 case (ie, replacement was not allowed). Characteristics, microbiology, and prognosis were then compared in the matched cohort.
Ethics
The data-responsible institution for this study was the Capital Region and the study was performed under approval number P-2019-191. In Denmark, retrospective cohort studies do not require approval from the Research Ethics Committee.
RESULTS
Population Characteristics
From 2010 to 2021, 273 (4.1%; median age: 82 y) patients with IE after TAVI, 1022 (15.3%; median age: 76 y) patients with IE after non-TAVI prosthetic valve, and 5376 (80.6%; median age: 71 y) patients with native valve were included (Table 1). The median time from TAVI to IE was 461 days (IQR: 154–933 d). Cardiac implantable electronic devices were present among 31.9% of IE after TAVI, 19.4% of IE after non-TAVI prosthetic valve, and 14.5% of native valve IE. Frailty and cardiovascular comorbidity burden were highest for IE after TAVI, while chronic obstructive pulmonary disease, diabetes, and cancer were comparable.
| Variable . | IE After TAVI (n = 273) . | IE After Non-TAVI Prosthetic Valve (n = 1022) . | Native Valve IE (n = 5376) . |
|---|---|---|---|
| Male, n (%) | 181 (66.3) | 740 (72.4) | 3580 (66.6) |
| Age, median [IQR], y | 82 [78, 87] | 76 [68, 81] | 71 [61, 79] |
| Length of hospital stay, median [IQR], d | 43 [23, 48] | 44 [29.2, 51.0] | 36 [26, 48] |
| Living alone, n (%) | 133 (48.7) | 412 (40.5) | 2458 (46.0) |
| Missing | 0 | 4 | 27 |
| Calendar year of IE, n (%) | |||
| 2010–2012 | 21 (7.7) | 219 (21.4) | 1135 (21.1) |
| 2013–2015 | 40 (14.7) | 257 (25.1) | 1340 (24.9) |
| 2016–2018 | 102 (37.4) | 288 (28.2) | 1387 (25.8) |
| 2019–2021 | 110 (40.3) | 258 (25.2) | 1514 (28.2) |
| Frailty group, n (%) | |||
| Low | 106 (38.8) | 445 (43.5) | 2923 (54.4) |
| Intermediate | 140 (51.3) | 491 (48.0) | 1976 (36.8) |
| High | 27 (9.9) | 86 (8.4) | 477 (8.9) |
| Comorbidities, n (%) | |||
| Stroke/systemic embolism | 64 (23.4) | 182 (17.8) | 643 (12.0) |
| Myocardial infarction | 37 (13.6) | 110 (10.8) | 380 (7.1) |
| Ischemic heart disease | 126 (46.2) | 511 (50.0) | 1169 (21.7) |
| Heart failure | 109 (39.9) | 370 (36.2) | 915 (17.0) |
| Peripheral artery disease | 52 (19.0) | 139 (13.6) | 599 (11.1) |
| Previous PCI | 65 (23.8) | 88 (8.6) | 355 (6.6) |
| Previous CABG | 6 (2.2) | 237 (23.2) | 135 (2.5) |
| Atrial fibrillation | 142 (52.0) | 508 (49.7) | 1244 (23.1) |
| Cardiac implantable electric device | 87 (31.9) | 198 (19.4) | 777 (14.5) |
| Diabetes | 45 (16.5) | 180 (17.6) | 835 (15.5) |
| Chronic kidney disease | 57 (20.9) | 155 (15.2) | 822 (15.3) |
| Liver disease | 6 (2.2) | 37 (3.6) | 372 (6.9) |
| COPD | 38 (13.9) | 142 (13.9) | 600 (11.2) |
| Cancer | 29 (10.6) | 96 (9.4) | 605 (11.3) |
| Co-medication, n (%) | |||
| Oral anticoagulants | 120 (44.0) | 531 (52.0) | 1003 (18.7) |
| ADP receptor antagonist | 82 (30.0) | 78 (7.6) | 412 (7.7) |
| NSAIDs | 15 (5.5) | 87 (8.5) | 817 (15.2) |
| Beta-blockers | 122 (44.7) | 497 (48.6) | 1531 (28.5) |
| Statins | 120 (44.0) | 520 (50.9) | 1547 (28.8) |
| Calcium channel blockers | 58 (21.2) | 220 (21.5) | 1027 (19.1) |
| Renin-angiotensin system inhibitors | 109 (39.9) | 465 (45.5) | 1803 (33.5) |
| Diuretics | 63 (23.1) | 292 (28.6) | 1242 (23.1) |
| Variable . | IE After TAVI (n = 273) . | IE After Non-TAVI Prosthetic Valve (n = 1022) . | Native Valve IE (n = 5376) . |
|---|---|---|---|
| Male, n (%) | 181 (66.3) | 740 (72.4) | 3580 (66.6) |
| Age, median [IQR], y | 82 [78, 87] | 76 [68, 81] | 71 [61, 79] |
| Length of hospital stay, median [IQR], d | 43 [23, 48] | 44 [29.2, 51.0] | 36 [26, 48] |
| Living alone, n (%) | 133 (48.7) | 412 (40.5) | 2458 (46.0) |
| Missing | 0 | 4 | 27 |
| Calendar year of IE, n (%) | |||
| 2010–2012 | 21 (7.7) | 219 (21.4) | 1135 (21.1) |
| 2013–2015 | 40 (14.7) | 257 (25.1) | 1340 (24.9) |
| 2016–2018 | 102 (37.4) | 288 (28.2) | 1387 (25.8) |
| 2019–2021 | 110 (40.3) | 258 (25.2) | 1514 (28.2) |
| Frailty group, n (%) | |||
| Low | 106 (38.8) | 445 (43.5) | 2923 (54.4) |
| Intermediate | 140 (51.3) | 491 (48.0) | 1976 (36.8) |
| High | 27 (9.9) | 86 (8.4) | 477 (8.9) |
| Comorbidities, n (%) | |||
| Stroke/systemic embolism | 64 (23.4) | 182 (17.8) | 643 (12.0) |
| Myocardial infarction | 37 (13.6) | 110 (10.8) | 380 (7.1) |
| Ischemic heart disease | 126 (46.2) | 511 (50.0) | 1169 (21.7) |
| Heart failure | 109 (39.9) | 370 (36.2) | 915 (17.0) |
| Peripheral artery disease | 52 (19.0) | 139 (13.6) | 599 (11.1) |
| Previous PCI | 65 (23.8) | 88 (8.6) | 355 (6.6) |
| Previous CABG | 6 (2.2) | 237 (23.2) | 135 (2.5) |
| Atrial fibrillation | 142 (52.0) | 508 (49.7) | 1244 (23.1) |
| Cardiac implantable electric device | 87 (31.9) | 198 (19.4) | 777 (14.5) |
| Diabetes | 45 (16.5) | 180 (17.6) | 835 (15.5) |
| Chronic kidney disease | 57 (20.9) | 155 (15.2) | 822 (15.3) |
| Liver disease | 6 (2.2) | 37 (3.6) | 372 (6.9) |
| COPD | 38 (13.9) | 142 (13.9) | 600 (11.2) |
| Cancer | 29 (10.6) | 96 (9.4) | 605 (11.3) |
| Co-medication, n (%) | |||
| Oral anticoagulants | 120 (44.0) | 531 (52.0) | 1003 (18.7) |
| ADP receptor antagonist | 82 (30.0) | 78 (7.6) | 412 (7.7) |
| NSAIDs | 15 (5.5) | 87 (8.5) | 817 (15.2) |
| Beta-blockers | 122 (44.7) | 497 (48.6) | 1531 (28.5) |
| Statins | 120 (44.0) | 520 (50.9) | 1547 (28.8) |
| Calcium channel blockers | 58 (21.2) | 220 (21.5) | 1027 (19.1) |
| Renin-angiotensin system inhibitors | 109 (39.9) | 465 (45.5) | 1803 (33.5) |
| Diuretics | 63 (23.1) | 292 (28.6) | 1242 (23.1) |
Abbreviations: ADP, adenosine diphosphate; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; IE, infective endocarditis; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
| Variable . | IE After TAVI (n = 273) . | IE After Non-TAVI Prosthetic Valve (n = 1022) . | Native Valve IE (n = 5376) . |
|---|---|---|---|
| Male, n (%) | 181 (66.3) | 740 (72.4) | 3580 (66.6) |
| Age, median [IQR], y | 82 [78, 87] | 76 [68, 81] | 71 [61, 79] |
| Length of hospital stay, median [IQR], d | 43 [23, 48] | 44 [29.2, 51.0] | 36 [26, 48] |
| Living alone, n (%) | 133 (48.7) | 412 (40.5) | 2458 (46.0) |
| Missing | 0 | 4 | 27 |
| Calendar year of IE, n (%) | |||
| 2010–2012 | 21 (7.7) | 219 (21.4) | 1135 (21.1) |
| 2013–2015 | 40 (14.7) | 257 (25.1) | 1340 (24.9) |
| 2016–2018 | 102 (37.4) | 288 (28.2) | 1387 (25.8) |
| 2019–2021 | 110 (40.3) | 258 (25.2) | 1514 (28.2) |
| Frailty group, n (%) | |||
| Low | 106 (38.8) | 445 (43.5) | 2923 (54.4) |
| Intermediate | 140 (51.3) | 491 (48.0) | 1976 (36.8) |
| High | 27 (9.9) | 86 (8.4) | 477 (8.9) |
| Comorbidities, n (%) | |||
| Stroke/systemic embolism | 64 (23.4) | 182 (17.8) | 643 (12.0) |
| Myocardial infarction | 37 (13.6) | 110 (10.8) | 380 (7.1) |
| Ischemic heart disease | 126 (46.2) | 511 (50.0) | 1169 (21.7) |
| Heart failure | 109 (39.9) | 370 (36.2) | 915 (17.0) |
| Peripheral artery disease | 52 (19.0) | 139 (13.6) | 599 (11.1) |
| Previous PCI | 65 (23.8) | 88 (8.6) | 355 (6.6) |
| Previous CABG | 6 (2.2) | 237 (23.2) | 135 (2.5) |
| Atrial fibrillation | 142 (52.0) | 508 (49.7) | 1244 (23.1) |
| Cardiac implantable electric device | 87 (31.9) | 198 (19.4) | 777 (14.5) |
| Diabetes | 45 (16.5) | 180 (17.6) | 835 (15.5) |
| Chronic kidney disease | 57 (20.9) | 155 (15.2) | 822 (15.3) |
| Liver disease | 6 (2.2) | 37 (3.6) | 372 (6.9) |
| COPD | 38 (13.9) | 142 (13.9) | 600 (11.2) |
| Cancer | 29 (10.6) | 96 (9.4) | 605 (11.3) |
| Co-medication, n (%) | |||
| Oral anticoagulants | 120 (44.0) | 531 (52.0) | 1003 (18.7) |
| ADP receptor antagonist | 82 (30.0) | 78 (7.6) | 412 (7.7) |
| NSAIDs | 15 (5.5) | 87 (8.5) | 817 (15.2) |
| Beta-blockers | 122 (44.7) | 497 (48.6) | 1531 (28.5) |
| Statins | 120 (44.0) | 520 (50.9) | 1547 (28.8) |
| Calcium channel blockers | 58 (21.2) | 220 (21.5) | 1027 (19.1) |
| Renin-angiotensin system inhibitors | 109 (39.9) | 465 (45.5) | 1803 (33.5) |
| Diuretics | 63 (23.1) | 292 (28.6) | 1242 (23.1) |
| Variable . | IE After TAVI (n = 273) . | IE After Non-TAVI Prosthetic Valve (n = 1022) . | Native Valve IE (n = 5376) . |
|---|---|---|---|
| Male, n (%) | 181 (66.3) | 740 (72.4) | 3580 (66.6) |
| Age, median [IQR], y | 82 [78, 87] | 76 [68, 81] | 71 [61, 79] |
| Length of hospital stay, median [IQR], d | 43 [23, 48] | 44 [29.2, 51.0] | 36 [26, 48] |
| Living alone, n (%) | 133 (48.7) | 412 (40.5) | 2458 (46.0) |
| Missing | 0 | 4 | 27 |
| Calendar year of IE, n (%) | |||
| 2010–2012 | 21 (7.7) | 219 (21.4) | 1135 (21.1) |
| 2013–2015 | 40 (14.7) | 257 (25.1) | 1340 (24.9) |
| 2016–2018 | 102 (37.4) | 288 (28.2) | 1387 (25.8) |
| 2019–2021 | 110 (40.3) | 258 (25.2) | 1514 (28.2) |
| Frailty group, n (%) | |||
| Low | 106 (38.8) | 445 (43.5) | 2923 (54.4) |
| Intermediate | 140 (51.3) | 491 (48.0) | 1976 (36.8) |
| High | 27 (9.9) | 86 (8.4) | 477 (8.9) |
| Comorbidities, n (%) | |||
| Stroke/systemic embolism | 64 (23.4) | 182 (17.8) | 643 (12.0) |
| Myocardial infarction | 37 (13.6) | 110 (10.8) | 380 (7.1) |
| Ischemic heart disease | 126 (46.2) | 511 (50.0) | 1169 (21.7) |
| Heart failure | 109 (39.9) | 370 (36.2) | 915 (17.0) |
| Peripheral artery disease | 52 (19.0) | 139 (13.6) | 599 (11.1) |
| Previous PCI | 65 (23.8) | 88 (8.6) | 355 (6.6) |
| Previous CABG | 6 (2.2) | 237 (23.2) | 135 (2.5) |
| Atrial fibrillation | 142 (52.0) | 508 (49.7) | 1244 (23.1) |
| Cardiac implantable electric device | 87 (31.9) | 198 (19.4) | 777 (14.5) |
| Diabetes | 45 (16.5) | 180 (17.6) | 835 (15.5) |
| Chronic kidney disease | 57 (20.9) | 155 (15.2) | 822 (15.3) |
| Liver disease | 6 (2.2) | 37 (3.6) | 372 (6.9) |
| COPD | 38 (13.9) | 142 (13.9) | 600 (11.2) |
| Cancer | 29 (10.6) | 96 (9.4) | 605 (11.3) |
| Co-medication, n (%) | |||
| Oral anticoagulants | 120 (44.0) | 531 (52.0) | 1003 (18.7) |
| ADP receptor antagonist | 82 (30.0) | 78 (7.6) | 412 (7.7) |
| NSAIDs | 15 (5.5) | 87 (8.5) | 817 (15.2) |
| Beta-blockers | 122 (44.7) | 497 (48.6) | 1531 (28.5) |
| Statins | 120 (44.0) | 520 (50.9) | 1547 (28.8) |
| Calcium channel blockers | 58 (21.2) | 220 (21.5) | 1027 (19.1) |
| Renin-angiotensin system inhibitors | 109 (39.9) | 465 (45.5) | 1803 (33.5) |
| Diuretics | 63 (23.1) | 292 (28.6) | 1242 (23.1) |
Abbreviations: ADP, adenosine diphosphate; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; IE, infective endocarditis; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
Microbiological Etiology of Infective Endocarditis
For IE after TAVI, Streptococcus spp. (33.0%), Enterococcus spp. (28.9%), and S. aureus (22.7%) were most common. Notably, blood culture–negative IE was rare. In comparison, polymicrobial IE and blood culture–negative IE were more common for the other 2 groups (chi-square P < .05) (Figure 1; see Supplementary Table 3 for further granularity).
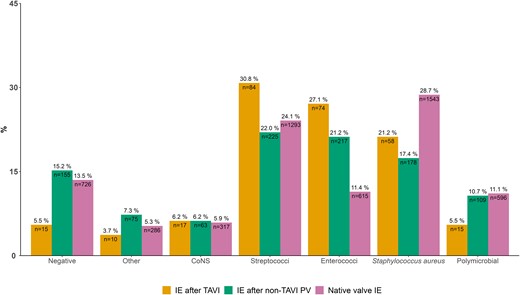
Microbiological etiology of 273 patients with IE after TAVI and 5376 with native valve IE. Percentages above the bars sum to 100% within groups. Numbers inside the bars sum to the total amount of cases within groups. Abbreviations: CoNS, coagulase-negative staphylococci; IE, infective endocarditis; PV, prosthetic valve; TAVI, transcatheter aortic valve implantation.
Microbiological Etiology According to Time From Valve Implantation/Substitution
Of the 273 patients with IE after TAVI, 119 (43.6%) were categorized as early IE after TAVI (≤12 m after TAVI) and the remaining 154 (56.4%) as late IE after TAVI (>12 m after TAVI). When comparing microbiological distribution, there was no significant difference (P = .686). However, among early IE after TAVI, Streptococcus spp. were numerically more prevalent and S. aureus was lower (Figure 2).
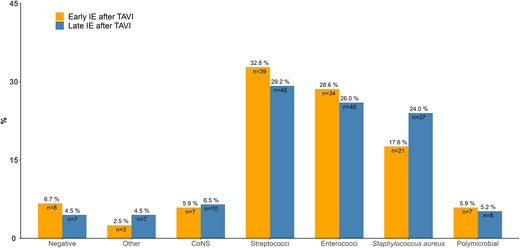
Microbiological etiology of 119 cases of early (≤12 m) IE after TAVI versus 153 cases of late (>12 m) IE after TAVI. Abbreviations: CoNS, coagulase-negative staphylococci; IE, infective endocarditis; TAVI, transcatheter aortic valve implantation.
In-Hospital Cardiac Surgery and Mortality
In-hospital cardiac surgery was performed on 3.7% of patients with IE after TAVI, 17.8% with IE after non-TAVI prosthetic valve, and 19.5% with native valve IE. The 90-day mortality was comparable between groups (Figure 3). For patients with IE after TAVI, the 5-year risk of death was higher compared with IE after non-TAVI prosthetic valve and native valve IE (Figure 4). In general, mortality was highest among patients with S. aureus regardless of in-hospital cardiac surgery (Supplementary Figures 1 and 2). In adjusted analysis, there was no interaction between exposure group and bacterial etiology on mortality (P-interaction = .274). Moreover, the 90-day mortality hazard ratio from the adjusted analysis was 1.13 (95% confidence interval [CI]: .86 to 1.49) for IE after non-TAVI prosthetic valve and 1.24 (95% CI: .97 to 1.60) for native valve IE compared with IE after TAVI. In 91-days–5-years analyses the numbers were .82 (95% CI: .65 to 1.04) and .97 (95% CI: .78 to 1.20), respectively.
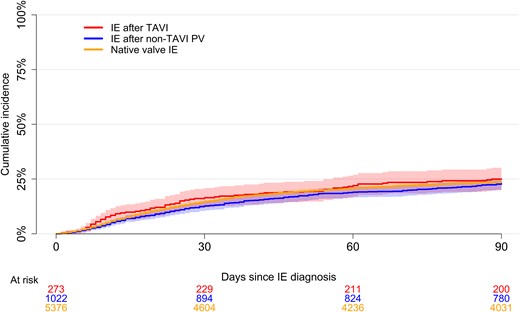
Short-term mortality. Ninety-day unadjusted mortality following IE after TAVI and native valve IE based on a reverse Kaplan-Meier curve. The shaded areas represent the 95% confidence interval for the IE after TAVI, IE after non-TAVI PV, and native valve IE groups, respectively. Abbreviations: IE, infective endocarditis; PV, prosthetic valve; TAVI, transcatheter aortic valve implantation.
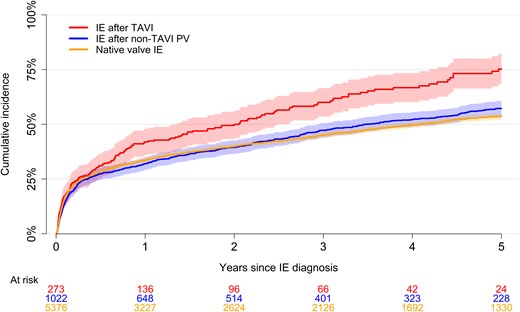
Long-term mortality. Five-year unadjusted mortality following IE after TAVI and native valve IE based on a reverse Kaplan-Meier curve. The shaded areas represent the 95% confidence interval for the IE after TAVI, IE after non-TAVI PV, and native valve IE groups, respectively. Abbreviations: IE, infective endocarditis; PV, prosthetic valve; TAVI, transcatheter aortic valve implantation.
Matched Population
The matched population included the same 273 patients with IE after TAVI and 476 and 819 sex-, age-, and calendar year–matched patients with IE after non-TAVI prosthetic valve and native valve IE, respectively. Matching was balanced with standardized mean differences less than 0.05 for all variables except for calendar year of IE in 2021 (standardized mean difference = 0.07). Due to a low sample size, matching balance was less accurate for the IE after non-TAVI prosthetic valve group. Cardiovascular comorbidity burden remained higher for patients with IE after TAVI (Supplementary Table 4). When comparing bacterial etiology, Streptococcus spp. remained highest in the IE after TAVI group and Enterococcus spp. was high for both IE after TAVI and IE after non-TAVI prosthetic valve. Staphylococcus aureus remained high for patients with matched native valve IE (Figure 5). The short-term mortality was highest for matched patients with native valve IE, with curves crossing at around 3 years (Supplementary Figure 3).
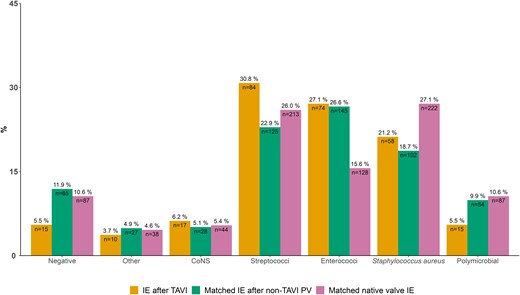
Microbiological etiology of 273 patients with IE after TAVI and 819 matched patients with native valve IE. Matching criteria were age, sex, and calendar year of IE. Percentages above the bars sum to 100% within groups. Numbers inside the bars sum to the total amount of cases within groups. Abbreviations: CoNS, coagulase-negative staphylococci; IE, infective endocarditis; PV, prosthetic valve; TAVI, transcatheter aortic valve implantation.
DISCUSSION
In this contemporary nationwide cohort study comparing patient characteristics, microbiology, and prognosis of patients with IE after TAVI with patients with native valve IE, the main findings can be summarized as follows: (1) patients with IE after TAVI are older and more comorbid, (2) IE after TAVI is more often associated with Enterococcus spp. and Streptococcus spp., (3) the bacterial etiology of early IE after TAVI is comparable to that of late IE after TAVI, and (4) unadjusted mortality is higher for IE after TAVI, but there was no difference in adjusted mortality.
Patient Characteristics
Stortecky et al [11] used Swiss data (February 2011 to July 2018) and identified 149 cases of IE after TAVI. Here, patients with IE after TAVI were slightly younger at a mean age of 80.1 years compared with a median age of 82 years in our study population. Data from international TAVI registries (June 2005 to October 2015) reported a median age of 80 years but also found that 25% of patients with IE after TAVI were younger than 59 years and 25% were older than 91 years [9]. However, this was based only on data from selected centers that accepted to report data to the registry. As such, the generalizability of patient characteristics of this study is limited. Bjursten et al [12] (January 2008 to September 2018) included 103 cases of IE after TAVI among 4336 patients from Swedish data with a mean age in the IE after TAVI group of 82 years, which is in accordance with our findings. Altogether, patients with IE after TAVI are markedly older and more comorbid than patients with other IE. Regardless of age, comorbidity burden is significant in all populations of IE after TAVI, which is important to consider as the prognosis and available treatment options (ie, valve surgery) may be limited due to the patient’s age and degree of frailty.
Microbiological Etiology
Previous studies have reported the microbiological etiology of patients with IE after TAVI [9–15]. Overall, only few studies were nationwide with data on blood cultures, which limits generalizability [11, 12, 15]. Thus, our large nationwide sample size represents a valuable contribution. Moreover, we had access to all blood cultures and the results of these directly from all Departments of Clinical Microbiology in Denmark. We found Streptococcus spp. and Enterococcus spp. to be more prevalent among patients with IE after TAVI compared with native valve IE. Streptococcus spp. were the most common bacteria in IE after TAVI. Other studies have found Streptococcus spp. to be prevalent in 28.8% to 34.0% of cases [10–12]. However, Regueiro et al [9] only found Streptococcus spp. in 15.5% of patients with IE after TAVI. The proportion of Enterococcus spp. associated with IE after TAVI in previous larger studies ranges from 20.4% to 26.2% [3].
Notably, we found that the differences in bacterial etiology remained even after matching on sex, age, and calendar year of IE. This suggests that other factors also contribute to the differences in bacterial etiology, despite previous studies highlighting that the incidence of Enterococcus spp. endocarditis increases with age [30]. A possible explanation for the higher proportion of Enterococcus spp. could be that most TAVI procedures are performed using a transfemoral access [31]. However, we also found a high proportion of Enterococcus spp. among patients with late IE after TAVI. Comorbidity burden remained higher after matching in the IE after TAVI group. Thus, factors such as poor bladder function, which may lead to an increased use of urinary catheterization, or procedures involving the gastrointestinal system both could predispose to infections leading to IE. The prevalence of S. aureus among patients with IE after TAVI was 22.7%. This is in line with findings from previous studies, with estimates ranging from 21.5% to 23.3% [9–12].
Data on early versus late IE after TAVI remain sparse; we found no significant difference in bacterial etiology of early versus late IE after TAVI. Stortecky et al [11] reported a higher prevalence of Enterococcus spp. in early versus late IE after TAVI and a lower prevalence of S. aureus among early versus late IE after TAVI. However, fewer Streptococcus spp. were present in that study in early versus late TAVI IE, although this was estimated among 56 late IE after TAVI cases compared with 154 in our study. Bjursten et al [12] reported data on 51 early and 52 late IE after TAVI cases. Here, more S. aureus and similar proportions of Enterococcus spp. and Streptococcus spp. were reported in early versus late IE after TAVI [12]. Nevertheless, Enterococcus spp. and Streptococcus spp. are still common pathogens in late IE after TAVI, which has implications on the empirical antibiotic treatment strategy.
Surgery and Mortality Associated With Infective Endocarditis After Transcatheter Aortic Valve Implantation
The higher comorbidity and advanced age of patients with IE after TAVI found in this study were also reflected in the low number of patients with IE after TAVI undergoing in-hospital cardiac surgery (3.5%) compared with other IE groups. This is despite a previous review highlighting that 50.0% or more of patients with IE after TAVI have an indication for surgical intervention, yet only 16.4% or fewer eventually undergo surgery [3]. The 90-day mortality was high, yet comparable between groups, with mortality curves separating at around half a year after diagnosis of IE. While long-term unadjusted mortality rates were higher for IE after TAVI, the adjusted models showed no increased mortality rates of IE after TAVI compared with other IE groups. Moreover, the unadjusted mortality rates were initially higher for matched patients with native valve IE compared with those with IE after TAVI, with the curves converging at around 3 years. This could be due to a higher prevalence of S. aureus–associated IE among matched patients with native valve IE initially, while the long-term prognosis is poor for those with IE after TAVI, possibly due to a higher comorbidity burden and thus higher competing risk of death.
Clinical Implications
In the 2017 American College of Cardiology expert consensus, prophylactic antibiotics are recommended prior to TAVI procedures, although no specific regimen is suggested [32], and previous studies report a high use of periprocedural antibiotics [11]. Importantly, although often administered, only about half of patients received antibiotics targeting the causative microorganisms [11]. Our study adds valuable information on the pathogens associated with overall, early, and late IE after TAVI. These results, in addition to those published, may guide future decision making on antibiotic prophylaxis—especially in the absence of randomized controlled trials. As we have shown, the pathogens associated with IE after TAVI differ from those of native valve IE. Finally, these data represent an important contribution to the epidemiological monitoring of disease, which has been poorly examined. With TAVI procedure rates increasing worldwide, the clinical impact of IE after TAVI will only grow in the future.
Strengths and Limitations
The main strength of the study is its large sample size, the possibility of virtually complete follow-up, and the nationwide data sources, which did not rely on voluntary data reporting, thus greatly improving the generalizability of the study.
However, some limitations apply. Although blood cultures were available, we were unable to determine the anatomical location of the endocarditis (eg, prosthetic valve endocarditis, endocarditis in a cardiac implantable electronic device). We lacked data on procedural characteristics of the TAVI group. Data on some factors associated with IE were unavailable (eg, dental status and long-term catheter use). Data on the type of valve prosthesis and echocardiographs and positron emission tomography scans were unavailable.
Conclusions
Patients with IE after TAVI are older and more comorbid, Enterococcus spp. and Streptococcus spp. are often present, and they are rarely blood culture negative compared with other groups of IE. While long-term unadjusted mortality rates were higher for IE after TAVI, the adjusted mortality rates were comparable. Future studies regarding antibiotic prophylaxis strategies covering enterococci should be considered in this setting.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
Author notes
Potential conflicts of interest. L. K.: Lecture fees from AstraZeneca, Bayer, Boehringer, Novartis, and Novo. H. B.: Lecture fees from Amgen, MSD, Bristol-Myers Squibb, and Sanofi. M. V.: Lecture fees from MyLab Oy. J. B. O.: Speaker’s honoraria or consultancy fees from Bayer, Bristol-Myers Squibb, and Pfizer. E. L. F.: Independent research grant from Novo Nordisk Foundation. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




