-
PDF
- Split View
-
Views
-
Cite
Cite
Saoussen Ben Abdallah, Yosra Mhalla, Imen Trabelsi, Adel Sekma, Rim Youssef, Khaoula Bel Haj Ali, Houda Ben Soltane, Hajer Yacoubi, Mohamed Amine Msolli, Nejla Stambouli, Kaouthar Beltaief, Mohamed Habib Grissa, Meriem Khrouf, Zied Mezgar, Chawki Loussaief, Wahid Bouida, Rabie Razgallah, Karima Hezbri, Asma Belguith, Naouel Belkacem, Zohra Dridi, Hamdi Boubaker, Riadh Boukef, Semir Nouira, Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial, Clinical Infectious Diseases, Volume 76, Issue 2, 15 January 2023, Pages 185–191, https://doi.org/10.1093/cid/ciac807
Close - Share Icon Share
Abstract
Zinc supplementation has been considered a potential therapy for coronavirus disease 2019 (COVID-19). We aimed to examine zinc efficacy in adult patients with COVID-19 infection.
We conducted a prospective, randomized, double-blind, placebo-controlled multicenter trial. Patients who were tested positive for COVID-19 without end-organ failure were randomized to oral zinc (n = 231) or matching placebo (n = 239) for 15 days. The primary combined outcome was death due to COVID-19 or intensive care unit (ICU) admission ≤30 days after randomization. Secondary outcomes included length of hospital stay for inpatients and duration of COVID-19 symptoms with COVID-19–related hospitalization for outpatients.
190 patients (40.4%) were ambulatory and 280 patients (59.6%) were hospitalized. Mortality at 30 days was 6.5% in the zinc group and 9.2% in the placebo group (OR: .68; 95% CI .34–1.35); ICU admission rates were, respectively, 5.2% and 11.3% (OR: .43; 95% CI .21–.87). Combined outcome was lower in the zinc group versus the placebo group (OR: .58; 95% CI .33–.99). Consistent results were observed in prespecified subgroups of patients aged <65 years, those with comorbidity, and those who needed oxygen therapy at baseline. Length of hospital stay was shorter in the zinc group versus the placebo group (difference: 3.5 days; 95% CI 2.76–4.23) in the inpatient group; duration of COVID-19 symptoms decreased with zinc treatment versus placebo in outpatients (difference: 1.9 days; 95% CI .62–2.6). No severe adverse events were observed during the study.
Our results showed that, in COVID-19 patients, oral zinc can decrease 30-day death, ICU admission rate and can shorten symptom duration.
Clinical Trials Registration. ClinicalTrials.gov, NCT05212480.
The coronavirus disease 2019 (COVID-19) pandemic has created a global health crisis since its first emergence in China in late December 2019. Its high transmissibility and increased morbidity have made COVID-19 a serious public health threat and burden [1]. Various drugs were initially proclaimed effective against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of COVID-19; they were later disapproved [2]. Like in many other diseases, the regulation of white blood cell production using immuno-nutrition is a novel concept that could be applied to COVID-19. Some molecules and nutrients such as zinc play central roles in keeping the function and integrity of the immune system [3, 4]. It has been shown that serum zinc was inversely correlated with outcome in sepsis, highlighting the potential value of the zinc approach in COVID-19 treatment [5–8]. Nevertheless, there is still limited evidence to support such a role of zinc in COVID-19, with the exception of observational studies and a few randomized clinical trials with small sample sizes [9–11]. The objective of the present trial was to evaluate the effect of zinc supplementation in non–critically ill patients with COVID-19. Our a priori hypothesis was that supplementation with zinc would reduce 30-day mortality and need for intensive care unit (ICU) admission.
METHODS
Study Design
VIZIR (viral infection treatment with oral zinc: a randomized controlled trial in COVID-19) was a prospective, randomized, double-blind, placebo-controlled multicenter trial, conducted from 15 February 2022 to 4 May 2022. The protocol was approved by the institutional review board of all of the participating centers. The VIZIR study was carried out in Tunisia, in 3 Tunisian university referral hospitals (Fattouma Bourguiba Hospital Monastir, Sahloul Hospital Sousse, Farhat Hached Hospital Sousse) and 2 nonuniversity hospitals (Ksar Hlel Hospital, Teboulba Hospital). Patients were first screened in the COVID-19 triage unit of each participating center. Written informed consent was obtained from all patients before enrollment.
Study Participants
Patients were eligible if they were 18 years or older and had a diagnosis of COVID-19. In all included patients, the diagnosis of COVID-19 was performed by reverse transcriptase–polymerase chain reaction (RT-PCR) or rapid antigen test. Diagnostic imaging (computed tomography scan) was performed in patients who had a negative first RT-PCR test result with concern for a false-negative RT-PCR (observer errors and low viral RNA levels). In all cases, a diagnostic confirmation by repeated RT-PCR test was carried out.
Exclusion Criteria
Patients were excluded if symptoms started beyond 7 days before inclusion. Patients under zinc treatment or with known hypersensitivity to zinc; severe comorbid conditions, including heart, liver, or renal failure (estimated glomerular filtration rate ≤30 mL/min/1.73 m2); malignancies; unsuitability for oral administration; swallowing disorders; cognitive impairments or poor mental status; and need for immediate ICU admission justified by the need for use of respiratory or cardiovascular organ support (oxygen delivered by high-flow nasal cannula, noninvasive or invasive mechanical ventilation, or the use of vasopressors or inotropes) were exclusion criteria. Refusal or inability to consent or to communicate were also exclusion criteria.
Randomization and Interventions
For all included patients we collected the following data at the first medical visit: demographic and clinical data including age, gender, comorbidities, completed vaccination status (currently 1, 2, or 3 doses of COVID-19 vaccine depending on the vaccine), symptoms, current treatment and physical examination findings, and severity grades (asymptomatic [grade I], symptomatic without oxygen support [grade II], and symptomatic with oxygen support [grade III]). Masked randomization was centralized and done electronically through an automated interactive web-response system (Dacima Software). Participants were randomly assigned (1:1) to either zinc treatment (zinc group) or placebo (placebo group). Allocation sequence was not stratified. Patients enrolled in the zinc group received 25 mg of elemental zinc (Zinc plus; Opalia Recordati, Tunisia) twice a day for 15 days. Patients enrolled in the placebo group received 1 capsule twice a day for 15 days. Zinc and placebo capsules were prepared, packed, and specified by code numbers in similar shapes. The trial team, site staff, and patients were unaware of the randomized assignments. All patients received supportive care according to national guidelines. Standard-of-care background therapy included corticosteroids, prophylactic anticoagulation, supplemental oxygen, and other treatments as clinically indicated. Trained research coordinators followed patients prospectively at home (outpatients were contacted via telephone calls) or in the hospital documenting compliance with study treatment and outcomes. For outpatients, we assessed at the 15-day and 30-day follow-up the evolution of clinical symptoms, duration and appearance of new symptoms, the need for hospitalization, need for ICU admission, and survival status. All outpatients were encouraged to report all adverse events and symptom evolution during the study period. For inpatients, we recorded death, need for ICU admission and length of ICU and hospital stay at 30-day evaluation. The database was a validated electronic case report form (eCRF). All eCRF users were trained as per completion guidelines and the data entry was done directly by the study staff with the patients.
Outcome Criteria
The primary outcomes measures were death rate, ICU admission rate, and combined outcome within 30 days after randomization. Secondary outcomes included length of stay in the hospital and protocol treatment safety. In outpatients, secondary outcomes also included duration of COVID-19 symptoms, need for hospitalization and oxygen therapy. We assessed all of the secondary outcomes through 30 days after randomization.
Statistical Analysis
Sample Size Calculation
Based on a projected combined event rate (death and ICU admission) at 30 days estimated at 30%, and using an ɑ-value of 0.05, a study of 460 patients will have 80% power to detect a 10% decrease in absolute risk in the zinc treatment group compared with the placebo group. The sample size was increased by 5% to compensate for the number of patients randomized but lost to follow-up, for a total sample size of 485 patients. We report mean and standard deviation, median and interquartile range, and frequency and percentages, depending on the nature and distribution of variables. We compared continuous variables using Student's t test. We compared categorical variables using Fisher's exact test. We included odds ratio (ORs) and 95% confidence intervals (CIs) in the comparison of 30-day primary and secondary outcomes for each intervention, both overall and in prespecified subgroups, defined according to characteristics at randomization: age, gender, comorbidity (obesity, history of arterial hypertension, cardiac disease, diabetes mellitus, chronic obstructive pulmonary disease, asthma, or kidney disease), and baseline clinical severity grading. The differences in the distributions of the severity grades between the zinc group and the placebo group at 15 days and at 30 days were analyzed with the use of Wilcoxon–Mann–Whitney test generalized ORs. Frequencies of adverse events and complications were compared with the chi-square test. All analyses were done according to the intention-to-treat principle, without adjustment for multiple comparisons. Two-sided P values of less than .05 were considered to indicate statistical significance. All analyses were done using the Statistical Package for Social Sciences software (SPSS version 20). This study is registered with ClinicalTrials.gov (NCT05212480).
RESULTS
We screened 1200 patients; 513 were eligible and 482 were enrolled (Figure 1). Twelve randomly assigned patients could not be evaluated, 8 withdrew consent and 4 because we could not ascertain their 30-day vital status; thus, there were 470 patients in the final intention-to-treat analysis. These patients were randomly assigned to either the zinc group (231 patients) or the placebo group (239 patients). Baseline characteristics of the included patients are summarized in Table 1. One hundred ninety patients (40.4%) were treated as outpatients and 280 patients (59.6%) were hospitalized. The mean duration between the first COVID-19 symptom and inclusion in the study was 4.6 ± 1.1 days. The mean age of patients was 54.2 ± 17.3 years, 53% were men, and the mean body mass index was 27.3 ± 3.8 kg/m². The most frequent comorbidities were hypertension (23.4%) and diabetes (19.4%). Approximately 20% of patients had a history of complete anti–COVID-19 vaccination (n = 94) and 23% received at least 1 dose (n = 108). Asthenia (56.4%), cough (47%), and fever (38.9%) were the most common signs at initial presentation; 38.9% of patients had oxygen saturation of less than 92%. Adjunctive treatment included paracetamol (54.3%), steroids (37.7%), anticoagulants (48.3%), and oxygen via facial mask (42.9%). Patients did not receive any antivirals during the trial. There were no significant differences in baseline characteristics between the 2 groups. Specifically, the distribution of severity grades in the 2 groups was similar: 5.6% and 5.9% of the zinc and placebo groups, respectively, were grade I, 34.2% and 35.1% were grade II, and 60.2% and 59% were grade III. Overall, 37 deaths were reported, 30 (81.1%) occurred in the ICU, and 7 (18.9%) outside the ICU. In the zinc group, 30-day mortality was 6.5% (95% CI 3.3–9.6), ICU admission rate was 5.2% (95% CI 2.3–8.0), and the rate of combined outcome was 10.4% (95% CI 6.4–14.3). In the placebo group, 30-day mortality was 9.2% (95% CI 5.5–12.8), ICU admission rate was 11.3% (95% CI 5.5–12.8), and the rate of combined outcome was 16.7% (95% CI 12.0–21.4) (Table 2). The combined outcome was reduced in the zinc group compared with the placebo group (OR: .58; 95% CI .33–.99) (Table 2); this difference was mainly related to the decrease in ICU admissions (OR: .43; 95% CI .21–.87), whereas the death rate was not different between study groups (OR: .68; 95% CI .34–1.35). In prespecified subgroup analysis, there was evidence of a treatment positive effect with zinc, as compared with placebo, in inpatients, patients aged more than 65 years, patients with comorbidity, and those requiring oxygen at baseline regarding combined primary outcome (Figure 2). In the inpatient subgroup, the length of hospital stay was reduced in the zinc group compared with the placebo group (7.1 ± 3.4 days vs 10.6 ± 2.8 days; difference: 3.5 days; 95% CI 2.76–4.23 days). In the outpatient subgroup, the duration of COVID-19 symptoms was shorter in the zinc group compared with the placebo group (9.6 ± 4.1 days vs 12.8 ± 6.7 days; difference: 1.9 days; 95% CI .62–2.6 days), whereas the hospital admission rate was similar in both groups (1.2% vs 3.8%, respectively) (OR: .30; 95% CI .03–2.8). The distributions of patients in the 2 groups on the basis of severity grade at baseline and at 15- and 30-day evaluation are shown in Figure 3. The percentage of grade I patients increased to 22.1% and 64.3% in the zinc group, respectively, at 15 and 30 days compared with 9.7% (difference: 12.7%; 95% CI 2.4–22.4) and 49.6% in the placebo group (difference: 15%; 95% CI 1.1–28.3). The percentage of grade III patients decreased to 46.8% (–13.4%) in the zinc group and increased to 60.5% (+1.5%) in the placebo group at 15 days (difference: 14.9%; 95% CI .01–27.4); at 30 days, the percentage of grade III patients decreased to 13.5% (−46.7%) in the zinc group and to 23.1% (−35.9%) in the placebo group (difference: 10.8%; 95% CI −1.03% to 20.2). With regard to adverse events, there were no important between-group differences in the incidence of adverse events during the treatment period. Minor adverse events were observed in 9 patients (3.9%) in the zinc group and 17 patients (7.1%) in the placebo group (OR: .52; 95% CI .23–1.12). Most of them were digestive symptoms. No treatment-related serious adverse events were reported in either treatment group.
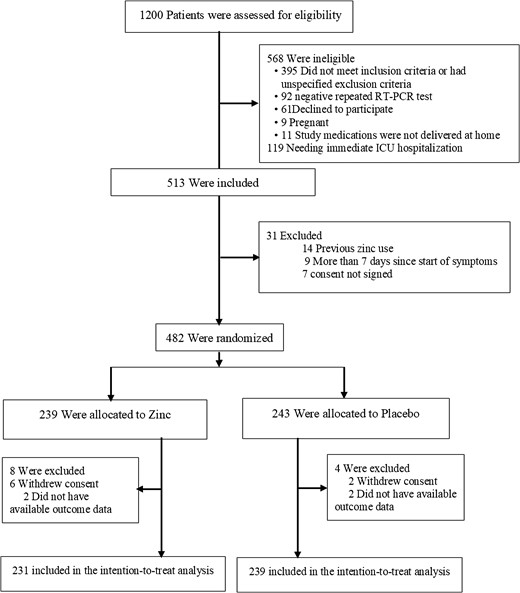
Flow diagram. Abbreviations: ICU, intensive care unit; RT-PCR, reverse transcriptase–polymerase chain reaction.
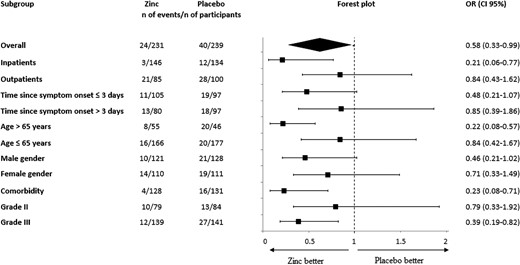
Forest plot of analyses of zinc as compared with placebo for the primary combined outcome including COVID-19–related death or intensive care unit admission at 30-day follow-up. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio.
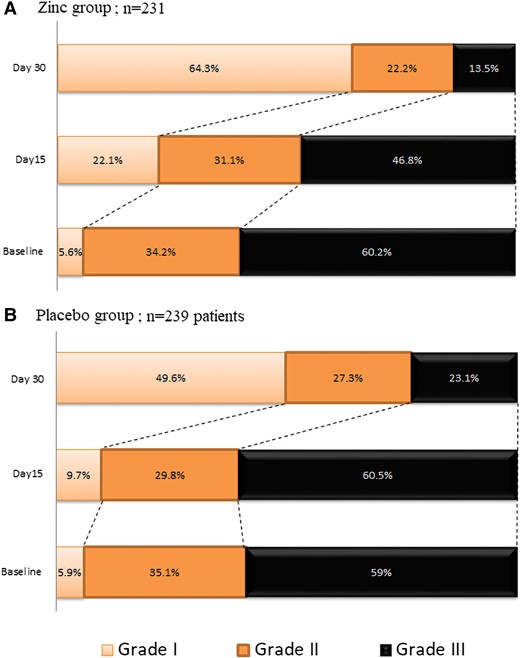
Shown is the distribution of severity grades in the zinc (A) and placebo (B) groups at baseline, at day 15, and at day 30. The severity grades indicate the following: I, no symptoms at all; II, COVID-19 symptoms without oxygen requirement; III, COVID-19 symptoms with oxygen requirement. At baseline, there was not a significant difference between the zinc group and the placebo group in the overall distribution of severity grades. At 15 days, a significant difference between the study groups was observed (P = .01). The distribution of severity grades at 30 days did not show significant differences between study groups. Abbreviation: COVID-19, coronavirus disease 2019.
| . | Zinc (n = 231) . | Placebo (n = 239) . |
|---|---|---|
| Age, mean (SD), y | 54.6 (17.4) | 53.7 (17.2) |
| Male gender, n (%) | 121 (52.4) | 128 (53.6) |
| BMI, mean (SD), kg/m2 | 27.2 (3.4) | 27.4 (4.2) |
| Active smoking, n (%) | 36 (15.6) | 36 (15.1) |
| Type 2 diabetes, n (%) | 37 (16) | 54 (22.6) |
| Hypertension, n (%) | 51 (22.1) | 59 (24.7) |
| Coronary artery disease, n (%) | 5 (2.2) | 9 (3.8) |
| COPD, n (%) | 7 (3) | 4 (1.7) |
| Asthma, n (%) | 6 (2.6) | 5 (2.1) |
| Renal failure, n (%) | 4 (1.7) | 1 (0.4) |
| Clinical signs, n (%) | ||
| ȃAsthenia | 128 (55.4) | 137 (57.3) |
| ȃCough | 116 (50.2) | 105 (43.9) |
| ȃFever | 94 (40.7) | 89 (37.2) |
| ȃPolypnea | 70 (30.3) | 73 (30.5) |
| ȃHeadache | 62 (26.8) | 72 (30.1) |
| ȃChest pain | 33 (14.3) | 30 (12.6) |
| ȃDiarrhea | 29 (12.6) | 33 (13.8) |
| ȃJoint pain | 25 (10.8) | 16 (6.7) |
| ȃAnosmia | 19 (8.2) | 28 (11.7) |
| ȃVomiting | 17 (7.4) | 17 (7.1) |
| ȃTaste loss | 13 (5.6) | 20 (8.4) |
| ȃAbdominal pain | 10 (4.3) | 18 (7.5) |
| Vital signs, mean (SD) | ||
| ȃSystolic blood pressure, mmHg | 114 (41) | 119 (38) |
| ȃDiastolic blood pressure, mmHg | 75 (25) | 76 (22) |
| ȃRespiratory rate, cycle/min | 22 (4) | 21 (3) |
| ȃHeart rate, beats/min | 95 (16) | 94 (15) |
| ȃPulse oxygen saturation, % | 92 (5) | 93 (3) |
| ȃHospitalization, n (%) | 146 (63.2) | 134 (56.1) |
| Medication for COVID-19 other than the study drug, n (%) | ||
| ȃDexamethasone or other steroids | 87 (37.7) | 90 (37.7) |
| ȃOxygen | 106 (45.9) | 96 (40.2) |
| ȃAntibiotics | 52 (22.5) | 45 (18.8) |
| ȃParacetamol | 132 (57.1) | 123 (51.5) |
| ȃVitamin C | 26 (11.3) | 26 (10.9) |
| ȃAnticoagulants | 119 (51.5) | 108 (45.2) |
| . | Zinc (n = 231) . | Placebo (n = 239) . |
|---|---|---|
| Age, mean (SD), y | 54.6 (17.4) | 53.7 (17.2) |
| Male gender, n (%) | 121 (52.4) | 128 (53.6) |
| BMI, mean (SD), kg/m2 | 27.2 (3.4) | 27.4 (4.2) |
| Active smoking, n (%) | 36 (15.6) | 36 (15.1) |
| Type 2 diabetes, n (%) | 37 (16) | 54 (22.6) |
| Hypertension, n (%) | 51 (22.1) | 59 (24.7) |
| Coronary artery disease, n (%) | 5 (2.2) | 9 (3.8) |
| COPD, n (%) | 7 (3) | 4 (1.7) |
| Asthma, n (%) | 6 (2.6) | 5 (2.1) |
| Renal failure, n (%) | 4 (1.7) | 1 (0.4) |
| Clinical signs, n (%) | ||
| ȃAsthenia | 128 (55.4) | 137 (57.3) |
| ȃCough | 116 (50.2) | 105 (43.9) |
| ȃFever | 94 (40.7) | 89 (37.2) |
| ȃPolypnea | 70 (30.3) | 73 (30.5) |
| ȃHeadache | 62 (26.8) | 72 (30.1) |
| ȃChest pain | 33 (14.3) | 30 (12.6) |
| ȃDiarrhea | 29 (12.6) | 33 (13.8) |
| ȃJoint pain | 25 (10.8) | 16 (6.7) |
| ȃAnosmia | 19 (8.2) | 28 (11.7) |
| ȃVomiting | 17 (7.4) | 17 (7.1) |
| ȃTaste loss | 13 (5.6) | 20 (8.4) |
| ȃAbdominal pain | 10 (4.3) | 18 (7.5) |
| Vital signs, mean (SD) | ||
| ȃSystolic blood pressure, mmHg | 114 (41) | 119 (38) |
| ȃDiastolic blood pressure, mmHg | 75 (25) | 76 (22) |
| ȃRespiratory rate, cycle/min | 22 (4) | 21 (3) |
| ȃHeart rate, beats/min | 95 (16) | 94 (15) |
| ȃPulse oxygen saturation, % | 92 (5) | 93 (3) |
| ȃHospitalization, n (%) | 146 (63.2) | 134 (56.1) |
| Medication for COVID-19 other than the study drug, n (%) | ||
| ȃDexamethasone or other steroids | 87 (37.7) | 90 (37.7) |
| ȃOxygen | 106 (45.9) | 96 (40.2) |
| ȃAntibiotics | 52 (22.5) | 45 (18.8) |
| ȃParacetamol | 132 (57.1) | 123 (51.5) |
| ȃVitamin C | 26 (11.3) | 26 (10.9) |
| ȃAnticoagulants | 119 (51.5) | 108 (45.2) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; SD, standard deviation.
| . | Zinc (n = 231) . | Placebo (n = 239) . |
|---|---|---|
| Age, mean (SD), y | 54.6 (17.4) | 53.7 (17.2) |
| Male gender, n (%) | 121 (52.4) | 128 (53.6) |
| BMI, mean (SD), kg/m2 | 27.2 (3.4) | 27.4 (4.2) |
| Active smoking, n (%) | 36 (15.6) | 36 (15.1) |
| Type 2 diabetes, n (%) | 37 (16) | 54 (22.6) |
| Hypertension, n (%) | 51 (22.1) | 59 (24.7) |
| Coronary artery disease, n (%) | 5 (2.2) | 9 (3.8) |
| COPD, n (%) | 7 (3) | 4 (1.7) |
| Asthma, n (%) | 6 (2.6) | 5 (2.1) |
| Renal failure, n (%) | 4 (1.7) | 1 (0.4) |
| Clinical signs, n (%) | ||
| ȃAsthenia | 128 (55.4) | 137 (57.3) |
| ȃCough | 116 (50.2) | 105 (43.9) |
| ȃFever | 94 (40.7) | 89 (37.2) |
| ȃPolypnea | 70 (30.3) | 73 (30.5) |
| ȃHeadache | 62 (26.8) | 72 (30.1) |
| ȃChest pain | 33 (14.3) | 30 (12.6) |
| ȃDiarrhea | 29 (12.6) | 33 (13.8) |
| ȃJoint pain | 25 (10.8) | 16 (6.7) |
| ȃAnosmia | 19 (8.2) | 28 (11.7) |
| ȃVomiting | 17 (7.4) | 17 (7.1) |
| ȃTaste loss | 13 (5.6) | 20 (8.4) |
| ȃAbdominal pain | 10 (4.3) | 18 (7.5) |
| Vital signs, mean (SD) | ||
| ȃSystolic blood pressure, mmHg | 114 (41) | 119 (38) |
| ȃDiastolic blood pressure, mmHg | 75 (25) | 76 (22) |
| ȃRespiratory rate, cycle/min | 22 (4) | 21 (3) |
| ȃHeart rate, beats/min | 95 (16) | 94 (15) |
| ȃPulse oxygen saturation, % | 92 (5) | 93 (3) |
| ȃHospitalization, n (%) | 146 (63.2) | 134 (56.1) |
| Medication for COVID-19 other than the study drug, n (%) | ||
| ȃDexamethasone or other steroids | 87 (37.7) | 90 (37.7) |
| ȃOxygen | 106 (45.9) | 96 (40.2) |
| ȃAntibiotics | 52 (22.5) | 45 (18.8) |
| ȃParacetamol | 132 (57.1) | 123 (51.5) |
| ȃVitamin C | 26 (11.3) | 26 (10.9) |
| ȃAnticoagulants | 119 (51.5) | 108 (45.2) |
| . | Zinc (n = 231) . | Placebo (n = 239) . |
|---|---|---|
| Age, mean (SD), y | 54.6 (17.4) | 53.7 (17.2) |
| Male gender, n (%) | 121 (52.4) | 128 (53.6) |
| BMI, mean (SD), kg/m2 | 27.2 (3.4) | 27.4 (4.2) |
| Active smoking, n (%) | 36 (15.6) | 36 (15.1) |
| Type 2 diabetes, n (%) | 37 (16) | 54 (22.6) |
| Hypertension, n (%) | 51 (22.1) | 59 (24.7) |
| Coronary artery disease, n (%) | 5 (2.2) | 9 (3.8) |
| COPD, n (%) | 7 (3) | 4 (1.7) |
| Asthma, n (%) | 6 (2.6) | 5 (2.1) |
| Renal failure, n (%) | 4 (1.7) | 1 (0.4) |
| Clinical signs, n (%) | ||
| ȃAsthenia | 128 (55.4) | 137 (57.3) |
| ȃCough | 116 (50.2) | 105 (43.9) |
| ȃFever | 94 (40.7) | 89 (37.2) |
| ȃPolypnea | 70 (30.3) | 73 (30.5) |
| ȃHeadache | 62 (26.8) | 72 (30.1) |
| ȃChest pain | 33 (14.3) | 30 (12.6) |
| ȃDiarrhea | 29 (12.6) | 33 (13.8) |
| ȃJoint pain | 25 (10.8) | 16 (6.7) |
| ȃAnosmia | 19 (8.2) | 28 (11.7) |
| ȃVomiting | 17 (7.4) | 17 (7.1) |
| ȃTaste loss | 13 (5.6) | 20 (8.4) |
| ȃAbdominal pain | 10 (4.3) | 18 (7.5) |
| Vital signs, mean (SD) | ||
| ȃSystolic blood pressure, mmHg | 114 (41) | 119 (38) |
| ȃDiastolic blood pressure, mmHg | 75 (25) | 76 (22) |
| ȃRespiratory rate, cycle/min | 22 (4) | 21 (3) |
| ȃHeart rate, beats/min | 95 (16) | 94 (15) |
| ȃPulse oxygen saturation, % | 92 (5) | 93 (3) |
| ȃHospitalization, n (%) | 146 (63.2) | 134 (56.1) |
| Medication for COVID-19 other than the study drug, n (%) | ||
| ȃDexamethasone or other steroids | 87 (37.7) | 90 (37.7) |
| ȃOxygen | 106 (45.9) | 96 (40.2) |
| ȃAntibiotics | 52 (22.5) | 45 (18.8) |
| ȃParacetamol | 132 (57.1) | 123 (51.5) |
| ȃVitamin C | 26 (11.3) | 26 (10.9) |
| ȃAnticoagulants | 119 (51.5) | 108 (45.2) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; SD, standard deviation.
| . | Zinc (n = 231) . | Placebo (n = 239) . | P . | OR [95% CI] . |
|---|---|---|---|---|
| Death, n (%) | 15 (6.5) | 22 (9.2) | .27 | .68 [.34–1.35] |
| ICU admission, n (%) | 12 (5.2) | 27 (11.3) | .01 | .43 [.21–.87] |
| Composite outcome, n (%) | 24 (10.4) | 40 (16.7) | .04 | .58 [.33–.99] |
| . | Zinc (n = 231) . | Placebo (n = 239) . | P . | OR [95% CI] . |
|---|---|---|---|---|
| Death, n (%) | 15 (6.5) | 22 (9.2) | .27 | .68 [.34–1.35] |
| ICU admission, n (%) | 12 (5.2) | 27 (11.3) | .01 | .43 [.21–.87] |
| Composite outcome, n (%) | 24 (10.4) | 40 (16.7) | .04 | .58 [.33–.99] |
Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
| . | Zinc (n = 231) . | Placebo (n = 239) . | P . | OR [95% CI] . |
|---|---|---|---|---|
| Death, n (%) | 15 (6.5) | 22 (9.2) | .27 | .68 [.34–1.35] |
| ICU admission, n (%) | 12 (5.2) | 27 (11.3) | .01 | .43 [.21–.87] |
| Composite outcome, n (%) | 24 (10.4) | 40 (16.7) | .04 | .58 [.33–.99] |
| . | Zinc (n = 231) . | Placebo (n = 239) . | P . | OR [95% CI] . |
|---|---|---|---|---|
| Death, n (%) | 15 (6.5) | 22 (9.2) | .27 | .68 [.34–1.35] |
| ICU admission, n (%) | 12 (5.2) | 27 (11.3) | .01 | .43 [.21–.87] |
| Composite outcome, n (%) | 24 (10.4) | 40 (16.7) | .04 | .58 [.33–.99] |
Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
DISCUSSION
In this multicenter, randomized, double-blind trial involving patients with COVID-19, a significant and clinically meaningful decrease in 30-day ICU admission rate and shorter duration of COVID-19 symptoms were observed with oral zinc administered for 15 days. In our prespecified subgroup analyses, we found that this benefit was especially observed in aged patients and those with comorbid conditions or those who need oxygen.
The activity of zinc against infectious pathogens has been demonstrated in a variety of viral species [12–16]. However, there is very scant information available on the role and effect of zinc in coronavirus disease [17–21]. Jothimani et al [18] in a recently published study including 47 patients with COVID-19 demonstrated that zinc deficiency was present in more than half (57.4%) of their cohort and was associated with prolonged hospitalization and increased mortality compared with a control group. In 4 outpatients with COVID-19, Finzi [19] reported that treatment with zinc reduced disease symptoms within 24 hours. Several clinical trials are currently investigating the effects of various doses of zinc either alone or with ionophores on the initiation and the progression of COVID-19 in patients.
To our knowledge, this study is the first well-powered, placebo-controlled clinical trial to report results of zinc for the treatment of patients with COVID-19. When administered orally to patients hospitalized with COVID-19 without end-organ failure, zinc demonstrated its efficacy to prevent ICU admission and to reduce hospital length of stay; for outpatients, zinc reduced symptom duration. Zinc should be considered for the treatment of patients with COVID-19.
Major strengths of our trial include being a randomized controlled trial and the large sample size. Moreover, subgroup analyses showed consistent results across both low- and high-risk patients. For inpatients, although the risk of death among patients receiving zinc and placebo was similar, the absolute risk reduction in ICU admission was approximately 10% and the length of hospital stay was reduced by 3 days. These results suggest that, for survivors, the disease trajectory is milder in zinc-group patients, with reduced need for hospital care and organ support. In outpatients, zinc treatment was associated with a reduction in COVID-19 symptom duration by approximately 3 days; in addition, there was a trend for fewer hospital admissions in patients in the zinc group, but the difference was not significant because of the small sample size of this category of patients. Our results should be highlighted with regard to not only the magnitude of a zinc positive effect but also the fact that this product is readily available at a low price to a larger percentage of the population, with minimal risk.
This trial has several limitations. First, generalizability is limited beyond patients with moderate clinical severity. Second, whether using zinc at higher doses than those prescribed in this trial would lead to different results is a question that needs to be investigated. Of note, the dose of zinc used in our study is within the range that is currently recommended [22]. Of relevance, 50 mg of zinc per day was found to be tolerable and unlikely to induce toxicity. Third, one could argue that longer treatment with zinc (>15 days) could add more clinical benefit. It is an important question, and we probably need specific data on long-term outcome and the possible preventive effect of zinc against the risk of long COVID [23]. The success of zinc treatment may be dependent on zinc serum levels; we did not assess serum zinc in the present study, and so we could not verify this relationship. Finally, although follow-up via telephone contact does not replace face-to-face visits, it was possible for us to assess outcomes in almost all of our included patients.
In summary, oral zinc treatment for 15 days is associated with a nearly 40% reduction in death and ICU admission, with shortening of symptom duration, in patients with COVID-19. Our results have very important clinical relevance in the absence of specific effective curative treatment.
Notes
Disclaimer. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Also, author Ben Abdallah participated in critical revision of intellectual content of the article, and final approval of the version of the article to be published. Additionally, they had substantial contribution in the original draft preparation.
Financial support. This work was supported by Opalia Recordati, Tunisia.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.




