-
PDF
- Split View
-
Views
-
Cite
Cite
Maura Manion, Afroditi Boulougoura, Nuha Naqvi, Silvia Lucena Lage, Elizabeth Richards, Christopher Grivas, Elizabeth Laidlaw, Safia Kuriakose, Ana M Ortega-Villa, Saber Tadros, Gregg Roby, Adam Rupert, France Galindo, Megan Anderson, Alice Pau, George Deepe, Virginia Sheikh, Irini Sereti, Polyfunctional Antigen Specific CD4+ T cell Responses in Patients With Human Immunodeficiency Virus/AIDS and Histoplasmosis Immune Reconstitution Inflammatory Syndrome, Clinical Infectious Diseases, Volume 76, Issue 3, 1 February 2023, Pages 531–534, https://doi.org/10.1093/cid/ciac514
Close - Share Icon Share
Abstract
In the combination antiretroviral era, there are limited data regarding the pathogenesis of histoplasmosis immune reconstitution inflammatory syndrome (IRIS) in people with human immunodeficiency virus (HIV). We immunologically characterized 10 cases of histoplasmosis, 4 of whom developed histoplasmosis IRIS. CD4+ T cells in histoplasmosis IRIS demonstrated a significant polyfunctional cytokine response to histoplasma antigen.
Histoplasma capsulatum is the most common endemic mycosis in patients with AIDS [1] and almost always manifests as disseminated disease in persons with human immunodeficiency virus (HIV. PWH) with a CD4 T-cell count <150 cells/µL [2]. Globally, endemic areas of this fungus include Central and South America, central regions of sub-Saharan Africa, Asia, and within the United States, the Mississippi, Ohio, and St. Lawrence River valleys. Earlier in the epidemic, histoplasmosis affected up to 5% of patients with AIDS in North America and was reported recently to have an estimated incidence of 1.48 cases per 100 PWH in Latin America [2, 3].
Immune reconstitution inflammatory syndrome (IRIS) represents the incongruous clinical deterioration that could be the result of either worsening (paradoxical) or uncovering (unmasking) of an infection or malignancy after initiation of antiretroviral therapy (ART) despite control of HIV viremia. The pathogenesis of IRIS is a complicated interplay between the myeloid compartment and CD4 T cells following ART initiation. Most studies investigating IRIS pathogenesis have been in mycobacterial disease, more specifically in Mycobacterium tuberculosis or Mycobacterium avium. In these studies, activation and priming of myeloid cells occurs without full maturation in the absence of functional interferon γ (IFN-γ) producing CD4 T cells prior to ART [4, 5]. After reconstitution of CD4 T cells, primed macrophages become functional releasing pathogenic amounts of inflammatory mediators leading to IRIS.
In the era of effective ART, histoplasmosis IRIS has been described in case reports and series, but its pathogenesis remains elusive [6]. H. capsulatum is, however, an intracellular pathogen with similar host targeted Th1 immunity as mycobacterial diseases, leading us to hypothesize that there could be similar myeloid and T-cell activation in patients with histoplasmosis IRIS [7]. Here we characterize our experience with 10 patients with HIV/AIDS and histoplasmosis within prospective clinical trials, out of whom 8 developed IRIS, and 4 of those were specifically related to histoplasmosis. Our goal was to describe the clinical and immunologic characteristics in those patients who developed histoplasmosis IRIS compared to the patients who did not.
METHODS
PWH who were naive to ART with a CD4 T-cell count <100 cells/µL were enrolled in 2 prospective observational National Institutes of Health (NIH) institutional review board (IRB) approved protocols (NCT00286767, NCT02147405) following participants from ART initiation up to 96 weeks. IRIS was defined following AIDS Clinical Trials Group IRIS definition criteria [8]. Healthy volunteer blood samples were obtained on a National Cancer Institute, NIH IRB approved protocol 99-CC-0168 and were de-identified prior to distribution. All participants provided written informed consent in accordance with the Declaration of Helsinki.
Cryopreserved plasma was obtained from study participants at baseline and 2–8 weeks after initiation of ART. Biomarkers were measured by electrochemiluminescence with custom multiplex kits (Meso Scale Discovery, R&D, Aviscera). Cryopreserved peripheral blood mononuclear cells (PBMCs) were collected at the IRIS timepoint and at matched timepoints for the non-IRIS samples (2–8 weeks post ART) and were used for immunophenotyping and in vitro stimulations. Data were grouped into 4 categories: histoplasma IRIS (Histo IRIS), non-histoplasma IRIS (Other IRIS), no IRIS (No IRIS), and healthy controls (HCs) (Supplementary Figure 1 and Supplementary Methods).
RESULTS
From 271 patients, we identified 10 patients with HIV/AIDS and histoplasmosis representing a prevalence of 3.6%. The median age of these patients was 38 years old, 8 were men, and 8 were Hispanic, from Mexico and Central America. Prior to starting ART, the median CD4 T-cell count was 41 (interquartile range [IQR], 11–60) cells/µL and plasma viral load was 239 914 copies/mL. Nine of the 10 patients had coinfections with non-tuberculous mycobacteria (NTM), herpes viruses, pneumocystis pneumonia, or parasites (Supplementary Table 1).
Clinical descriptions are provided in the Supplementary Methods. The predominant initial clinical presentations of histoplasmosis were pulmonary infiltrates on imaging and gastrointestinal involvement with ulcerative lesions. Four of the 10 patients developed Histo IRIS, 4 developed Other IRIS, and 2 did not develop IRIS. Of the 4 patients who had Histo IRIS, 3 developed IRIS only to histoplasmosis, and 1 developed IRIS to both histoplasmosis and NTM. The 4 patients with IRIS to other pathogens included varicella zoster virus (VZV), tuberculosis (TB), and NTMs. The main clinical presentations of Histo IRIS were paradoxical with worsening lymphadenopathy and recrudescent pulmonary findings. Two patients with Histo IRIS and 3 patients with Other IRIS required corticosteroids (Figure 1A).
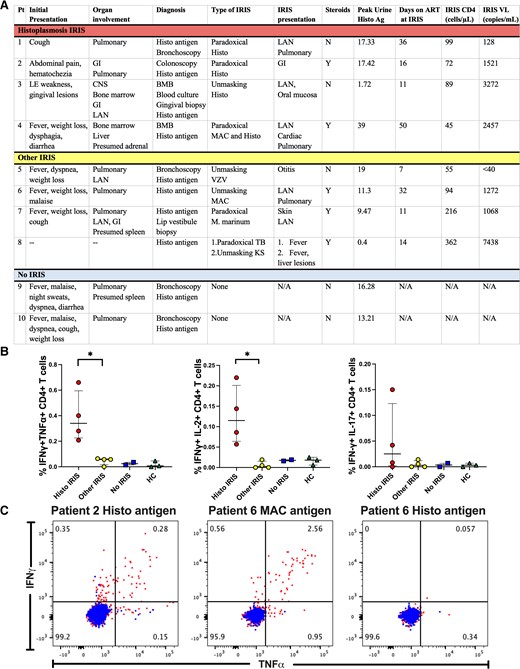
Patient comparisons. Abbreviations: CNS, central nervous system; GI, gastrointestinal; HC, healthy control; IFNγ, functional interferon γ; IL, interleukin; INF, interferon; IRIS, immune reconstitution inflammatory syndrome; LAN, lymphadenopathy; MAC, Mycobacterium avium complex; N/A, not applicable; TB, tuberculosis; tnfα, tumor necrosis factor alpha; VZV, varicella zoster virus.
Inflammatory Biomarker Data
Inflammatory biomarkers were evaluated prior to ART and 2–8 weeks after ART, and no significant differences were found between the Histo IRIS, Other IRIS, and No IRIS groups (Supplementary Table 2). Similarly, no differences were found when the Histo IRIS and Other IRIS groups were combined for analysis and compared to the No IRIS group.
T-cell Phenotypes
No significant differences were found between CD4+ and CD8+ T-cell memory subsets among patients with Histo IRIS, Other IRIS, and No IRIS patients. No significant differences were found in cycling and activation/inhibitory receptors (Ki-67 and PD-1) between Histo IRIS, Other IRIS, and No IRIS in CD4 and CD8 T cells. In addition, no significant differences were found in regulatory T cells between Histo IRIS, Other IRIS, and No IRIS (Supplementary Tables 3 and 4).
When Histo IRIS and Other IRIS groups were combined for analysis, increased CD4 and CD8 T-cell effector memory cells (P = .012, P = .024, respectively) were observed in IRIS patients compared to HCs, as well as a lower proportion of CD4+ central memory cells and naive cells in IRIS compared to No IRIS (P = .044) and HCs (P = .024), respectively. The combined IRIS group also demonstrated increased Ki-67 expression in CD4 and CD8 T cells compared to HCs (P = .012 and P = .012) and No IRIS (in CD4s, P = .044), and increased PD-1 expression in CD4 T cells (P = .012) compared to HCs (Supplementary Tables 3 and 4).
Monocyte Phenotypes
No significant differences were found in monocyte subsets between Histo IRIS, Other IRIS, and No IRIS. In the combined IRIS group, intermediate monocytes decreased in the IRIS subset compared to No IRIS (P = .044). No other significant differences were found among monocyte subset distribution between IRIS, No IRIS, and HCs (Supplementary Table 3).
Assessment of Antigen-Specific Responses
PBMCs stimulated with histoplasma cell wall/membrane extract had a significantly higher percentage of CD4+IFN-γ+tumor necrosis factor α (TNFα+) and CD4+IFN-γ+interleukin 2 (IL-2+) producing T cells in Histo IRIS patients compared to Other IRIS patients (P = .029) (Figure 1B). Single gating showed IFN-γ remained significantly elevated in patients with Histo IRIS compared to Other IRIS when stimulated with histoplasma extract (Supplementary Figure 2). In vitro stimulations performed with antigens related to the IRIS trigger polyfunctional cytokine responses with IFN-γ and TNFα (Figure 1C).
No significant differences were found in cytokine production by CD8 T cells in the presence of histoplasma antigen between Histo IRIS, Other IRIS, and No IRIS (Supplementary Figure 3).
DISCUSSION
According to published reports, histoplasmosis IRIS presents mostly with gastrointestinal, hematologic, pulmonary, and lymphatic involvement and can frequently be unmasking IRIS [6]. In our 4 patients with histoplasmosis IRIS, we saw similar presentations ranging from small bowel obstruction to worsening lymphadenopathy and pulmonary symptoms. Interestingly, the majority of patients with histoplasmosis in our cohort had IRIS, either to Histoplasma or another pathogen or both. Three of the 4 patients with histoplasmosis IRIS presented with paradoxical IRIS, as compared to the predominance of unmasking IRIS in other cohorts. This difference may be due to the availability of rapid diagnostic tests in the United States, highlighting and reinforcing the importance of rapid diagnostic testing in endemic areas before ART initiation [6].
To our knowledge, this is the first study characterizing immunologic responses during histoplasma IRIS. Our findings of a dysregulated myeloid and T-cell response in histoplasmosis IRIS, most salient an increased polyfunctional cytokine response to histoplasma antigen by CD4+ T cells in the histoplasma IRIS group as compared to the other IRIS group, were consistent with previously described findings in mycobacterial IRIS [9, 10]. When IRIS groups were combined, we also observed increased effector memory and T-cell activation at the IRIS timepoint and a decreased frequency of intermediate monocytes compared to those patients who did not develop IRIS as previously described [4, 11]. We did not find significant differences in the inflammatory biomarkers, however, given this was a small cohort we may not have had the power to detect differences.
To conclude, we have found that histoplasmosis in PWH in the United States is still an opportunistic infection that should be considered in people with severe CD4 lymphopenia especially in those from endemic areas and it can present with a wide range of clinical manifestations including IRIS. The pathogenesis of histoplasmosis-associated IRIS is consistent with what has been observed in the mycobacterial IRIS literature with evidence of increased T-cell activation and polyfunctional (TNFα, IFN-γ, and IL-2) CD4 responses in response to histoplasma antigen at the time of IRIS. Histoplasmosis in PWH was frequently seen with other coinfections suggesting that apart from IRIS, other coinfections are common in these patients. As such, providers treating patients from endemic areas should be alert about the possibility that histoplasmosis can present with IRIS and also be aware of the need for thorough evaluation for other pathogens.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants and the staff of the outpatient clinic 8 and the inpatient ward team of the NIH Clinical Center.
Financial support. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Disease.
References
Author notes
A. B. and N. N. contributed equally to this work.
Potential conflicts of interest. G. S. D. reports NIH funded grants NIH R01 AI 106269, NIH R01 AI 133797, and NIH R21 AI 160722. All other authors report no potential conflicts. erest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




