-
PDF
- Split View
-
Views
-
Cite
Cite
Ruth Namazzi, Robert Opoka, Dibyadyuti Datta, Paul Bangirana, Anthony Batte, Zachary Berrens, Michael J Goings, Andrew L Schwaderer, Andrea L Conroy, Chandy C John, Acute Kidney Injury Interacts With Coma, Acidosis, and Impaired Perfusion to Significantly Increase Risk of Death in Children With Severe Malaria, Clinical Infectious Diseases, Volume 75, Issue 9, 1 November 2022, Pages 1511–1519, https://doi.org/10.1093/cid/ciac229
Close - Share Icon Share
Abstract
Mortality in severe malaria remains high in children treated with intravenous artesunate. Acute kidney injury (AKI) is a common complication of severe malaria, but the interactions between AKI and other complications on the risk of mortality in severe malaria are not well characterized.
Between 2014 and 2017, 600 children aged 6–48 months to 4 years hospitalized with severe malaria were enrolled in a prospective clinical cohort study evaluating clinical predictors of mortality in children with severe malaria.
The mean age of children in this cohort was 2.1 years (standard deviation, 0.9 years) and 338 children (56.3%) were male. Mortality was 7.3%, and 52.3% of deaths occurred within 12 hours of admission. Coma, acidosis, impaired perfusion, AKI, elevated blood urea nitrogen (BUN), and hyperkalemia were associated with increased mortality (all P < .001). AKI interacted with each risk factor to increase mortality (P < .001 for interaction). Children with clinical indications for dialysis (14.4% of all children) had an increased risk of death compared with those with no indications for dialysis (odds ratio, 6.56; 95% confidence interval, 3.41-12.59).
AKI interacts with coma, acidosis, or impaired perfusion to significantly increase the risk of death in severe malaria. Among children with AKI, those who have hyperkalemia or elevated BUN have a higher risk of death. A better understanding of the causes of these complications of severe malaria, and development and implementation of measures to prevent and treat them, such as dialysis, are needed to reduce mortality in severe malaria.
Malaria is an important cause of child mortality globally, with two-thirds of malaria deaths in 2020 occurring in children younger than 5 years of age [1]. Although artesunate treatment reduces mortality in children with severe malaria (SM) compared with quinine treatment [2], mortality remains at around 8% [2]. In the AQUAMAT study, which evaluated artesunate versus quinine in the treatment of severe falciparum malaria in African children, the presence of acidosis, coma, elevated blood urea nitrogen (BUN), convulsions, and chronic illness predicted mortality, but acute kidney injury (AKI) was not assessed [3]. A subsequent systematic review evaluating predictors of mortality in African children with SM identified coma, renal failure, hypoglycemia, shock, and deep breathing as independent predictors of mortality [4]. More recent studies evaluating AKI using consensus definitions show that AKI is common, occurring in 24%–59% of children with SM [5–10]. However, the relationship between AKI and other predictors of mortality in African children with SM, and the combined risk of other known factors with AKI, remain poorly defined. A better understanding of the interactions of clinical and laboratory risk factors for mortality in pediatric SM could lead to new interventions to decrease mortality.
In this multi-site study of Ugandan children with SM, we evaluated clinical and laboratory risk factors for mortality in children 6 months to 4 years of age and assessed the interactions between AKI and other clinical predictors of mortality, including metabolic and electrolyte abnormalities amenable to clinical intervention.
METHODS
Study Population
Between 2014 and 2017, 600 children with SM were enrolled in the study designed to assess cognition in children < 5 years of age at 12 months’ follow-up [11]. Children were eligible if they: (1) were between 6 months and 4 years of age; (2) had diagnostic evidence of malaria with either a positive rapid diagnostic test for Plasmodium falciparum histidine-rich protein-2 or direct visualization of parasites by Giemsa microscopy; (3) required hospitalization; and (4) had 1 of more of the following SM criteria: coma (Blantyre coma score < 3), respiratory distress (deep acidotic breathing or lower chest wall retractions), multiple seizures (≥ 2 generalized seizures in 24 hours or a seizure > 30 minutes in duration), severe anemia (hemoglobin < 5 g/dL), or prostration (≥ 1 year, unable to sit unsupported or stand; < 1 year, unable to breastfeed). Children were recruited at 2 referral hospitals in Central and Eastern Uganda: Mulago National Referral Hospital in Kampala and Jinja Regional Referral Hospital in Jinja. Exclusion criteria included the following: known chronic illness requiring medical care, history of coma, head trauma, known developmental delay, cerebral palsy, or prior hospitalization for malnutrition. Delayed exclusion criteria included an elevated cerebrospinal fluid white blood cell count in a child with coma.
Clinical Care and Participant Follow-up
Clinical care was supervised by study pediatricians. On enrollment, a medical history and physical examination were recorded. Children were managed according to the Uganda National Guidelines for the treatment of SM [12] with detailed management protocols summarized in Table S1. Fluids were administered intravenously as clinically indicated based on the degree of hypovolemia and shock with fluid administered as a slow infusion over 1 hour unless a bolus was indicated (shock with fluid loss). Children requiring blood transfusion were given packed red cells at a dose of 10 mL/kg over 4 hours or whole blood at 20 mL/kg if packed red blood cells were not available. Indications for blood transfusion included a hemoglobin ≤ 5 g/dL or < 6 g/dL with signs of respiratory distress. Malaria was treated with intravenous artesunate at a dose of 2.4 mg/kg if ≥ 20-kg body weight or 3 mg/kg for children < 20 kg at 0, 12, and 24 hours after enrollment followed by once-daily intravenous artesunate until the child was able tolerate oral artemether-lumefantrine. Participants with suspected concomitant bacteriemia received intravenous antibiotics unless blood culture results were negative or the patient improved clinically. All children in coma received intravenous ceftriaxone at 100 mg/kg until the cerebrospinal fluid analysis results revealed no laboratory evidence of meningitis. A pediatric nephrologist was consulted for children with AKI for symptomatic management and referral for dialysis as indicated. At the time the study was conducted, hemodialysis was available as a fee-based service on adult wards on a limited basis because of availability.
Assessment of Clinical Complications
Features of SM were defined according to the World Health Organization SM criteria [13]. Kidney function was evaluated using the World Health Organization definition of renal impairment (creatinine ≥ 3 mg/dL) and the Kidney Disease: Improving Global Outcomes criteria for AKI. AKI was defined as a 1.5-fold increase in serum creatinine over estimated baseline [14]. AKI staging was as follows: stage 1, 1.5- to 1.9-fold increase in creatinine over baseline; stage 2, 2.0- to 2.9-fold increase over baseline; and stage 3, ≥3.0-fold increase over baseline [14]. Baseline creatinine was estimated using a height-independent approach validated in the population [15]. Strict urine output monitoring was not routinely performed in either facility, so urine-based Kidney Disease: Improving Global Outcomes criteria were not applied to define AKI. Multiorgan dysfunction was assessed based on the presence of complications in the following organ systems: cerebral (coma, multiple seizures), respiratory (respiratory distress, deep breathing; hypoxemia), circulatory (cold peripheries, delayed capillary refill, shock), hematological (severe anemia, thrombocytopenia, platelet count < 150 × 103/µL, hemoglobinuria, abnormal bleeding), kidney (AKI, elevated BUN), and hepatic (jaundice, albumin < 3.5 mg/dL). Multiorgan dysfunction was defined as 2 or more organ systems affected. Additional complications assessed included hyperkalemia (potassium ≥ 6.0 mmol/L), hyponatremia (sodium < 130), and acidosis (base excess < –8 mmol/L or bicarbonate < 15 mmol/L if base excess was missing or lactate > 5 mmol/L if base excess and bicarbonate were missing).
Clinical Laboratory Assessments
Peripheral blood smears were used to quantify parasite density using Giemsa staining using the white blood cell count. Malaria parasite biomass was assessed by enzyme-linked immunosorbent assay quantifying plasma histidine-rich protein 2 levels (Malaria Ag Cellabs, Brookvale, Australia). Lithium heparin whole blood was used for an i-STAT handheld blood analyzer to assess a basic metabolic panel and blood gas values (Abbott Point of Care Inc., Princeton, New Jersey). Lactate and glucose levels were assessed using point-of-care analyzers (Accutrend Plus, Roche Diagnostics). Creatinine was assessed using a Beckman Coulter AU5822 chemistry analyzer using the modified Jaffe colorimetric method (Beckman Coulter, Brea, California), which is traceable to an isotope dilution mass spectrometry reference method using the US National Institute of Standards and Technology Standard Reference material SRM967. To assess for bacteremia, 1–3 mL of whole blood was inoculated into pediatric blood culture bottles (Peds Plus/F), which were incubated in an automatic BACTEC 9050 Blood culture system for up to 5 days. Positive samples were Gram stained and subcultured on blood agar, chocolate agar, or MacConkey agar plates. Human immunodeficiency virus serology was performed using antibody rapid diagnostic kits (Determine Alere, STAT-PAK, and Uni-Gold) using standard diagnostic criteria, with all seropositive samples in children < 18 months and indeterminate samples in children of any age confirmed by polymerase chain reaction (Abbott Laboratories Ltd.).
Ethics Approval and Consent to Participate
Initial verbal consent from the parents or legal guardians of study participants was obtained for children fulfilling inclusion criteria because most participants were critically ill and required emergency stabilization. Written informed consent was obtained once the participant was clinically stabilized. Ethical approval was granted by the institutional review boards at Makerere University School of Medicine, the University of Minnesota, and Indiana University, and regulatory approval granted by the Uganda National Council for Science and Technology.
Statistical Analysis
Data were analyzed using STATA v14.0 (StataCorp) and GraphPad Prism version 7.03 for Windows (GraphPad Software, La Jolla, California). Demographic and clinical characteristics of the study participants were summarized as descriptive statistics. Characteristics were compared using Student t test or Pearson χ2 test. Logistic regression was used to evaluate predictors of mortality and adjusted models included participant age, sex, and site. To adjust for multiple comparisons, the Benjamini-Hochberg false discovery procedure was applied at a threshold of 0.05.
RESULTS
Description of Baseline Demographic Characteristics of the Study Population
Between March 2014 and March 2017, 600 children with SM were enrolled in the study (Figure 1, Figure S1). The mean age of children was 2.1 years of age (standard deviation, 0.9) with 287 (47.8%) younger than 2 years of age. The most common features of SM were prostration (71.8%), acidosis (46.3%), AKI (45.3%), severe anemia (37.6%), and multiple seizures (42.2%) (Table 1). Multiorgan involvement was common, with 526 (88.1%) of children having more than 1 organ system affected.
Baseline Demographic and Laboratory Characteristics of Children Enrolled With Severe Malaria
| Demographics . | N . | Combined . | N . | Died . | N . | Survived . | P Value . |
|---|---|---|---|---|---|---|---|
| Age, y | 600 | 2.1 (0.9) | 44 | 2.1 (0.9) | 556 | 2.1 (0.9) | .884 |
| Sex, male, n (%) | 600 | 338 (56.3) | 44 | 19 (43.2) | 556 | 319 (57.4) | .068 |
| Weight-for-age z score | 592 | -1.1 (1.1) | 37 | -1.5 (1.1) | 555 | -1.0 (1.1) | .021 |
| Weight-for-height z score | 592 | -0.7 (1.1) | 37 | -1.0 (1.7) | 555 | -0.7 (1.1) | .073 |
| Height-for-age z score | 600 | -1.1 (1.3) | 44 | -1.4 (0.7) | 556 | -1.1 (1.3) | .147 |
| Site of enrollment, n (%) | |||||||
| Kampala | 600 | 332 (55.3) | 44 | 21 (47.7) | 556 | 311 (55.9) | .292 |
| Jinja | 268 (44.7) | 23 (52.3) | 245 (44.1) | ||||
| Medical history | |||||||
| Duration of fever, d | 588 | 3.6 (2.3) | 41 | 3.1 (2.0) | 547 | 3.6 (2.3) | .153 |
| Antimalarial use, n (%) | 589 | 364 (61.8) | 43 | 29 (67.4) | 546 | 335 (61.4) | .429 |
| Prereferral artesunate, n (%) | 589 | 91 (15.5) | 43 | 11 (25.6) | 546 | 80 (14.7) | .056 |
| Antibiotic use, n (%) | 589 | 203 (34.5) | 43 | 22 (51.2) | 546 | 181 (33.2) | .017 |
| Blackwater fever, n (%) | 596 | 141 (23.7) | 43 | 11 (25.6) | 553 | 130 (23.5) | .758 |
| Examination findings | |||||||
| Axillary temperature, °C | 599 | 37.7 (1.2) | 43 | 37.3 (1.5) | 556 | 37.8 (1.2) | .023 |
| Respiratory rate, beats/min | 599 | 51 (13) | 43 | 59 (14) | 556 | 50 (13) | <.0001 |
| Systolic blood pressure, mm Hg | 590 | 98 (13) | 38 | 102 (16) | 552 | 97 (13) | .046 |
| BCS | 598 | 4.2 (1.3) | 43 | 2. (1.6) | 555 | 4.3 (1.2) | <.0001 |
| Coma (BCS < 3), n (%) | 598 | 73 (12.2) | 43 | 20 (46.5) | 555 | 53 (9.6) | <.0001 |
| Multiple seizures, n (%) | 600 | 253 (42.2) | 44 | 20 (45.5) | 556 | 233 (41.9) | .646 |
| Deep breathing, n (%) | 599 | 129 (21.5) | 43 | 23 (53.5) | 556 | 106 (19.1) | <.0001 |
| Respiratory distress, n (%) | 599 | 175 (29.2) | 43 | 30 (69.8) | 556 | 145 (26.1) | <.0001 |
| Hypoxemia, n (%) | 598 | 63 (10.5) | 42 | 10 (23.8) | 556 | 53 (9.5) | .004 |
| Prostration, n (%) | 600 | 431 (71.8) | 44 | 43 (97.7) | 556 | 388 (69.8) | <.0001 |
| Jaundice, n (%) | 598 | 137 (22.9) | 42 | 16 (38.1) | 556 | 121 (21.8) | .015 |
| Cold peripheries, n (%) | 597 | 24 (4.0) | 43 | 9 (20.9) | 554 | 15 (2.7) | <.0001 |
| Delayed capillary refill, n (%) | 598 | 34 (5.7) | 43 | 9 (20.9) | 555 | 25 (4.5) | <.0001 |
| Shocka, n (%) | 589 | 49 (8.3) | 38 | 10 (26.3) | 551 | 39 (7.1) | <.0001 |
| Abnormal bleeding, n (%) | 599 | 9 (1.5) | 43 | 3 (7.0) | 556 | 6 (1.1) | .002 |
| Laboratory findings | |||||||
| WBC ×103/µL | 596 | 16.1 (12.0) | 43 | 21.6 (16.5) | 553 | 15.7 (11.5) | .002 |
| Neutrophil ×103/µL | 596 | 8.3 (6.3) | 43 | 11.4 (7.6) | 553 | 8.0 (6.1) | .0006 |
| Platelet count ×109/µL | 596 | 153 (121) | 43 | 139 (115) | 553 | 154 (122) | .426 |
| Hemoglobin, g/dL | 596 | 6.3 (3.0) | 43 | 6.3 (2.8) | 553 | 6.3 (3.0) | .933 |
| Parasite density, parasites/µL | 593 | 152210 (385973) | 33 | 342560 (715673) | 406 | 196723 (406703) | .066 |
| Plasma HRP-2, ng/mL | 593 | 4775 (7017) | 43 | 6033 (7834) | 550 | 4677 (6948) | .222 |
| Base excess, mmol/L | 571 | -9.0 (8.0) | 43 | -18.4 (7.7) | 528 | -8.3 (7.5) | <.0001 |
| Lactate, mmol/L | 574 | 5.7 (4.5) | 43 | 8.4 (5.7) | 531 | 5.5 (4.3) | <.0001 |
| Acidosisb, n (%) | 598 | 277(46.3) | 43 | 39 (90.7) | 555 | 238 (42.9) | <.0001 |
| BUN, mg/dL | 596 | 18 (20) | 43 | 36 (32) | 553 | 17 (18) | <.0001 |
| Creatinine, mg/dL | 598 | 0.5 (0.5) | 43 | 1.0 (1.3) | 555 | 0.4 (0.4) | <.0001 |
| Severe anemiac, n (%) | 600 | 271 (45.2) | 44 | 15 (34.1) | 556 | 256 (46.0) | .125 |
| Thrombocytopenia, n (%) | 596 | 363 (60.9) | 43 | 27 (62.8) | 553 | 336 (60.8) | .793 |
| AKId, n (%) | 598 | 271 (45.3) | 43 | 37 (86.1) | 555 | 234 (42.2) | <.0001 |
| Elevated BUNe, n (%) | 596 | 151 (25.3) | 43 | 31 (72.1) | 553 | 120 (21.7) | <.0001 |
| WHO Renal Impairment, n (%) | 598 | 6 (1.0) | 43 | 2 (4.7) | 555 | 4 (0.7) | .013 |
| Hyponatremia, n (%) | 559 | 46 (8.2) | 42 | 9 (21.4) | 517 | 37 (7.2) | .001 |
| Hyperkalemia, n (%) | 558 | 35 (6.3) | 42 | 12 (28.6) | 516 | 23 (4.5) | <.0001 |
| Hypoglycemiaf, n (%) | 595 | 24 (4.0) | 43 | 5 (11.6) | 552 | 19 (3.4) | .009 |
| Co-infections | |||||||
| HIV infection, n (%) | 595 | 13 (2.2) | 42 | 0 (0) | 553 | 13 (2.4) | .315 |
| Bacteremia, n (%) | 585 | 22 (3.8) | 41 | 2 (4.9) | 544 | 20 (3.7) | .697 |
| Antibiotic pretreatmentg | 200 | 6 (3.0) | 22 | 2 (9.1) | 178 | 4 (2.3) | .076 |
| No antibiotic pretreatment | 375 | 16 (4.3) | 19 | 0 (0.0) | 356 | 16 (4.5) | |
| Demographics . | N . | Combined . | N . | Died . | N . | Survived . | P Value . |
|---|---|---|---|---|---|---|---|
| Age, y | 600 | 2.1 (0.9) | 44 | 2.1 (0.9) | 556 | 2.1 (0.9) | .884 |
| Sex, male, n (%) | 600 | 338 (56.3) | 44 | 19 (43.2) | 556 | 319 (57.4) | .068 |
| Weight-for-age z score | 592 | -1.1 (1.1) | 37 | -1.5 (1.1) | 555 | -1.0 (1.1) | .021 |
| Weight-for-height z score | 592 | -0.7 (1.1) | 37 | -1.0 (1.7) | 555 | -0.7 (1.1) | .073 |
| Height-for-age z score | 600 | -1.1 (1.3) | 44 | -1.4 (0.7) | 556 | -1.1 (1.3) | .147 |
| Site of enrollment, n (%) | |||||||
| Kampala | 600 | 332 (55.3) | 44 | 21 (47.7) | 556 | 311 (55.9) | .292 |
| Jinja | 268 (44.7) | 23 (52.3) | 245 (44.1) | ||||
| Medical history | |||||||
| Duration of fever, d | 588 | 3.6 (2.3) | 41 | 3.1 (2.0) | 547 | 3.6 (2.3) | .153 |
| Antimalarial use, n (%) | 589 | 364 (61.8) | 43 | 29 (67.4) | 546 | 335 (61.4) | .429 |
| Prereferral artesunate, n (%) | 589 | 91 (15.5) | 43 | 11 (25.6) | 546 | 80 (14.7) | .056 |
| Antibiotic use, n (%) | 589 | 203 (34.5) | 43 | 22 (51.2) | 546 | 181 (33.2) | .017 |
| Blackwater fever, n (%) | 596 | 141 (23.7) | 43 | 11 (25.6) | 553 | 130 (23.5) | .758 |
| Examination findings | |||||||
| Axillary temperature, °C | 599 | 37.7 (1.2) | 43 | 37.3 (1.5) | 556 | 37.8 (1.2) | .023 |
| Respiratory rate, beats/min | 599 | 51 (13) | 43 | 59 (14) | 556 | 50 (13) | <.0001 |
| Systolic blood pressure, mm Hg | 590 | 98 (13) | 38 | 102 (16) | 552 | 97 (13) | .046 |
| BCS | 598 | 4.2 (1.3) | 43 | 2. (1.6) | 555 | 4.3 (1.2) | <.0001 |
| Coma (BCS < 3), n (%) | 598 | 73 (12.2) | 43 | 20 (46.5) | 555 | 53 (9.6) | <.0001 |
| Multiple seizures, n (%) | 600 | 253 (42.2) | 44 | 20 (45.5) | 556 | 233 (41.9) | .646 |
| Deep breathing, n (%) | 599 | 129 (21.5) | 43 | 23 (53.5) | 556 | 106 (19.1) | <.0001 |
| Respiratory distress, n (%) | 599 | 175 (29.2) | 43 | 30 (69.8) | 556 | 145 (26.1) | <.0001 |
| Hypoxemia, n (%) | 598 | 63 (10.5) | 42 | 10 (23.8) | 556 | 53 (9.5) | .004 |
| Prostration, n (%) | 600 | 431 (71.8) | 44 | 43 (97.7) | 556 | 388 (69.8) | <.0001 |
| Jaundice, n (%) | 598 | 137 (22.9) | 42 | 16 (38.1) | 556 | 121 (21.8) | .015 |
| Cold peripheries, n (%) | 597 | 24 (4.0) | 43 | 9 (20.9) | 554 | 15 (2.7) | <.0001 |
| Delayed capillary refill, n (%) | 598 | 34 (5.7) | 43 | 9 (20.9) | 555 | 25 (4.5) | <.0001 |
| Shocka, n (%) | 589 | 49 (8.3) | 38 | 10 (26.3) | 551 | 39 (7.1) | <.0001 |
| Abnormal bleeding, n (%) | 599 | 9 (1.5) | 43 | 3 (7.0) | 556 | 6 (1.1) | .002 |
| Laboratory findings | |||||||
| WBC ×103/µL | 596 | 16.1 (12.0) | 43 | 21.6 (16.5) | 553 | 15.7 (11.5) | .002 |
| Neutrophil ×103/µL | 596 | 8.3 (6.3) | 43 | 11.4 (7.6) | 553 | 8.0 (6.1) | .0006 |
| Platelet count ×109/µL | 596 | 153 (121) | 43 | 139 (115) | 553 | 154 (122) | .426 |
| Hemoglobin, g/dL | 596 | 6.3 (3.0) | 43 | 6.3 (2.8) | 553 | 6.3 (3.0) | .933 |
| Parasite density, parasites/µL | 593 | 152210 (385973) | 33 | 342560 (715673) | 406 | 196723 (406703) | .066 |
| Plasma HRP-2, ng/mL | 593 | 4775 (7017) | 43 | 6033 (7834) | 550 | 4677 (6948) | .222 |
| Base excess, mmol/L | 571 | -9.0 (8.0) | 43 | -18.4 (7.7) | 528 | -8.3 (7.5) | <.0001 |
| Lactate, mmol/L | 574 | 5.7 (4.5) | 43 | 8.4 (5.7) | 531 | 5.5 (4.3) | <.0001 |
| Acidosisb, n (%) | 598 | 277(46.3) | 43 | 39 (90.7) | 555 | 238 (42.9) | <.0001 |
| BUN, mg/dL | 596 | 18 (20) | 43 | 36 (32) | 553 | 17 (18) | <.0001 |
| Creatinine, mg/dL | 598 | 0.5 (0.5) | 43 | 1.0 (1.3) | 555 | 0.4 (0.4) | <.0001 |
| Severe anemiac, n (%) | 600 | 271 (45.2) | 44 | 15 (34.1) | 556 | 256 (46.0) | .125 |
| Thrombocytopenia, n (%) | 596 | 363 (60.9) | 43 | 27 (62.8) | 553 | 336 (60.8) | .793 |
| AKId, n (%) | 598 | 271 (45.3) | 43 | 37 (86.1) | 555 | 234 (42.2) | <.0001 |
| Elevated BUNe, n (%) | 596 | 151 (25.3) | 43 | 31 (72.1) | 553 | 120 (21.7) | <.0001 |
| WHO Renal Impairment, n (%) | 598 | 6 (1.0) | 43 | 2 (4.7) | 555 | 4 (0.7) | .013 |
| Hyponatremia, n (%) | 559 | 46 (8.2) | 42 | 9 (21.4) | 517 | 37 (7.2) | .001 |
| Hyperkalemia, n (%) | 558 | 35 (6.3) | 42 | 12 (28.6) | 516 | 23 (4.5) | <.0001 |
| Hypoglycemiaf, n (%) | 595 | 24 (4.0) | 43 | 5 (11.6) | 552 | 19 (3.4) | .009 |
| Co-infections | |||||||
| HIV infection, n (%) | 595 | 13 (2.2) | 42 | 0 (0) | 553 | 13 (2.4) | .315 |
| Bacteremia, n (%) | 585 | 22 (3.8) | 41 | 2 (4.9) | 544 | 20 (3.7) | .697 |
| Antibiotic pretreatmentg | 200 | 6 (3.0) | 22 | 2 (9.1) | 178 | 4 (2.3) | .076 |
| No antibiotic pretreatment | 375 | 16 (4.3) | 19 | 0 (0.0) | 356 | 16 (4.5) | |
Data presented are mean (standard deviation) unless otherwise indicated.
Abbreviations: AKI, acute kidney infection; BCS, Blantyre coma scale; BUN, blood urea nitrogen; HIV, human immunodeficiency virus; HRP-2, histidine-rich protein-2; KDIGO, Kidney Disease: Improving Global Outcomes; WBC, white blood cell; WHO, World Health Organization.
Compensated and decompensated shock.
Base excess < –8 mmol/L or bicarbonate < 15 mmol/L if base excess was missing or lactate > 5 mmol/L if base excess and bicarbonate were missing.
Hemoglobin ≤ 5 g/dL.
KDIGO guidelines (1.5× increase in creatinine from estimated baseline or 0.3 mg/dL increase in creatinine within 24 hours of admission).
BUN > 20 mg/dL.
Glucose < 2.2 mmol/dL.
Antibiotic pretreatment missing in 10 participants with culture results (n = 575).
Baseline Demographic and Laboratory Characteristics of Children Enrolled With Severe Malaria
| Demographics . | N . | Combined . | N . | Died . | N . | Survived . | P Value . |
|---|---|---|---|---|---|---|---|
| Age, y | 600 | 2.1 (0.9) | 44 | 2.1 (0.9) | 556 | 2.1 (0.9) | .884 |
| Sex, male, n (%) | 600 | 338 (56.3) | 44 | 19 (43.2) | 556 | 319 (57.4) | .068 |
| Weight-for-age z score | 592 | -1.1 (1.1) | 37 | -1.5 (1.1) | 555 | -1.0 (1.1) | .021 |
| Weight-for-height z score | 592 | -0.7 (1.1) | 37 | -1.0 (1.7) | 555 | -0.7 (1.1) | .073 |
| Height-for-age z score | 600 | -1.1 (1.3) | 44 | -1.4 (0.7) | 556 | -1.1 (1.3) | .147 |
| Site of enrollment, n (%) | |||||||
| Kampala | 600 | 332 (55.3) | 44 | 21 (47.7) | 556 | 311 (55.9) | .292 |
| Jinja | 268 (44.7) | 23 (52.3) | 245 (44.1) | ||||
| Medical history | |||||||
| Duration of fever, d | 588 | 3.6 (2.3) | 41 | 3.1 (2.0) | 547 | 3.6 (2.3) | .153 |
| Antimalarial use, n (%) | 589 | 364 (61.8) | 43 | 29 (67.4) | 546 | 335 (61.4) | .429 |
| Prereferral artesunate, n (%) | 589 | 91 (15.5) | 43 | 11 (25.6) | 546 | 80 (14.7) | .056 |
| Antibiotic use, n (%) | 589 | 203 (34.5) | 43 | 22 (51.2) | 546 | 181 (33.2) | .017 |
| Blackwater fever, n (%) | 596 | 141 (23.7) | 43 | 11 (25.6) | 553 | 130 (23.5) | .758 |
| Examination findings | |||||||
| Axillary temperature, °C | 599 | 37.7 (1.2) | 43 | 37.3 (1.5) | 556 | 37.8 (1.2) | .023 |
| Respiratory rate, beats/min | 599 | 51 (13) | 43 | 59 (14) | 556 | 50 (13) | <.0001 |
| Systolic blood pressure, mm Hg | 590 | 98 (13) | 38 | 102 (16) | 552 | 97 (13) | .046 |
| BCS | 598 | 4.2 (1.3) | 43 | 2. (1.6) | 555 | 4.3 (1.2) | <.0001 |
| Coma (BCS < 3), n (%) | 598 | 73 (12.2) | 43 | 20 (46.5) | 555 | 53 (9.6) | <.0001 |
| Multiple seizures, n (%) | 600 | 253 (42.2) | 44 | 20 (45.5) | 556 | 233 (41.9) | .646 |
| Deep breathing, n (%) | 599 | 129 (21.5) | 43 | 23 (53.5) | 556 | 106 (19.1) | <.0001 |
| Respiratory distress, n (%) | 599 | 175 (29.2) | 43 | 30 (69.8) | 556 | 145 (26.1) | <.0001 |
| Hypoxemia, n (%) | 598 | 63 (10.5) | 42 | 10 (23.8) | 556 | 53 (9.5) | .004 |
| Prostration, n (%) | 600 | 431 (71.8) | 44 | 43 (97.7) | 556 | 388 (69.8) | <.0001 |
| Jaundice, n (%) | 598 | 137 (22.9) | 42 | 16 (38.1) | 556 | 121 (21.8) | .015 |
| Cold peripheries, n (%) | 597 | 24 (4.0) | 43 | 9 (20.9) | 554 | 15 (2.7) | <.0001 |
| Delayed capillary refill, n (%) | 598 | 34 (5.7) | 43 | 9 (20.9) | 555 | 25 (4.5) | <.0001 |
| Shocka, n (%) | 589 | 49 (8.3) | 38 | 10 (26.3) | 551 | 39 (7.1) | <.0001 |
| Abnormal bleeding, n (%) | 599 | 9 (1.5) | 43 | 3 (7.0) | 556 | 6 (1.1) | .002 |
| Laboratory findings | |||||||
| WBC ×103/µL | 596 | 16.1 (12.0) | 43 | 21.6 (16.5) | 553 | 15.7 (11.5) | .002 |
| Neutrophil ×103/µL | 596 | 8.3 (6.3) | 43 | 11.4 (7.6) | 553 | 8.0 (6.1) | .0006 |
| Platelet count ×109/µL | 596 | 153 (121) | 43 | 139 (115) | 553 | 154 (122) | .426 |
| Hemoglobin, g/dL | 596 | 6.3 (3.0) | 43 | 6.3 (2.8) | 553 | 6.3 (3.0) | .933 |
| Parasite density, parasites/µL | 593 | 152210 (385973) | 33 | 342560 (715673) | 406 | 196723 (406703) | .066 |
| Plasma HRP-2, ng/mL | 593 | 4775 (7017) | 43 | 6033 (7834) | 550 | 4677 (6948) | .222 |
| Base excess, mmol/L | 571 | -9.0 (8.0) | 43 | -18.4 (7.7) | 528 | -8.3 (7.5) | <.0001 |
| Lactate, mmol/L | 574 | 5.7 (4.5) | 43 | 8.4 (5.7) | 531 | 5.5 (4.3) | <.0001 |
| Acidosisb, n (%) | 598 | 277(46.3) | 43 | 39 (90.7) | 555 | 238 (42.9) | <.0001 |
| BUN, mg/dL | 596 | 18 (20) | 43 | 36 (32) | 553 | 17 (18) | <.0001 |
| Creatinine, mg/dL | 598 | 0.5 (0.5) | 43 | 1.0 (1.3) | 555 | 0.4 (0.4) | <.0001 |
| Severe anemiac, n (%) | 600 | 271 (45.2) | 44 | 15 (34.1) | 556 | 256 (46.0) | .125 |
| Thrombocytopenia, n (%) | 596 | 363 (60.9) | 43 | 27 (62.8) | 553 | 336 (60.8) | .793 |
| AKId, n (%) | 598 | 271 (45.3) | 43 | 37 (86.1) | 555 | 234 (42.2) | <.0001 |
| Elevated BUNe, n (%) | 596 | 151 (25.3) | 43 | 31 (72.1) | 553 | 120 (21.7) | <.0001 |
| WHO Renal Impairment, n (%) | 598 | 6 (1.0) | 43 | 2 (4.7) | 555 | 4 (0.7) | .013 |
| Hyponatremia, n (%) | 559 | 46 (8.2) | 42 | 9 (21.4) | 517 | 37 (7.2) | .001 |
| Hyperkalemia, n (%) | 558 | 35 (6.3) | 42 | 12 (28.6) | 516 | 23 (4.5) | <.0001 |
| Hypoglycemiaf, n (%) | 595 | 24 (4.0) | 43 | 5 (11.6) | 552 | 19 (3.4) | .009 |
| Co-infections | |||||||
| HIV infection, n (%) | 595 | 13 (2.2) | 42 | 0 (0) | 553 | 13 (2.4) | .315 |
| Bacteremia, n (%) | 585 | 22 (3.8) | 41 | 2 (4.9) | 544 | 20 (3.7) | .697 |
| Antibiotic pretreatmentg | 200 | 6 (3.0) | 22 | 2 (9.1) | 178 | 4 (2.3) | .076 |
| No antibiotic pretreatment | 375 | 16 (4.3) | 19 | 0 (0.0) | 356 | 16 (4.5) | |
| Demographics . | N . | Combined . | N . | Died . | N . | Survived . | P Value . |
|---|---|---|---|---|---|---|---|
| Age, y | 600 | 2.1 (0.9) | 44 | 2.1 (0.9) | 556 | 2.1 (0.9) | .884 |
| Sex, male, n (%) | 600 | 338 (56.3) | 44 | 19 (43.2) | 556 | 319 (57.4) | .068 |
| Weight-for-age z score | 592 | -1.1 (1.1) | 37 | -1.5 (1.1) | 555 | -1.0 (1.1) | .021 |
| Weight-for-height z score | 592 | -0.7 (1.1) | 37 | -1.0 (1.7) | 555 | -0.7 (1.1) | .073 |
| Height-for-age z score | 600 | -1.1 (1.3) | 44 | -1.4 (0.7) | 556 | -1.1 (1.3) | .147 |
| Site of enrollment, n (%) | |||||||
| Kampala | 600 | 332 (55.3) | 44 | 21 (47.7) | 556 | 311 (55.9) | .292 |
| Jinja | 268 (44.7) | 23 (52.3) | 245 (44.1) | ||||
| Medical history | |||||||
| Duration of fever, d | 588 | 3.6 (2.3) | 41 | 3.1 (2.0) | 547 | 3.6 (2.3) | .153 |
| Antimalarial use, n (%) | 589 | 364 (61.8) | 43 | 29 (67.4) | 546 | 335 (61.4) | .429 |
| Prereferral artesunate, n (%) | 589 | 91 (15.5) | 43 | 11 (25.6) | 546 | 80 (14.7) | .056 |
| Antibiotic use, n (%) | 589 | 203 (34.5) | 43 | 22 (51.2) | 546 | 181 (33.2) | .017 |
| Blackwater fever, n (%) | 596 | 141 (23.7) | 43 | 11 (25.6) | 553 | 130 (23.5) | .758 |
| Examination findings | |||||||
| Axillary temperature, °C | 599 | 37.7 (1.2) | 43 | 37.3 (1.5) | 556 | 37.8 (1.2) | .023 |
| Respiratory rate, beats/min | 599 | 51 (13) | 43 | 59 (14) | 556 | 50 (13) | <.0001 |
| Systolic blood pressure, mm Hg | 590 | 98 (13) | 38 | 102 (16) | 552 | 97 (13) | .046 |
| BCS | 598 | 4.2 (1.3) | 43 | 2. (1.6) | 555 | 4.3 (1.2) | <.0001 |
| Coma (BCS < 3), n (%) | 598 | 73 (12.2) | 43 | 20 (46.5) | 555 | 53 (9.6) | <.0001 |
| Multiple seizures, n (%) | 600 | 253 (42.2) | 44 | 20 (45.5) | 556 | 233 (41.9) | .646 |
| Deep breathing, n (%) | 599 | 129 (21.5) | 43 | 23 (53.5) | 556 | 106 (19.1) | <.0001 |
| Respiratory distress, n (%) | 599 | 175 (29.2) | 43 | 30 (69.8) | 556 | 145 (26.1) | <.0001 |
| Hypoxemia, n (%) | 598 | 63 (10.5) | 42 | 10 (23.8) | 556 | 53 (9.5) | .004 |
| Prostration, n (%) | 600 | 431 (71.8) | 44 | 43 (97.7) | 556 | 388 (69.8) | <.0001 |
| Jaundice, n (%) | 598 | 137 (22.9) | 42 | 16 (38.1) | 556 | 121 (21.8) | .015 |
| Cold peripheries, n (%) | 597 | 24 (4.0) | 43 | 9 (20.9) | 554 | 15 (2.7) | <.0001 |
| Delayed capillary refill, n (%) | 598 | 34 (5.7) | 43 | 9 (20.9) | 555 | 25 (4.5) | <.0001 |
| Shocka, n (%) | 589 | 49 (8.3) | 38 | 10 (26.3) | 551 | 39 (7.1) | <.0001 |
| Abnormal bleeding, n (%) | 599 | 9 (1.5) | 43 | 3 (7.0) | 556 | 6 (1.1) | .002 |
| Laboratory findings | |||||||
| WBC ×103/µL | 596 | 16.1 (12.0) | 43 | 21.6 (16.5) | 553 | 15.7 (11.5) | .002 |
| Neutrophil ×103/µL | 596 | 8.3 (6.3) | 43 | 11.4 (7.6) | 553 | 8.0 (6.1) | .0006 |
| Platelet count ×109/µL | 596 | 153 (121) | 43 | 139 (115) | 553 | 154 (122) | .426 |
| Hemoglobin, g/dL | 596 | 6.3 (3.0) | 43 | 6.3 (2.8) | 553 | 6.3 (3.0) | .933 |
| Parasite density, parasites/µL | 593 | 152210 (385973) | 33 | 342560 (715673) | 406 | 196723 (406703) | .066 |
| Plasma HRP-2, ng/mL | 593 | 4775 (7017) | 43 | 6033 (7834) | 550 | 4677 (6948) | .222 |
| Base excess, mmol/L | 571 | -9.0 (8.0) | 43 | -18.4 (7.7) | 528 | -8.3 (7.5) | <.0001 |
| Lactate, mmol/L | 574 | 5.7 (4.5) | 43 | 8.4 (5.7) | 531 | 5.5 (4.3) | <.0001 |
| Acidosisb, n (%) | 598 | 277(46.3) | 43 | 39 (90.7) | 555 | 238 (42.9) | <.0001 |
| BUN, mg/dL | 596 | 18 (20) | 43 | 36 (32) | 553 | 17 (18) | <.0001 |
| Creatinine, mg/dL | 598 | 0.5 (0.5) | 43 | 1.0 (1.3) | 555 | 0.4 (0.4) | <.0001 |
| Severe anemiac, n (%) | 600 | 271 (45.2) | 44 | 15 (34.1) | 556 | 256 (46.0) | .125 |
| Thrombocytopenia, n (%) | 596 | 363 (60.9) | 43 | 27 (62.8) | 553 | 336 (60.8) | .793 |
| AKId, n (%) | 598 | 271 (45.3) | 43 | 37 (86.1) | 555 | 234 (42.2) | <.0001 |
| Elevated BUNe, n (%) | 596 | 151 (25.3) | 43 | 31 (72.1) | 553 | 120 (21.7) | <.0001 |
| WHO Renal Impairment, n (%) | 598 | 6 (1.0) | 43 | 2 (4.7) | 555 | 4 (0.7) | .013 |
| Hyponatremia, n (%) | 559 | 46 (8.2) | 42 | 9 (21.4) | 517 | 37 (7.2) | .001 |
| Hyperkalemia, n (%) | 558 | 35 (6.3) | 42 | 12 (28.6) | 516 | 23 (4.5) | <.0001 |
| Hypoglycemiaf, n (%) | 595 | 24 (4.0) | 43 | 5 (11.6) | 552 | 19 (3.4) | .009 |
| Co-infections | |||||||
| HIV infection, n (%) | 595 | 13 (2.2) | 42 | 0 (0) | 553 | 13 (2.4) | .315 |
| Bacteremia, n (%) | 585 | 22 (3.8) | 41 | 2 (4.9) | 544 | 20 (3.7) | .697 |
| Antibiotic pretreatmentg | 200 | 6 (3.0) | 22 | 2 (9.1) | 178 | 4 (2.3) | .076 |
| No antibiotic pretreatment | 375 | 16 (4.3) | 19 | 0 (0.0) | 356 | 16 (4.5) | |
Data presented are mean (standard deviation) unless otherwise indicated.
Abbreviations: AKI, acute kidney infection; BCS, Blantyre coma scale; BUN, blood urea nitrogen; HIV, human immunodeficiency virus; HRP-2, histidine-rich protein-2; KDIGO, Kidney Disease: Improving Global Outcomes; WBC, white blood cell; WHO, World Health Organization.
Compensated and decompensated shock.
Base excess < –8 mmol/L or bicarbonate < 15 mmol/L if base excess was missing or lactate > 5 mmol/L if base excess and bicarbonate were missing.
Hemoglobin ≤ 5 g/dL.
KDIGO guidelines (1.5× increase in creatinine from estimated baseline or 0.3 mg/dL increase in creatinine within 24 hours of admission).
BUN > 20 mg/dL.
Glucose < 2.2 mmol/dL.
Antibiotic pretreatment missing in 10 participants with culture results (n = 575).
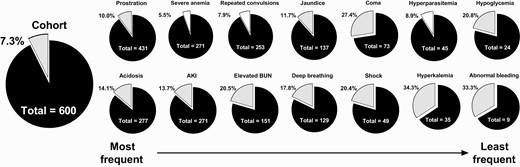
Pie charts of mortality by clinical complication. Pie charts showing the frequency of children death in children with severe malaria (gray) for the entire cohort (left) and specific clinical complications organized from the most common complications to the least frequent. Complications associated with increased mortality are shaded in gray while complications not associated with mortality (severe anemia, repeated convulsions, hyperparasitemia) are shaded in white. Abbreviation: AKI, acute kidney injury.
Mortality in Children Admitted With SM
Overall, 44 children died during admission, corresponding to a case fatality rate of 7.3%. Mortality was comparable between Kampala (6.3%) and Jinja (8.6%) (P = .292). Most deaths occurred within 12 hours of admission (52.3%) with a median (interquartile range) time to death of 9.4 (2.5-24.3) hours from admission, and 72.7% of deaths occurred within 24 hours. To identify risk factors for mortality, we compared the baseline demographic, clinical, and laboratory characteristics of children who died with those who survived (Table 1).
Ten clinical and laboratory variables predicted mortality in children with SM after adjusting for participant age, sex, and site of enrollment and correcting for multiple comparisons. Clinically defined variables included coma (adjusted odds ratio [aOR], 8.3; 95% confidence interval [CI], 4.2–16.3), cold peripheries (aOR, 9.6; 95% CI, 3.8–23.8), delayed capillary refill time (aOR, 6.0; 95% CI, 2.5–14.0), shock (aOR, 4.8; 95% CI, 2.2–10.8), respiratory distress (aOR, 7.4; 95% CI, 3.6–15.1), and deep breathing (aOR, 5.2; 95% CI, 2.7–10.1). Laboratory defined variables included AKI (aOR, 8.6; 95% CI ,3.5–20.9), elevated BUN (aOR, 9.3; 95% CI, 4.6–18.7), acidosis (aOR, 12.5; 95% CI, 4.4–35.7), and hyperkalemia (aOR, 9.3; 95% CI, 3.8–22.6) (Figure 2).
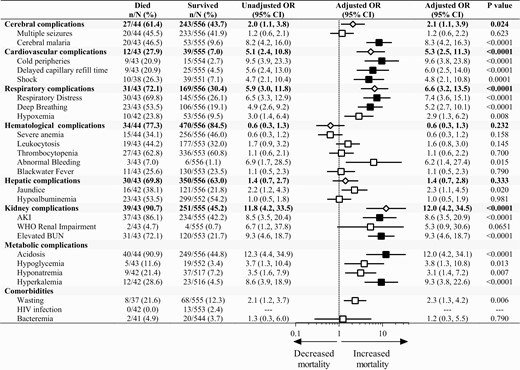
Clinical features in severe malaria associated with increased mortality. Forest plot depicting the adjusted odds ratio from a logistic regression adjusting for child age, sex, and site. Results significant following adjustment for multiple comparisons (n = 24) are shaded in black. Composite variables of organ dysfunction are presented as a diamond based on the following definitions: cerebral complications, the presence of coma or multiple seizures on admission; cardiovascular complications, cold peripheries, delayed capillary refill, shock; respiratory complications, deep breathing, respiratory distress, hypoxemia; hematological complications, leukocytosis, severe anemia, thrombocytopenia, abnormal bleeding, blackwater fever; liver complications, jaundice, hypoalbuminemia; kidney complications, AKI, elevated blood urea nitrogen. Abbreviations: AKI, acute kidney injury; OR, odds ratio.
Persistent organ dysfunction after clinical stabilization was associated with increased mortality. Children who died had higher lactate levels at 12 and 24 hours of hospitalization compared with children who survived (12 hours, 5.9 [died] vs 3.3 [survived]; 24 hours, 7.1 [died] vs 2.6 [survived]; P < .0001 for both). Further, mortality was 7.2% in children with AKI at 24 hours compared with 0.6% in children without AKI at 24 hours (P < .0001).
Acute Kidney Injury Interacts With Other Complications to Increase Mortality Risk
Although AKI is recognized as a risk factor for mortality in SM, the interrelationships between AKI and other risk factors for mortality are not well described. Among the predictors of mortality, we conducted additional analyses to evaluate the interaction between AKI and other clinical predictors in Figure 3. Among the highly correlated variables that reflected impairment of perfusion (cold peripheries, shock, delayed capillary refill time), we selected the single variable with the strongest correlation with mortality (cold peripheries) to evaluate the interaction with AKI. AKI was present in 60% in children with coma and associated with 9.35-fold increased odds of mortality (95% CI, 1.97-44.34). When evaluating mortality of children with SM, the presence of AKI had a synergistic effect, increasing mortality in children with the 5 other risk factors: coma, acidosis, impaired perfusion (cold peripheries), elevated BUN, or hyperkalemia (Figure 3, Table S2; P < .001 interaction term). Because AKI strongly interacted with all other factors predictive of mortality, a multivariate model including all factors and adjusting for each was not appropriate.
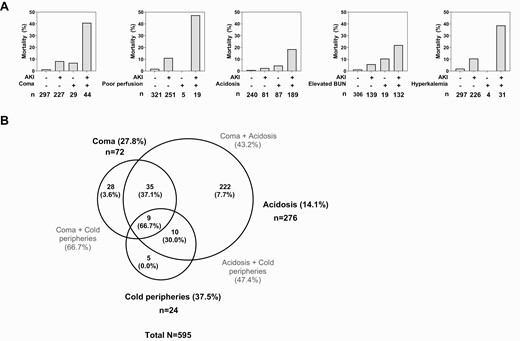
Interrelationship between AKI and clinical complications associated with severe malaria mortality. A, top row, Bar graphs showing that mortality in children with or without AKI and the presence of coma, impaired or poor perfusion (cold peripheries), acidosis, elevated BUN, or hyperkalemia. For each complication there is evidence of interaction between the clinical complication and AKI with mortality substantially higher in children with both complications (P < .0001 in clinical model based on the interaction term). B, Venn diagram showing the intersection of clinical complications with mortality with the number of complications in each area indicated and the frequency of mortality in the specified groups presented in the brackets as a percentage. Abbreviations: AKI, acute kidney injury; BUN, blood urea nitrogen; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; WHO, World Health Organization.
Mortality was markedly higher in children with a clinical indication for kidney support therapy (KST) defined as life-threatening BUN (1.5%, BUN > 100 mg/dL), acidosis (11.5%, bicarbonate < 8 mmol/L), or hyperkalemia (4.5%, potassium > 7.1 mmol/L) compared with children without (24.4% vs 4.3%; OR, 6.56; 95% CI, 3.41–12.59; P < .0001). Overall, 14.2% of children (85/598) had a clinical indication for KST and multiple indications for dialysis resulted in a substantial increase in mortality (Figure 4). Among children who died, 47% had an indication for dialysis and died faster than children without a KST indication (median, 5.6 hours vs 19.6 hours; P = .036, respectively). Although hemodialysis was available in a limited capacity at the Mulago site, none of the children survived long enough to be transferred and receive dialysis.
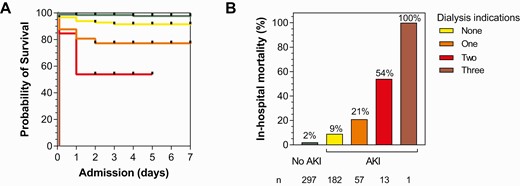
Mortality increases substantially in children with severe malaria according to the number of indications for dialysis present on admission. A, Kaplan-Meier survival graph showing percent survival during hospitalization based on admission kidney function. Children with no AKI are in green, children with AKI but no clinical indications for dialysis in yellow, children with 1 indication for dialysis (blood urea nitrogen > 100 mg/dL, bicarbonate < 8 mmol/L, potassium > 7.1 mmol/L) in orange, children with 2 indications in red, and 3 indications for dialysis in dark red. B, Bar graph showing the frequency of death based on the number of clinical indications for dialysis. Abbreviation: AKI, acute kidney injury.
DISCUSSION
In the present study, we demonstrate that AKI substantially increases mortality in children with SM and interacts with other clinical complications to synergistically increase the risk of death. We also show that hyperkalemia, a previously unstudied risk factor, was not uncommon in children with SM (6.3% of children in this cohort), and when seen with AKI was associated with very high mortality (38.7%). We further demonstrate that indications for dialysis strongly correlate with mortality, and that mortality increases with the number of dialysis indications present.
Mortality in the present study was compatible with estimates from the AQUAMAT randomized clinical trial (8.5%) [2]. Because mortality frequently occurred in < 12 hours following admission, this study highlights the limited therapeutic window in children presenting with underlying circulatory, neurological, metabolic, and kidney complications [3]. Our results are consistent with previous studies reporting most SM deaths occur within 24 hours of hospitalization [2, 16, 17]. Our study findings suggest that a better understanding of the causes of coma, impaired perfusion, acidosis, AKI, elevated BUN, and hyperkalemia will be critical to the design of interventions to successfully reduce mortality in SM. While we investigate the causes of these complications, interventions such as dialysis have the potential to reduce mortality in children with SM who present with severe elevated BUN, hyperkalemia, or acidosis.
AKI was associated with severe metabolic and electrolyte abnormalities with the finding of hyperkalemia as a risk factor for mortality a novel finding. Few prior studies measured serum potassium. Hyperkalemia is an independent risk factor for mortality, primarily from the effects of severe hyperkalemia on cardiac function [18]. In the present study, hyperkalemia in the presence of AKI was associated with high mortality, suggesting that potassium levels should be measured when feasible. Interventions to manage hyperkalemia are needed including limiting potassium intake, use of calcium gluconate, kayexalate (sodium polystyrene sulfonate), salbutamol nebulization, or sodium bicarbonate in the context of acidosis. KST is an effective methodology to normalize the serum potassium in children who do not respond to medical therapy [19].
There is increasing evidence that acidosis may contribute to AKI by reducing renal blood flow and driving inflammatory responses that may contribute to structural kidney injury [20]. This is supported by the findings in our cohort, in which the presence of acidosis and AKI were associated with substantially higher mortality than either complication alone. Acidosis not responsive to medical management can be corrected by KST using peritoneal dialysis or hemodialysis. However, in children with multiorgan failure, lactic acidosis remains a mortality risk factor despite KST [21]. Acidosis was one of the most common complications associated with increased mortality and frequently occurs in the context of AKI. Acidosis in SM may occur because of infected red blood cell sequestration in end organs and consequent local ischemia and hypoxia or from end-organ and peripheral hypoperfusion. The kidney is the primary organ responsible for maintaining acid-base homeostasis and electrolytes, and impaired kidney function leads to impaired excretion of acids and regulation of electrolytes [22]. Interventions to decrease or reverse sequestration have been proposed, but none has yet been successful in randomized clinical trials.
Consistent with previous studies, elevated BUN was a risk factor for mortality [3]. In the AQUAMAT study, elevated BUN was associated with multiple SM complications, including anemia and shock, which suggests dehydration and hypovolemia may contribute to elevated BUN [23]. BUN could be used to risk-stratify children at increased risk of mortality. The development of an affordable dipstick-based saliva urea nitrogen test that correlates with BUN [24] represents a promising tool to identify children with elevated BUN and AKI at risk of mortality (reviewed in [10]).
Shock and impaired perfusion are known complications of SM, but only shock has been previously described as a risk factor for mortality, and the origin of shock or impaired perfusion in SM is still unclear. Bacteremia may have contributed to sepsis or septic shock in a larger number of children than those with detected bacteremia because 203 children with severe malaria (34.5%) had antibiotic treatment before admission, which may have decreased yield of blood bacterial cultures. We cannot rule out sepsis as an underlying contributor to both mortality and AKI in the cohort. Myocarditis and parasitized red blood cells in the myocardium have been documented in SM, and clinical studies of children have noted abnormal echocardiogram findings in 4%–9% of children [25]. Further studies evaluating cardiac function in children with SM may help to elucidate the contribution of cardiac dysfunction to shock or impaired perfusion in SM.
Cerebral malaria is one of the most severe manifestations of SM with mortality occurring in 13%–30% of children [3, 26]. Cerebral malaria also represents a risk factor for long-term neurocognitive, behavioral, and mental health problems in surviving children, making it a leading cause of acquired neurodisability in sub-Saharan Africa [27–37]. Mortality in children with coma was substantially higher in the presence of AKI, suggesting that clinically silent complications may contribute to the high mortality observed in children with cerebral malaria.
On admission, 14.2% of children with SM had clinical indications for KST, and these children had very high mortality. This finding supports the need for expanded access to KST to reduce mortality in SM. KST may consist of peritoneal dialysis, hemodialysis, and continuous venous–venous hemofiltration. Despite availability of hemodialysis in our setting, access was limited. Peritoneal dialysis is technically feasible in children across a range of settings, can be initiated emergently at the bedside, is an acceptable KST modality for AKI, and is more cost-effective than other forms of KST [38]. The effectiveness of KST may depend on the primary underlying metabolic anomaly.
In addition to expanding access to KST, adaptation of guidelines to prevent AKI progression and improve AKI management should be strengthened across malaria endemic areas [14, 39]. AKI progression may be reduced with prompt restoration of kidney perfusion, correction of metabolic abnormalities, discontinuation of nonessential nephrotoxic medications, and administration of potential kidney-protective medications (eg, acetaminophen) [11, 40].
CONCLUSIONS
Despite the introduction of highly effective antimalarial therapies, mortality in malaria remains high in the context of multiorgan dysfunction. This study suggests that supportive care alone may be insufficient to reduce SM mortality and increased access to diagnostics and therapeutics to identify and treat life-threatening acid-base and electrolyte abnormalities will be needed to improve outcomes. The striking findings regarding increased mortality with increasing indications for dialysis highlight an urgent need for better access to KST in sub-Saharan Africa.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
NOTES
Acknowledgments. The authors thank Mulago Hospital, Jinja Regional Referral Hospital Children’s ward, and Global Health Uganda for conducting the study; study participants and their caregivers; and our dedicated study teams for the excellent clinical care and follow-up provided to all children. The authors specifically acknowledge Gloria Kyarisima and Robert Twine for data management; Quaraish Serwanja, Tukei Robert, Alex Kitwamuwesi, and Peter Wambi for clinical care; Friday Rose for study coordination and nursing care; and Leticia Murungi for quality control and regulatory oversight.
Author Contributions. R. N., R. O. O., A. L. C., P.B., and C. C. J. designed the study, supervised the study, analyzed the results, and wrote the first draft of the manuscript. D. D. and A. L. C. performed laboratory analyses, supervised laboratory processes, analyzed the results, and monitored the study. A. B. supervised clinical care of study participants. All authors participated in the editing of the manuscript and approved the final version.
Financial support. This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke (R01NS055349, D43 NS078280 to C. C. J.) and the Fogarty International Center (D43 TW010928 to C. C. J.), and a Ralph W. and Grace M. Showalter Young Investigator Award to ALC. The funders had no role in data collection, analysis, interpretation, or the decision to publish.
References
Author notes
A. L. C. and C. C. J. contributed equally to this work.
Potential conflicts of interest. A. L. C. reports being a member of the XXV pediatric Acute Dialysis Quality Initiative on Setting the Landscape and Designing the Future of Pediatric Critical Care Nephrology. C. C. J. reports participation on FEVER DSMB (Birbeck, principal investigator) and Treating Brain Welling in Cerebral Malaria (Taylor, principal investigator). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




