-
PDF
- Split View
-
Views
-
Cite
Cite
Gordon Chun Kau Chan, Grace Chung Yan Lui, Candy Ngai Sze Wong, Sindy Sin Ting Yip, Timothy Chun Man Li, Catherine Siu King Cheung, Ryan Kin Ho Sze, Cheuk Chun Szeto, Kai Ming Chow, Safety Profile and Clinical and Virological Outcomes of Nirmatrelvir-Ritonavir Treatment in Patients With Advanced Chronic Kidney Disease and Coronavirus Disease 2019, Clinical Infectious Diseases, Volume 77, Issue 10, 15 November 2023, Pages 1406–1412, https://doi.org/10.1093/cid/ciad371
Close - Share Icon Share
Abstract
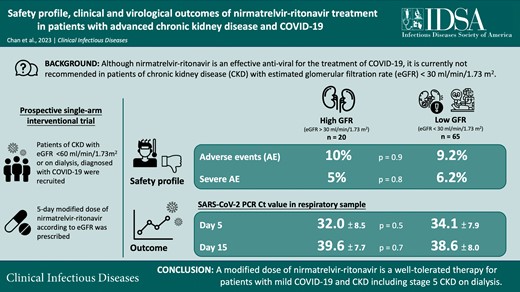
Nirmatrelvir-ritonavir is currently not recommended in patients with an estimated glomerular filtration rate (eGFR) <30 mL/minute/1.73 m2.
To determine the safety profile and clinical and virological outcomes of nirmatrelvir-ritonavir use at a modified dosage in adults with chronic kidney disease (CKD), a prospective, single-arm, interventional trial recruited patients with eGFR <30 mL/minute/1.73 m2 and on dialysis. Primary outcomes included safety profile, adverse/serious adverse events, and events leading to drug discontinuation. Disease symptoms, virological outcomes by serial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral polymerase chain reaction (PCR) tests, rapid antigen tests, and virological and symptomatic rebound were also recorded.
Fifty-nine (69.4%) of the 85 participants had stage 5 CKD and were on dialysis. Eighty (94.1%) completed the full treatment course; 9.4% and 5.9% had adverse and serious adverse events, and these were comparable between those with eGFR < or >30 mL/minute/1.73 m2. The viral load significantly decreased on days 5, 15, and 30 (P < .001 for all), and the reduction was consistent in the subgroup with eGFR <30 mL/minute/1.73 m2. Ten patients had virological rebound, which was transient and asymptomatic.
Among patients with CKD, a modified dose of nirmatrelvir-ritonavir is a well-tolerated therapy in mild COVID-19 as it can effectively suppress the SARS-CoV-2 viral load with a favorable safety profile. Virological and symptomatic rebound, although transient with low infectivity, may occur after treatment. Nirmatrelvir-ritonavir should be considered for use in patients with CKD, including stage 5 CKD on dialysis.
Clinical Trials Registration. Clinical Trials.gov; identifier: NCT05624840.
Treatment for coronavirus disease 2019 (COVID-19) treatment is not immune to the phenomena of “renalism,” indicative of therapeutic nihilism that causes patients with kidney disease to wait longer for effective interventions. Patients with chronic kidney disease (CKD), especially those on dialysis, are at higher risk of severe COVID-19. Antivirals such as nirmatrelvir-ritonavir can effectively prevent disease progression [1] and is the only approved oral antiviral treatment for COVID-19 in some countries. However, most trials have excluded patients with CKD. Accordingly, nirmatrelvir-ritonavir has not received approval in patients with an estimated glomerular filtration rate (eGFR) of less than 30 mL/minute/1.75 m2. Pharmacokinetics-pharmacodynamics data support a dose-adjusted regimen due to a reduced clearance of nirmatrelvir with impaired kidney function [2]. Nevertheless, the clinical effectiveness and safety of such regimens at advanced stages of CKD have not been well explored. In the present study, we aimed to investigate the safety and effectiveness of dose-adjusted nirmatrelvir-ritonavir treatment in patients with CKD and an eGFR of less than 30 mL/minute/1.75 m2.
METHODS
This is a prospective, single-arm interventional study. Adult patients with CKD (with eGFR <60 mL/min/1.75 m2) who had COVID-19 infection with symptom onset at less than 5 days were recruited from 1 November 2022 to 31 January 2023. Patients who had severe disease, including patients who require supplemental oxygen therapy, and those with contraindications to nirmatrelvir-ritonavir were excluded. Patients with an eGFR above 60 mL/minute/1.75 m2 were recruited as controls. Written consent was obtained from all recruited participants. The study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (reference number CREC-2022.361). All study procedures were in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice. The study was registered at ClinicalTrials.gov (NCT05624840).
The diagnosis of COVID-19 was established by a positive rapid antigen test (RAT) (INDICAID rapid test; PHASE Scientific International Ltd, Hong Kong) or detectable quantitative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by polymerase chain reaction (PCR) test in nasopharyngeal, nasal swab, or deep throat saliva. The clinical state was evaluated by the World Health Organization’s Clinical Progression Scale (WHO-CPS). The participants’ biochemical parameters, concurrent medical diseases, and medications were recorded. The Charlson Comorbidity Index was used to measure the comorbidity load. A modified treatment dose was prescribed for 5 days according to their eGFR calculated by the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation, as summarized in Figure 1.
![Flow diagram. Dosage recommendations from Hiremath et al [14]. Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/77/10/10.1093_cid_ciad371/2/m_ciad371f1.jpeg?Expires=1750185096&Signature=uetSBrAMmcEEQYr79WVKEhXgNAWXs1Cn0nPO-M62sTX6oq-JBpTNGBo1RXwVQXK-2FrLpNq9kyH5zgofikksSaRR4Nf~LKNWxV7bJsZ2qE3LTKuMV4lfMeRFgdVG1yfd1ccDMxoQievh8lLewATFvYK0SoCbT7rj6U8B-dy2ovbNIvpP~IYhpB1gl229xaNMjTx-FwG9kpLuWIBtdW6H5Mm2yhvfwEV-JTp68bKQOtbBFzOdTyJJ6ie2kCejokvxkr5gc7R~buUKMD~ABDn4qmZAkZ2FcVT5amRPnbtKLo8X0TVpAgWJdIY27PDkDXCp7VVxXanYb9xbreM12oEhjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Flow diagram. Dosage recommendations from Hiremath et al [14]. Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
All patients were followed for 30 days after recruitment. The RAT and PCR tests were repeated at days 5, 15, and 30, and when symptomatic. Clinically significant viral rebound was defined as a reduction in PCR cycle threshold (Ct) value by at least 3 and with a value less than 30 [3]. Disease symptoms were recorded daily by a structured symptom diary developed according to the Centers for Disease Control and Prevention (CDC) criteria [4].
Statistical analysis was performed by SPSS for Mac software version 27 (SPSS, IBM Corporation, Armonk, NY, USA). Descriptive data are presented as means ± standard deviations if normally distributed or medians (interquartile range) if non–normally distributed. Clinical parameters were compared by Student's t test or chi-square test, as appropriate. To explore the profile of nirmatrelvir-ritonavir beyond the approved eGFR range, participants were further classified into low- and high-eGFR groups based on the eGFR below and above 30 mL/minute/1.73 m2, respectively, for further subgroup analyses. To assess the variation in low-/high-eGFR groups with Ct values across time, with adjustment of potential confounding variables of age, sex, days of measurement from treatment initiation, duration of symptoms before treatment, WHO-CPS, COVID-19 vaccination doses, and days from the last COVID-19 vaccination, generalized estimating equation (GEE) linear models with autoregressive working correlation matrices were performed. Sensitivity analyses were performed with unstructured working correlation matrices in the GEE model. Multivariate regression models, with the inclusion of the covariates mentioned and the Charlson Comorbidity Index, were further built to identify predictors of PCR negativity, and moderate/severe disease by WHO-CPS at days 5, 15, and 30. All statistical tests were 2-sided, and P < .05 was considered statistically significant.
RESULTS
Eighty-six consecutive patients who met the inclusion criteria were screened. One patient refused to participate in the study. Thus, 85 patients consented and were recruited: 75 patients with CKD and 10 patients as controls. The study flow is summarized in Figure 1, and their baseline clinical and biochemical characteristics are summarized in Table 1 and Supplementary Table 1. The mean age was 64.2 ± 12.2 years, and 50 (58.8%) were male. The proportions of patients vaccinated with 0, 1, 2, and at least 3 doses of COVID-19 vaccines were 4.7%, 1.2%, 5.9%, and 88.2%, respectively. Fifty-nine patients (69.4%) had stage 5 CKD and were on dialysis. At baseline, patients from the low-GFR group had lower WHO-CPS scores and lymphocyte and hemoglobin levels compared with those in the high-GFR group (Table 1). They also had a greater comorbidity load (Supplementary Table 1).
Baseline and Serial Characteristics of Recruited Subjects According to Estimated Glomerular Filtration Rate
| . | Baseline . | Day 5 . | Day 15 . | Day 30 . | ||||
|---|---|---|---|---|---|---|---|---|
| . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . |
| SARS-CoV-2 RT-PCR Ct value | 21.5 ± 5.3 | 21.4 ± 5.2 | 32.0 ± 8.5 | 34.1 ± 7.9 | 39.6 ± 7.7 | 38.6 ± 8.0 | 44.1 ± 2.6 | 43.5 ± 4.3 |
| SARS-CoV-2 RT-PCR Ct value >30 | 0 (0%) | 0 (0%) | 8 (57.1%) | 29 (61.7%) | 11 (91.7%) | 46 (85.2%) | 17 (100%) | 56 (96.6%) |
| Undetectable SARS-CoV-2 PCR | 0 (0%) | 0 (0%) | 3 (21.4%) | 11 (23.4%) | 7 (58.3%) | 29 (53.7%) | 15 (88.2%) | 51 (87.9%) |
| Positive RAT | 20 (100%) | 65 (100%) | 6 (30.0%) | 26 (40.0%) | 1 (5.0%) | 1 (1.5%) | 0 (0%) | 0 (0%) |
| WHO-CPS classification | ||||||||
| Uninfected (WHO-CPS 0) | 0 (0%) | 0 (0%) | 3 (15%) | 5 (7.7%) | 7 (35%) | 25 (38.5%) | 15 (75%) | 49 (75.4%) |
| Ambulatory mild disease (WHO-CPS 1–3) | 3 (15%) | 50 (76.9%)* | 6 (30%) | 50 (76.9%)* | 13 (65%) | 36 (55.4%) | 5 (25%) | 16 (24.6%) |
| Hospitalized: moderate disease (WHO-CPS 4–5) | 17 (85%) | 15 (23.1%)* | 11 (55%) | 9 (13.8%)* | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hospitalized: severe disease (WHO-CPS 6–9) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 4 (6.2%) | 0 (0%) | 0 (0%) |
| Laboratory parameters | ||||||||
| Hemoglobin (g/dL) | 12.9 ± 1.5 | 9.8 ± 1.7* | 12.7 ± 1.7 | 9.9 ± 1.6* | 12.3 ± 1.3 | 9.9 ± 1.5* | 12.6 ± 1.3 | 9.8 ± 1.6* |
| White blood cell count (109/L) | 12.5 ± 27.1 | 5.3 ± 2.4** | 6.7 ± 3.3 | 5.6 ± 2.1 | 7.1 ± 1.4 | 7.3 ± 3.2 | 7.0 ± 1.5 | 6.9 ± 2.4 |
| Neutrophils (109/L) | 4.3 ± 2.6 | 3.7 ± 1.9 | 4.3 ± 3.3 | 3.9 ± 1.8 | 4.4 ± 1.1 | 5.2 ± 2.8 | 4.2 ± 1.3 | 4.7 ± 2.0 |
| Lymphocytes (109/L) | 1.2 ± 0.6 | 0.8 ± 0.4* | 1.6 ± 0.8 | 1.1 ± 0.4* | 1.9 ± 0.7 | 1.2 ± 0.6* | 1.9 ± 0.7 | 1.2 ± 0.8* |
| Platelets (109/L) | 172 ± 47 | 178 ± 71 | 168 ± 61 | 181 ± 74 | 279 ± 130 | 233 ± 100 | 209 ± 71 | 219 ± 88 |
| Urea (mmol/L) | 6.0 ± 2.5 | 24.5 ± 8.4* | 7.2 ± 3.1 | 28.2 ± 8.5* | 6.9 ± 2.9 | 24.4 ± 9.1* | 7.2 ± 2.4 | 23.1 ± 9.4* |
| Creatinine (µmol/L) | 103 ± 33 | 808 ± 305* | 107 ± 37 | 959 ± 368* | 94 ± 32 | 857 ± 320* | 95 ± 33 | 762 ± 318* |
| Albumin (g/L) | 32.4 ± 5.3 | 30.6 ± 5.4 | 32.1 ± 5.3 | 31.7 ± 7.7 | 32.9 ± 5.4 | 30.9 ± 4.7 | 35.2 ± 5.1 | 30.7 ± 4.5* |
| C-reactive protein (mg/L) | 26.3 ± 15.9 | 23.9 ± 27.5 | 14.9 ± 14.7 | 22.8 ± 36.1 | 7.7 ± 7.9 | 10.8 ± 15.4 | 5.0 ± 6.5 | 9.5 ± 14.2 |
| D-dimer (ng/mL) | 976 ± 1313 | 1242 ± 1323 | 1497 ± 2245 | 1109 ± 638 | 793 ± 529 | 1513 ± 1641 | 923 ± 1139 | 1432 ± 1151 |
| . | Baseline . | Day 5 . | Day 15 . | Day 30 . | ||||
|---|---|---|---|---|---|---|---|---|
| . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . |
| SARS-CoV-2 RT-PCR Ct value | 21.5 ± 5.3 | 21.4 ± 5.2 | 32.0 ± 8.5 | 34.1 ± 7.9 | 39.6 ± 7.7 | 38.6 ± 8.0 | 44.1 ± 2.6 | 43.5 ± 4.3 |
| SARS-CoV-2 RT-PCR Ct value >30 | 0 (0%) | 0 (0%) | 8 (57.1%) | 29 (61.7%) | 11 (91.7%) | 46 (85.2%) | 17 (100%) | 56 (96.6%) |
| Undetectable SARS-CoV-2 PCR | 0 (0%) | 0 (0%) | 3 (21.4%) | 11 (23.4%) | 7 (58.3%) | 29 (53.7%) | 15 (88.2%) | 51 (87.9%) |
| Positive RAT | 20 (100%) | 65 (100%) | 6 (30.0%) | 26 (40.0%) | 1 (5.0%) | 1 (1.5%) | 0 (0%) | 0 (0%) |
| WHO-CPS classification | ||||||||
| Uninfected (WHO-CPS 0) | 0 (0%) | 0 (0%) | 3 (15%) | 5 (7.7%) | 7 (35%) | 25 (38.5%) | 15 (75%) | 49 (75.4%) |
| Ambulatory mild disease (WHO-CPS 1–3) | 3 (15%) | 50 (76.9%)* | 6 (30%) | 50 (76.9%)* | 13 (65%) | 36 (55.4%) | 5 (25%) | 16 (24.6%) |
| Hospitalized: moderate disease (WHO-CPS 4–5) | 17 (85%) | 15 (23.1%)* | 11 (55%) | 9 (13.8%)* | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hospitalized: severe disease (WHO-CPS 6–9) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 4 (6.2%) | 0 (0%) | 0 (0%) |
| Laboratory parameters | ||||||||
| Hemoglobin (g/dL) | 12.9 ± 1.5 | 9.8 ± 1.7* | 12.7 ± 1.7 | 9.9 ± 1.6* | 12.3 ± 1.3 | 9.9 ± 1.5* | 12.6 ± 1.3 | 9.8 ± 1.6* |
| White blood cell count (109/L) | 12.5 ± 27.1 | 5.3 ± 2.4** | 6.7 ± 3.3 | 5.6 ± 2.1 | 7.1 ± 1.4 | 7.3 ± 3.2 | 7.0 ± 1.5 | 6.9 ± 2.4 |
| Neutrophils (109/L) | 4.3 ± 2.6 | 3.7 ± 1.9 | 4.3 ± 3.3 | 3.9 ± 1.8 | 4.4 ± 1.1 | 5.2 ± 2.8 | 4.2 ± 1.3 | 4.7 ± 2.0 |
| Lymphocytes (109/L) | 1.2 ± 0.6 | 0.8 ± 0.4* | 1.6 ± 0.8 | 1.1 ± 0.4* | 1.9 ± 0.7 | 1.2 ± 0.6* | 1.9 ± 0.7 | 1.2 ± 0.8* |
| Platelets (109/L) | 172 ± 47 | 178 ± 71 | 168 ± 61 | 181 ± 74 | 279 ± 130 | 233 ± 100 | 209 ± 71 | 219 ± 88 |
| Urea (mmol/L) | 6.0 ± 2.5 | 24.5 ± 8.4* | 7.2 ± 3.1 | 28.2 ± 8.5* | 6.9 ± 2.9 | 24.4 ± 9.1* | 7.2 ± 2.4 | 23.1 ± 9.4* |
| Creatinine (µmol/L) | 103 ± 33 | 808 ± 305* | 107 ± 37 | 959 ± 368* | 94 ± 32 | 857 ± 320* | 95 ± 33 | 762 ± 318* |
| Albumin (g/L) | 32.4 ± 5.3 | 30.6 ± 5.4 | 32.1 ± 5.3 | 31.7 ± 7.7 | 32.9 ± 5.4 | 30.9 ± 4.7 | 35.2 ± 5.1 | 30.7 ± 4.5* |
| C-reactive protein (mg/L) | 26.3 ± 15.9 | 23.9 ± 27.5 | 14.9 ± 14.7 | 22.8 ± 36.1 | 7.7 ± 7.9 | 10.8 ± 15.4 | 5.0 ± 6.5 | 9.5 ± 14.2 |
| D-dimer (ng/mL) | 976 ± 1313 | 1242 ± 1323 | 1497 ± 2245 | 1109 ± 638 | 793 ± 529 | 1513 ± 1641 | 923 ± 1139 | 1432 ± 1151 |
Data are expressed as means ± standard deviations or no. (%) and compared by Student's t-test or chi-square test as appropriate.
Abbreviations: Ct, cycle threshold; GFR, glomerular filtration rate; PCR, polymerase chain reaction; RAT, rapid antigen test; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO-CPS, World Health Organization's Clinical Progression Scale.
*P < .001 compared with the high-GFR group.
**P < .05 compared with the high-GFR group.
Baseline and Serial Characteristics of Recruited Subjects According to Estimated Glomerular Filtration Rate
| . | Baseline . | Day 5 . | Day 15 . | Day 30 . | ||||
|---|---|---|---|---|---|---|---|---|
| . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . |
| SARS-CoV-2 RT-PCR Ct value | 21.5 ± 5.3 | 21.4 ± 5.2 | 32.0 ± 8.5 | 34.1 ± 7.9 | 39.6 ± 7.7 | 38.6 ± 8.0 | 44.1 ± 2.6 | 43.5 ± 4.3 |
| SARS-CoV-2 RT-PCR Ct value >30 | 0 (0%) | 0 (0%) | 8 (57.1%) | 29 (61.7%) | 11 (91.7%) | 46 (85.2%) | 17 (100%) | 56 (96.6%) |
| Undetectable SARS-CoV-2 PCR | 0 (0%) | 0 (0%) | 3 (21.4%) | 11 (23.4%) | 7 (58.3%) | 29 (53.7%) | 15 (88.2%) | 51 (87.9%) |
| Positive RAT | 20 (100%) | 65 (100%) | 6 (30.0%) | 26 (40.0%) | 1 (5.0%) | 1 (1.5%) | 0 (0%) | 0 (0%) |
| WHO-CPS classification | ||||||||
| Uninfected (WHO-CPS 0) | 0 (0%) | 0 (0%) | 3 (15%) | 5 (7.7%) | 7 (35%) | 25 (38.5%) | 15 (75%) | 49 (75.4%) |
| Ambulatory mild disease (WHO-CPS 1–3) | 3 (15%) | 50 (76.9%)* | 6 (30%) | 50 (76.9%)* | 13 (65%) | 36 (55.4%) | 5 (25%) | 16 (24.6%) |
| Hospitalized: moderate disease (WHO-CPS 4–5) | 17 (85%) | 15 (23.1%)* | 11 (55%) | 9 (13.8%)* | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hospitalized: severe disease (WHO-CPS 6–9) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 4 (6.2%) | 0 (0%) | 0 (0%) |
| Laboratory parameters | ||||||||
| Hemoglobin (g/dL) | 12.9 ± 1.5 | 9.8 ± 1.7* | 12.7 ± 1.7 | 9.9 ± 1.6* | 12.3 ± 1.3 | 9.9 ± 1.5* | 12.6 ± 1.3 | 9.8 ± 1.6* |
| White blood cell count (109/L) | 12.5 ± 27.1 | 5.3 ± 2.4** | 6.7 ± 3.3 | 5.6 ± 2.1 | 7.1 ± 1.4 | 7.3 ± 3.2 | 7.0 ± 1.5 | 6.9 ± 2.4 |
| Neutrophils (109/L) | 4.3 ± 2.6 | 3.7 ± 1.9 | 4.3 ± 3.3 | 3.9 ± 1.8 | 4.4 ± 1.1 | 5.2 ± 2.8 | 4.2 ± 1.3 | 4.7 ± 2.0 |
| Lymphocytes (109/L) | 1.2 ± 0.6 | 0.8 ± 0.4* | 1.6 ± 0.8 | 1.1 ± 0.4* | 1.9 ± 0.7 | 1.2 ± 0.6* | 1.9 ± 0.7 | 1.2 ± 0.8* |
| Platelets (109/L) | 172 ± 47 | 178 ± 71 | 168 ± 61 | 181 ± 74 | 279 ± 130 | 233 ± 100 | 209 ± 71 | 219 ± 88 |
| Urea (mmol/L) | 6.0 ± 2.5 | 24.5 ± 8.4* | 7.2 ± 3.1 | 28.2 ± 8.5* | 6.9 ± 2.9 | 24.4 ± 9.1* | 7.2 ± 2.4 | 23.1 ± 9.4* |
| Creatinine (µmol/L) | 103 ± 33 | 808 ± 305* | 107 ± 37 | 959 ± 368* | 94 ± 32 | 857 ± 320* | 95 ± 33 | 762 ± 318* |
| Albumin (g/L) | 32.4 ± 5.3 | 30.6 ± 5.4 | 32.1 ± 5.3 | 31.7 ± 7.7 | 32.9 ± 5.4 | 30.9 ± 4.7 | 35.2 ± 5.1 | 30.7 ± 4.5* |
| C-reactive protein (mg/L) | 26.3 ± 15.9 | 23.9 ± 27.5 | 14.9 ± 14.7 | 22.8 ± 36.1 | 7.7 ± 7.9 | 10.8 ± 15.4 | 5.0 ± 6.5 | 9.5 ± 14.2 |
| D-dimer (ng/mL) | 976 ± 1313 | 1242 ± 1323 | 1497 ± 2245 | 1109 ± 638 | 793 ± 529 | 1513 ± 1641 | 923 ± 1139 | 1432 ± 1151 |
| . | Baseline . | Day 5 . | Day 15 . | Day 30 . | ||||
|---|---|---|---|---|---|---|---|---|
| . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . | High-GFR Group . | Low-GFR Group . |
| SARS-CoV-2 RT-PCR Ct value | 21.5 ± 5.3 | 21.4 ± 5.2 | 32.0 ± 8.5 | 34.1 ± 7.9 | 39.6 ± 7.7 | 38.6 ± 8.0 | 44.1 ± 2.6 | 43.5 ± 4.3 |
| SARS-CoV-2 RT-PCR Ct value >30 | 0 (0%) | 0 (0%) | 8 (57.1%) | 29 (61.7%) | 11 (91.7%) | 46 (85.2%) | 17 (100%) | 56 (96.6%) |
| Undetectable SARS-CoV-2 PCR | 0 (0%) | 0 (0%) | 3 (21.4%) | 11 (23.4%) | 7 (58.3%) | 29 (53.7%) | 15 (88.2%) | 51 (87.9%) |
| Positive RAT | 20 (100%) | 65 (100%) | 6 (30.0%) | 26 (40.0%) | 1 (5.0%) | 1 (1.5%) | 0 (0%) | 0 (0%) |
| WHO-CPS classification | ||||||||
| Uninfected (WHO-CPS 0) | 0 (0%) | 0 (0%) | 3 (15%) | 5 (7.7%) | 7 (35%) | 25 (38.5%) | 15 (75%) | 49 (75.4%) |
| Ambulatory mild disease (WHO-CPS 1–3) | 3 (15%) | 50 (76.9%)* | 6 (30%) | 50 (76.9%)* | 13 (65%) | 36 (55.4%) | 5 (25%) | 16 (24.6%) |
| Hospitalized: moderate disease (WHO-CPS 4–5) | 17 (85%) | 15 (23.1%)* | 11 (55%) | 9 (13.8%)* | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hospitalized: severe disease (WHO-CPS 6–9) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 4 (6.2%) | 0 (0%) | 0 (0%) |
| Laboratory parameters | ||||||||
| Hemoglobin (g/dL) | 12.9 ± 1.5 | 9.8 ± 1.7* | 12.7 ± 1.7 | 9.9 ± 1.6* | 12.3 ± 1.3 | 9.9 ± 1.5* | 12.6 ± 1.3 | 9.8 ± 1.6* |
| White blood cell count (109/L) | 12.5 ± 27.1 | 5.3 ± 2.4** | 6.7 ± 3.3 | 5.6 ± 2.1 | 7.1 ± 1.4 | 7.3 ± 3.2 | 7.0 ± 1.5 | 6.9 ± 2.4 |
| Neutrophils (109/L) | 4.3 ± 2.6 | 3.7 ± 1.9 | 4.3 ± 3.3 | 3.9 ± 1.8 | 4.4 ± 1.1 | 5.2 ± 2.8 | 4.2 ± 1.3 | 4.7 ± 2.0 |
| Lymphocytes (109/L) | 1.2 ± 0.6 | 0.8 ± 0.4* | 1.6 ± 0.8 | 1.1 ± 0.4* | 1.9 ± 0.7 | 1.2 ± 0.6* | 1.9 ± 0.7 | 1.2 ± 0.8* |
| Platelets (109/L) | 172 ± 47 | 178 ± 71 | 168 ± 61 | 181 ± 74 | 279 ± 130 | 233 ± 100 | 209 ± 71 | 219 ± 88 |
| Urea (mmol/L) | 6.0 ± 2.5 | 24.5 ± 8.4* | 7.2 ± 3.1 | 28.2 ± 8.5* | 6.9 ± 2.9 | 24.4 ± 9.1* | 7.2 ± 2.4 | 23.1 ± 9.4* |
| Creatinine (µmol/L) | 103 ± 33 | 808 ± 305* | 107 ± 37 | 959 ± 368* | 94 ± 32 | 857 ± 320* | 95 ± 33 | 762 ± 318* |
| Albumin (g/L) | 32.4 ± 5.3 | 30.6 ± 5.4 | 32.1 ± 5.3 | 31.7 ± 7.7 | 32.9 ± 5.4 | 30.9 ± 4.7 | 35.2 ± 5.1 | 30.7 ± 4.5* |
| C-reactive protein (mg/L) | 26.3 ± 15.9 | 23.9 ± 27.5 | 14.9 ± 14.7 | 22.8 ± 36.1 | 7.7 ± 7.9 | 10.8 ± 15.4 | 5.0 ± 6.5 | 9.5 ± 14.2 |
| D-dimer (ng/mL) | 976 ± 1313 | 1242 ± 1323 | 1497 ± 2245 | 1109 ± 638 | 793 ± 529 | 1513 ± 1641 | 923 ± 1139 | 1432 ± 1151 |
Data are expressed as means ± standard deviations or no. (%) and compared by Student's t-test or chi-square test as appropriate.
Abbreviations: Ct, cycle threshold; GFR, glomerular filtration rate; PCR, polymerase chain reaction; RAT, rapid antigen test; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO-CPS, World Health Organization's Clinical Progression Scale.
*P < .001 compared with the high-GFR group.
**P < .05 compared with the high-GFR group.
Adverse and Severe Adverse Events
The details of adverse and serious adverse events are summarized in Table 2. In short, 8 (9.4%) and 5 (5.9%) patients experienced adverse and serious adverse events, respectively. Three (3.5%) patients’ adverse events were gastrointestinal symptoms that were attributed to nirmatrelvir-ritonavir. Five of the patients (5.9%) discontinued nirmatrelvir-ritonavir at 2.2 ± 1.6 days: 3 because of severe COVID-19 pneumonia, which required switching to remdesivir according to local treatment guidelines, and 2 because of gastrointestinal symptoms.
Summary of Severe Adverse Events, Adverse Events, and Events Leading to Discontinuation of Drugs
| . | All (N = 85) . | High-GFR Group (n = 20) . | Low-GFR Group (n = 65) . | P . |
|---|---|---|---|---|
| Events that emerged during study period, patient no. (%) | ||||
| Any adverse events | 8 (9.4%) | 2 (10%) | 6 (9.2%) | .9 |
| Severe adverse eventsa | 5 (5.9%) | 1 (5%) | 4 (6.2%) | .8 |
| Discontinued drugs because of adverse eventb | 5 (5.9%) | 0 (0%) | 5 (7.7%) | |
| Events considered to be related to drug, patient no. (%) | ||||
| Any adverse events | 3 (3.5%) | 0 (0%) | 3 (4.6%) | |
| Severe adverse events | 0 (0%) | 0 (0%) | 0 (0%) | |
| Discontinued drugs because of adverse eventb | 2 (2.4%) | 0 (0%) | 2 (3.1%) | |
| . | All (N = 85) . | High-GFR Group (n = 20) . | Low-GFR Group (n = 65) . | P . |
|---|---|---|---|---|
| Events that emerged during study period, patient no. (%) | ||||
| Any adverse events | 8 (9.4%) | 2 (10%) | 6 (9.2%) | .9 |
| Severe adverse eventsa | 5 (5.9%) | 1 (5%) | 4 (6.2%) | .8 |
| Discontinued drugs because of adverse eventb | 5 (5.9%) | 0 (0%) | 5 (7.7%) | |
| Events considered to be related to drug, patient no. (%) | ||||
| Any adverse events | 3 (3.5%) | 0 (0%) | 3 (4.6%) | |
| Severe adverse events | 0 (0%) | 0 (0%) | 0 (0%) | |
| Discontinued drugs because of adverse eventb | 2 (2.4%) | 0 (0%) | 2 (3.1%) | |
Abbreviations: COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate.
aAll reported severe adverse events were related to severe COVID-19 pneumonia.
bAll reported events leading to discontinuation of drugs were related to gastrointestinal symptoms.
Summary of Severe Adverse Events, Adverse Events, and Events Leading to Discontinuation of Drugs
| . | All (N = 85) . | High-GFR Group (n = 20) . | Low-GFR Group (n = 65) . | P . |
|---|---|---|---|---|
| Events that emerged during study period, patient no. (%) | ||||
| Any adverse events | 8 (9.4%) | 2 (10%) | 6 (9.2%) | .9 |
| Severe adverse eventsa | 5 (5.9%) | 1 (5%) | 4 (6.2%) | .8 |
| Discontinued drugs because of adverse eventb | 5 (5.9%) | 0 (0%) | 5 (7.7%) | |
| Events considered to be related to drug, patient no. (%) | ||||
| Any adverse events | 3 (3.5%) | 0 (0%) | 3 (4.6%) | |
| Severe adverse events | 0 (0%) | 0 (0%) | 0 (0%) | |
| Discontinued drugs because of adverse eventb | 2 (2.4%) | 0 (0%) | 2 (3.1%) | |
| . | All (N = 85) . | High-GFR Group (n = 20) . | Low-GFR Group (n = 65) . | P . |
|---|---|---|---|---|
| Events that emerged during study period, patient no. (%) | ||||
| Any adverse events | 8 (9.4%) | 2 (10%) | 6 (9.2%) | .9 |
| Severe adverse eventsa | 5 (5.9%) | 1 (5%) | 4 (6.2%) | .8 |
| Discontinued drugs because of adverse eventb | 5 (5.9%) | 0 (0%) | 5 (7.7%) | |
| Events considered to be related to drug, patient no. (%) | ||||
| Any adverse events | 3 (3.5%) | 0 (0%) | 3 (4.6%) | |
| Severe adverse events | 0 (0%) | 0 (0%) | 0 (0%) | |
| Discontinued drugs because of adverse eventb | 2 (2.4%) | 0 (0%) | 2 (3.1%) | |
Abbreviations: COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate.
aAll reported severe adverse events were related to severe COVID-19 pneumonia.
bAll reported events leading to discontinuation of drugs were related to gastrointestinal symptoms.
Symptoms and Clinical Outcomes
Cough (n = 18; 21.2%) and fatigue (n = 10; 11.8%) were the most frequent symptoms reported during treatment. With treatment, the disease symptoms resolved in 18.3 ± 11.1 days, with no difference in resolution time between GFR groups (19.4 ± 11.1 vs 17.9 ± 11.2 days; P = .7). 48 (56.5%) and 85 (100%) patients were asymptomatic on day 15 and 30, respectively. The WHO-CPS disease severity distribution is summarized in Table 1 and Supplementary Figure 1. In essence, low-GFR-group participants had milder disease by WHO-CPS at day 5 (P = .001), although the difference was not significant at day 15 and 30. In the multivariate analysis, the baseline eGFR did not predict clinical disease severity at days 5, 15, and 30 (P = .09, .9, and .8, respectively). In this model, the number of COVID-19 vaccinations predicted the WHO-CPS score at day 15 (beta: −.35; 95% confidence interval [CI]: −.61 to −.08; P = .01), whereas the Charlson Comorbidity Index (beta: .04; 95% CI: .002 to .08; P = .04) and the time from the last COVID-19 vaccination (beta: .001; 95% CI: .001–.003; P = .006) predicted the WHO-CPS score at day 30.
Virological Outcomes
With treatment, the participants’ RATs were negative after a mean period of 5.4 ± 3.3 days. The PCR Ct value increased by 12.2 ± 7.4 to 33.6 ± 8.0 (P < .001), when over half of the participants (63.8%) had a PCR Ct value above 30 by day 5 (Supplementary Table 2). The Ct value further increased to 38.8 ± 7.9 at day 15 (P < .001) and to 43.7 ± 4.0 at day 30 (P < .001) (Figure 2 and Supplementary Table 2), with 84.6% and 97.2% of patients with PCR Ct values above 30, respectively. The low-GFR group had a similar RAT positivity rate and PCR Ct values at all time periods (Table 1). In the multivariate GEE models (Supplementary Table 3 and Supplementary Figure 2), there were no significant difference in the change in viral load between the low- and high-GFR group, whereas age, sex, and symptom duration were significant factors. With the same group of covariates, baseline eGFR did not predict PCR negativity at days 5, 15, and 30 in the multivariate regression models.
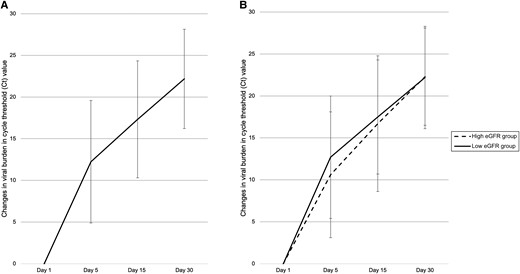
Changes in viral burden in Ct value with time in the (A) whole cohort and (B) eGFR subgroups. Abbreviations: Ct, cycle threshold; eGFR, glomerular filtration rate.
Virological and Symptom Rebound
Ten patients (11.8%) developed virological rebound: 4 (4.7%) and 6 (7.1%) patients were identified by PCR and RAT testing, respectively (Supplementary Table 4). The mean onset of RAT rebound was 2 ± 1 days after completion of nirmatrelvir-ritonavir treatment. Most of these patients (n = 9; 90%) were asymptomatic, whereas 1 patient reported mild cough during the rebound. In addition, 5 patients (5.9%) developed symptom rebound at a mean period of 4 ± 3 days after resolution of symptoms. Most symptoms were runny nose, cough, dry mouth, and myalgia, which lasted for a mean period of 2 ± 1 days. None of the symptom rebound was associated with virological rebound.
DISCUSSION
The present study is the first prospective study to document the safety and virological and clinical outcome in patients with CKD prescribed with a modified dose of nirmatrelvir-ritonavir after COVID-19. We showed that nirmatrelvir-ritonavir can effectively suppress the viral load with a favorable safety profile and low rates of viral and symptom rebound.
Patients with advanced CKD are vulnerable to severe COVID-19 and related hospitalization, intensive care unit admission, and mortality [5]. During the Omicron wave in Hong Kong, rates of 19.4 and 21.9 deaths out of 1000 peritoneal dialysis (PD) and hemodialysis (HD) populations, respectively, were attributed to COVID-19 [6]. While antiviral treatment can effectively treat COVID-19, the phenomenon of “renalism” was reflected by the observation that 219 of 484 COVID-19 trials (45.0%) registered in the WHO–International Clinical Trials Registry Platform (WHO-ICTRP) excluded patients with advanced CKD [7]. The landmark study of nirmatrelvir-ritonavir, Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, also excluded patients with an eGFR lower than 30 mL/minute/1.73 m2 [8]. A pharmacokinetic study has confirmed the significance of renal clearance in the metabolism of nirmatrelvir [9]. With that, a dose reduction is recommended for patients with CKD taking nirmatrelvir-ritonavir [2].
Hiremath et al [10] have recently reported data on nirmatrelvir-ritonavir in patients on maintenance dialysis in Canada. While they reported a low hospitalization and mortality rate, the virological outcomes and biochemical changes were not addressed. Nevertheless, our cohort consists of a more comprehensive dialysis population with more patients on PD.
The incidence of adverse events and serious adverse events in our cohort was comparable between patients with higher eGFR receiving a standard recommended dose and those with lower eGFR or on dialysis receiving an adjusted dose of nirmatrelvir-ritonavir. This observation supported the safety profile of a renally adjusted dose in patients with an eGFR of less than 30 mL/minute/1.73 m2. The most common drug-related adverse event leading to drug discontinuation was gastrointestinal symptoms, which were commonly reported in EPIC-HR trial [7].
In the present study, the rate of viral burden rebound was 4.7%, similar to the rate of 2.3–6.6% reported previously [11, 12]. Asymptomatic virological rebound occurred mainly in the patients undergoing dialysis, possibly due to more frequent routine testing before attending a dialysis center. Nonetheless, the viral rebound was transient and asymptomatic, and was not associated with adverse clinical outcomes or mortality. With regard to symptom rebound, our rate was lower than that reported in a large unvaccinated and untreated cohort [3]. Again, our results indicated that the symptoms were self-limiting and not associated with an increased infectivity demonstrated by RAT.
Post-COVID condition (PCC; or long COVID) is another debilitating sequela of COVID-19 infection. It affects up to 50% of the patients with COVID-19, and this condition is characterized by unresolved symptoms, such as fatigue, dyspnea, and palpitations, that last for several months after recovery from acute illness [13]. The role of nirmatrelvir-ritonavir in the treatment of PCC has been increasingly recognized, as studies have indicated that the persistent circulation of the residual viral spike antigen is a major cause [14]. For example, Xie et al [15] reported that individuals who were treated with nirmatrelvir-ritonavir during the acute phase of infection (ie, ≤5 d of viral positivity) had 36% lower odds of developing PCC. Case reports have also shown that nirmatrelvir-ritonavir treatment, even when the individuals are no longer infectious, helps improve symptoms of PCC [16, 17]. Currently, a randomized controlled trial is underway to systematically evaluate the efficacy of extended nirmatrelvir-ritonavir therapy for PCC [18].
There are several limitations to this study. Our sample size is small, and an untreated control group was unavailable for comparison. We also did not analyze the drug level of nirmatrelvir-ritonavir. A recent study that investigated the pharmacokinetics of more frequent dosing of nirmatrelvir/ritonavir in patients undergoing HD found a peak plasma concentration at the higher end of the level [19]. Because urinary excretion is the primary excretion route for nirmatrelvir, further dedicated pharmacokinetic studies should be undertaken to explore the optimal dosing to be used in patients at advanced stages of CKD. However, being the first prospective study to investigate the safety profile and clinical and virological outcomes of nirmatrelvir-ritonavir use in patients with CKD remains the key strength of our study. We also systematically evaluated viral and symptom rebound after treatment, which has not been addressed in patients with CKD undergoing COVID-19 treatment.
In conclusion, our results provide supportive evidence of nirmatrelvir-ritonavir use in patients with eGFR of less than 30 mL/minute/1.73 m2, including patients on dialysis who have COVID-19. Based on our preliminary data, modified doses of nirmatrelvir-ritonavir according to eGFR can be considered in this high-risk group of patients with advanced CKD, especially when an alternative treatment is unavailable.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by a research grant from the Charlie Lee Charitable Foundation. C. C. S. receives research grant and consultancy fees from Baxter Healthcare.
Data sharing statement. The data generated in this study are available from the corresponding author on a reasonable request, subject to approval from the local authority.
References
Author notes
The results presented in this paper have not been published previously in whole or part, except in abstract format.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




