-
PDF
- Split View
-
Views
-
Cite
Cite
Helena Huerga, Uzma Khan, Mathieu Bastard, Carole D Mitnick, Nathalie Lachenal, Palwasha Y Khan, Kwonjune J Seung, Nara Melikyan, Saman Ahmed, Michael L Rich, Francis Varaine, Elna Osso, Makhmujan Rashitov, Naseem Salahuddin, Gocha Salia, Epifanio Sánchez, Armine Serobyan, Muhammad Rafi Siddiqui, Dri Grium Tefera, Dmitry Vetushko, Lusine Yeghiazaryan, David Holtzman, Shirajul Islam, Andargachew Kumsa, Gamarly Jacques Leblanc, Olga Leonovich, Shahid Mamsa, Mohammad Manzur-ul-Alam, Zaw Myint, Shrivani Padayachee, Molly F Franke, Catherine Hewison, endTB study observational study team, Safety and Effectiveness Outcomes From a 14-Country Cohort of Patients With Multi-Drug Resistant Tuberculosis Treated Concomitantly With Bedaquiline, Delamanid, and Other Second-Line Drugs, Clinical Infectious Diseases, Volume 75, Issue 8, 15 October 2022, Pages 1307–1314, https://doi.org/10.1093/cid/ciac176
Close - Share Icon Share
Abstract
Concomitant use of bedaquiline (Bdq) and delamanid (Dlm) for multi-drug/rifampicin resistant tuberculosis (MDR/RR-TB) has raised concerns about a potentially poor risk-benefit ratio. Yet this combination is an important alternative for patients infected with strains of TB with complex drug resistance profiles or who cannot tolerate other therapies. We assessed safety and treatment outcomes of MDR/RR-TB patients receiving concomitant Bdq and Dlm, along with other second-line anti-TB drugs.
We conducted a multi-centric, prospective observational cohort study across 14 countries among patients receiving concomitant Bdq-Dlm treatment. Patients were recruited between April 2015 and September 2018 and were followed until the end of treatment. All serious adverse events and adverse events of special interest (AESI), leading to a treatment change, or judged significant by a clinician, were systematically monitored and documented.
Overall, 472 patients received Bdq and Dlm concomitantly. A large majority also received linezolid (89.6%) and clofazimine (84.5%). Nearly all (90.3%) had extensive disease; most (74.2%) had resistance to fluoroquinolones. The most common AESI were peripheral neuropathy (134, 28.4%) and electrolyte depletion (94, 19.9%). Acute kidney injury and myelosuppression were seen in 40 (8.5%) and 24 (5.1%) of patients, respectively. QT prolongation occurred in 7 patients (1.5%). Overall, 78.0% (358/458) had successful treatment outcomes, 8.9% died, and 7.2% experienced treatment failure.
Concomitant use of Bdq and Dlm, along with linezolid and clofazimine, is safe and effective for MDR/RR-TB patients with extensive disease. Using these drugs concomitantly is a good therapeutic option for patients with resistance to many anti-TB drugs.
Bedaquiline (Bdq) and delamanid (Dlm), are two of the world’s newest anti-tuberculosis agents. Concerns have been raised about a potentially poor risk-benefit ratio when used together for multi-drug/rifampicin resistant tuberculosis (MDR/RR-TB). Yet, the concomitant use of Bdq and Dlm along with other second-line drugs such as linezolid or clofazimine is an important alternative for patients infected with strains of TB with complex drug resistance profiles or who cannot tolerate other therapies [1].
The possibility of increased rates of cardiotoxicity have limited co-administration of Bdq and Dlm to situations where all other treatment options are exhausted [2]. Many clinicians and National TB Programs in the areas hardest hit by MDR/RR-TB continue to avoid using Bdq and Dlm in combination out of fear of their potential overlapping toxicity [3]. Widespread concomitant use is also hampered by limited access to the drugs, especially Dlm, in part related to its high cost, and to the restricted recommendations for its use in international guidelines for MDR/RR-TB treatment [4]. These recommendations were based on equivocal results of a phase 3 trial that aimed to evaluate the effect of Dlm when added to an already optimized regimen [5]. However, the limited concomitant use of Bdq and Dlm when needed may increase the use of other combinations that are less efficacious or have greater toxicity including QT prolongation.
Limited evidence from small studies have shown good efficacy and safety when Bdq and Dlm were used concomitantly [6–15] and a randomized trial in 84 patients found no evidence of grade 3 or 4 adverse QTc prolongation events and no deaths when using Bdq and Dlm in combination [16]. However, a low sample size results in limited precision in estimates and possibility that important, but rare adverse events did not occur. Moreover, these studies included small proportions of patients receiving linezolid and clofazimine, 2 top tier drugs in MDR/RR-TB treatment [4]. Here we assessed the safety and effectiveness outcomes from a large prospective cohort of patients treated concomitantly with Bdq and Dlm along with other second-line anti-TB drugs such as linezolid and clofazimine, across 14 countries.
METHODS
Study Design and Population
We conducted a multi-center, prospective observational study, that consecutively recruited MDR/RR-TB patients between 1 April 2015 and 30 September 2018 and followed them until end of treatment. In the present analysis, we included patients from 14 countries (Armenia, Bangladesh, Belarus, Ethiopia, Haiti, Indonesia, Kazakhstan, Kenya, Kyrgyzstan, Lesotho, Myanmar, Pakistan, Peru, and South Africa) who received concomitant treatment with Bdq and Dlm (defined as receiving both drugs for at least 1 day at any time during treatment). More details on the study methodology and recruitment procedures can be found in the published protocol for the endTB observational study [17].
Study Procedures
In each country, patients were treated and monitored according to national guidelines and the World Health Organization (WHO) guidance during the study period [18]. Upon initiating treatment (and then monthly thereafter), patients underwent a clinical examination, electrocardiogram (ECG), audiometry (for those receiving second-line, injectable medications), laboratory blood tests, sputum smear microscopy, and culture. Drug-susceptibility testing (DST) was performed at baseline and as indicated. As part of clinical monitoring, comorbidities were assessed at the start of treatment, as were any adverse events occurring during treatment. All serious adverse events (SAE) and adverse events of special interest (AESI) of any severity, as well as adverse events (AE) leading to a change in treatment or that were judged clinically significant by a clinician, were documented. AESIs were defined a priori due to their relevance to treatment. We defined clinically relevant AESIs as those severe enough to warrant at a change in TB regimen or electrolytes/thyroxine supplementation, according to the endTB Clinical Guide [19]. Supplementary Table 1 shows the defined severity thresholds for the clinically relevant AESIs. The clinician responsible for each patient assessed AE severity, event outcome, and causality, and data were entered into a local endTB electronic medical record [17]. In addition, SAEs were reported to the MSF pharmacovigilance (PV) unit in Geneva, Switzerland, to local authorities as applicable in each study country and, as appropriate, to drug manufacturers. A full description of the SAE cases, including demographics, relevant preexisting conditions, TB history, concomitant or prior drugs, tests results and investigations, and TB drugs was reported to the PV unit [20]. After preliminary medical review by the PV unit officers, difficult cases, particularly unexpected SAEs that were possibly drug-related, were reviewed by the medical review board (MRB), which included at least 2 PV officers and 2 experienced MDR-TB experts. The PV unit medical reviewers and the MRB reviewed the site causality assessment and provided support to sites in medical management. Drug causality assessment was conservative: drugs were classified as “related” unless clear other causal factors were identified, and relatedness could be excluded.
Statistical Analyses
We described patient and treatment characteristics using frequencies and percentages for categorical variables, and median and interquartile ranges (IQRs) for continuous variables. Extensive disease was defined as a positive baseline sputum smear of 3+ and cavitary disease on the chest X-ray [21].
We calculated the prevalence and incidence of clinically relevant AESI (first occurrence during concomitant use of Bdq and Dlm) as well as their 95% confidence intervals (CI). Median and IQRs described the number of months until the first event occurred. Incidence was calculated as the number of events per 1000 person-months of concomitant treatment. Person-months of exposure were counted from the start of concomitant treatment until the event or until 2 days after concomitant treatment was stopped, whichever came first. In addition, we described the frequency of SAEs (whether treatment related or not), the severity distribution of clinically relevant AESI (maximum severity grade is reported if patient experienced the event more than once) and the proportion of patients affected by each clinically relevant AESI who recovered without sequelae. We also provided additional clinical and treatment details on patients with fatal SAEs reported as “death (of undetermined cause)” or “sudden death.” Further information on the safety of receiving multidrug-resistant regimens containing Bdq or Dlm in the endTB observational study cohort, and exhaustive information on fatal events has been reported elsewhere [22, 23]. We examined safety and effectiveness stratified by timing of Bdq and Dlm introduction (at treatment initiation versus later in treatment). We described the frequency of temporary and permanent drug discontinuation due to AEs during the period of concomitant use of Bdq and Dlm among patients who received Bdq and Dlm at treatment initiation. Treatment outcomes were those assigned by the treating clinician as per the WHO 2013 recommendations [24]. As WHO treatment outcome definitions were interpreted differently across study sites, we standardized the outcomes by applying an algorithm that included treatment duration and bacteriological results. We conducted sensitivity analyses to calculate treatment outcomes using this algorithm.
Ethical Considerations
The endTB observational study protocol was approved by the MSF Ethics Review Board (Geneva, Switzerland), the Partners Healthcare Human Research Committee (Boston, Massachusetts, USA), IRD Institutional Review Board (Karachi, Pakistan), and the local ethics review boards in each of the countries where the study was conducted. Written informed consent was obtained from all participants. Minors (<18 years) provided assent, and consent was obtained from parents or guardians per local legal regulations.
RESULTS
Study Population
Of the 2731 patients included in the endTB cohort, a total of 472 (17.3%) patients received Bdq and Dlm concomitantly, 311 (65.9%) at MDR/RR-TB treatment initiation. A large majority of patients received linezolid (423, 89.6%) and clofazimine (399, 84.5%) in addition to Bdq and Dlm. Of the 311 patients who received Bdq and Dlm at MDR/RR-TB treatment initiation, 302 (97.1%) also received linezolid and 280 (90.0%) clofazimine. An injectable agent (aminoglycoside or polypeptide) was used concomitantly in 82/472 (17.4%) patients: 26/311 (8.4%) in whom Bdq and Dlm were started at initiation and in 56/161 (34.8%) patients in whom concomitant use started later.
The median age was 36 (IQR: 29–46) years and 289 (61.2%) were men. Many had comorbidities, including 15.5% with human immunodeficiency virus (HIV), 14.7% with hepatitis C virus (HCV), and 16.1% with diabetes, and 39.0% had a low body mass index (BMI <18.5 kg/m2). Nearly all patients (90.3%) had extensive disease. Most (85.5%) of the cohort had been previously treated with 2nd line drugs, including 92.9% of those who received Bdq and Dlm at MDR/RR-TB treatment initiation. Most of the patients (74.2%) had resistance to fluoroquinolones, and half (53.2%) had resistance to both fluoroquinolones and injectable medications. A detailed description of patient characteristics can be found in Table 1. The distribution of study participants by country can be found in Supplementary Table 2.
Baseline Characteristics of 472 MDR-TB Patients Receiving Concomitant Bedaquiline and Delamanid Therapy in 14 Countries.
| . | Concomitant Bdq and Dlm at MDR/RR-TB Treatment Initiation N = 311 . | Concomitant Bdq and Dlm during MDR/RR-TB Treatment N = 161 . | Total N = 472 . | |||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . |
| Demographics (N = 472) | ||||||
| Men | 200 | 64.3 | 89 | 55.3 | 289 | 61.2 |
| Median age [25th, 75th percentile] | 36 [2946] | 36 [29–46] | 36 [29–46] | |||
| Comorbidities and clinical status | ||||||
| Low body mass index (<18.5 Kg/m2) (N = 438) | 102 | 35.4 | 69 | 46.0 | 171 | 39.0 |
| HIV positive (N = 471)a | 33 | 10.6 | 40 | 24.8 | 73 | 15.5 |
| Hepatitis C positive antibody (N = 470)b | 54 | 17.4 | 15 | 9.4 | 69 | 14.7 |
| Hepatitis B positive surface antigen (N = 469) | 22 | 7.1 | 2 | 1.3 | 24 | 5.1 |
| Diabetes (N = 467) | 48 | 15.5 | 27 | 17.1 | 75 | 16.1 |
| Anemia (Hemoglobin <8 g/dl) (N = 462) | 5 | 1.6 | 7 | 4.5 | 12 | 2.6 |
| Other non-communicable diseasesc (N = 472) | 34 | 10.9 | 16 | 9.9 | 50 | 10.6 |
| Hospitalized at treatment initiation (N = 472) | 252 | 81.0 | 92 | 57.1 | 344 | 72.9 |
| Previous TB treatment (N = 472) | ||||||
| No previous TB treatment | 16 | 5.1 | 21 | 13.0 | 37 | 7.8 |
| Previously treated only with first line TB drugs | 6 | 1.9 | 26 | 16.1 | 32 | 6.8 |
| Previously treated with second line TB drugs | 289 | 92.9 | 114 | 70.8 | 403 | 85.4 |
| Disease site and severity (N = 472) | ||||||
| Extrapulmonary | 1 | 0.3 | 1 | 0.6 | 2 | 0.4 |
| Pulmonary | 310 | 99.7 | 160 | 99.4 | 470 | 99.6 |
| Extensive diseased (N = 432) | 270 | 90.6 | 120 | 89.6 | 390 | 90.3 |
| Resistance profiles (N = 472) | ||||||
| MDR/RR-TB with fluoroquinolone and injectable resistance | 193 | 62.1 | 58 | 36.0 | 251 | 53.2 |
| MDR/RR-TB with fluoroquinolone resistance | 54 | 17.4 | 45 | 28.0 | 99 | 21.0 |
| MDR/RR-TB with injectable medication resistance | 14 | 4.5 | 17 | 10.6 | 31 | 6.6 |
| MDR/RR-TB without fluoroquinolone or injectable resistance | 29 | 9.3 | 23 | 14.3 | 52 | 11.0 |
| No resistance test results | 21 | 6.7 | 18 | 11.2 | 39 | 8.3 |
| Anti-TB drugs received at treatment initiation (N = 472) | ||||||
| Linezolid | 302 | 97.1 | 121 | 75.2 | 423 | 89.6 |
| Clofazimine | 280 | 90.0 | 119 | 73.9 | 399 | 84.5 |
| Pyrazinamide | 107 | 34.4 | 101 | 62.7 | 208 | 44.1 |
| Carbapenem | 132 | 42.4 | 28 | 17.4 | 160 | 33.9 |
| Moxifloxacin | 54 | 17.4 | 102 | 63.4 | 156 | 33.1 |
| Cycloserine | 78 | 25.1 | 77 | 47.8 | 155 | 32.8 |
| Ethionamide or Prothionamide | 36 | 11.6 | 69 | 42.9 | 105 | 22.2 |
| Injectable medicatione | 26 | 8.4 | 56 | 34.8 | 82 | 17.4 |
| P-Aminosalicylic acid | 27 | 8.7 | 46 | 28.6 | 73 | 15.5 |
| . | Concomitant Bdq and Dlm at MDR/RR-TB Treatment Initiation N = 311 . | Concomitant Bdq and Dlm during MDR/RR-TB Treatment N = 161 . | Total N = 472 . | |||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . |
| Demographics (N = 472) | ||||||
| Men | 200 | 64.3 | 89 | 55.3 | 289 | 61.2 |
| Median age [25th, 75th percentile] | 36 [2946] | 36 [29–46] | 36 [29–46] | |||
| Comorbidities and clinical status | ||||||
| Low body mass index (<18.5 Kg/m2) (N = 438) | 102 | 35.4 | 69 | 46.0 | 171 | 39.0 |
| HIV positive (N = 471)a | 33 | 10.6 | 40 | 24.8 | 73 | 15.5 |
| Hepatitis C positive antibody (N = 470)b | 54 | 17.4 | 15 | 9.4 | 69 | 14.7 |
| Hepatitis B positive surface antigen (N = 469) | 22 | 7.1 | 2 | 1.3 | 24 | 5.1 |
| Diabetes (N = 467) | 48 | 15.5 | 27 | 17.1 | 75 | 16.1 |
| Anemia (Hemoglobin <8 g/dl) (N = 462) | 5 | 1.6 | 7 | 4.5 | 12 | 2.6 |
| Other non-communicable diseasesc (N = 472) | 34 | 10.9 | 16 | 9.9 | 50 | 10.6 |
| Hospitalized at treatment initiation (N = 472) | 252 | 81.0 | 92 | 57.1 | 344 | 72.9 |
| Previous TB treatment (N = 472) | ||||||
| No previous TB treatment | 16 | 5.1 | 21 | 13.0 | 37 | 7.8 |
| Previously treated only with first line TB drugs | 6 | 1.9 | 26 | 16.1 | 32 | 6.8 |
| Previously treated with second line TB drugs | 289 | 92.9 | 114 | 70.8 | 403 | 85.4 |
| Disease site and severity (N = 472) | ||||||
| Extrapulmonary | 1 | 0.3 | 1 | 0.6 | 2 | 0.4 |
| Pulmonary | 310 | 99.7 | 160 | 99.4 | 470 | 99.6 |
| Extensive diseased (N = 432) | 270 | 90.6 | 120 | 89.6 | 390 | 90.3 |
| Resistance profiles (N = 472) | ||||||
| MDR/RR-TB with fluoroquinolone and injectable resistance | 193 | 62.1 | 58 | 36.0 | 251 | 53.2 |
| MDR/RR-TB with fluoroquinolone resistance | 54 | 17.4 | 45 | 28.0 | 99 | 21.0 |
| MDR/RR-TB with injectable medication resistance | 14 | 4.5 | 17 | 10.6 | 31 | 6.6 |
| MDR/RR-TB without fluoroquinolone or injectable resistance | 29 | 9.3 | 23 | 14.3 | 52 | 11.0 |
| No resistance test results | 21 | 6.7 | 18 | 11.2 | 39 | 8.3 |
| Anti-TB drugs received at treatment initiation (N = 472) | ||||||
| Linezolid | 302 | 97.1 | 121 | 75.2 | 423 | 89.6 |
| Clofazimine | 280 | 90.0 | 119 | 73.9 | 399 | 84.5 |
| Pyrazinamide | 107 | 34.4 | 101 | 62.7 | 208 | 44.1 |
| Carbapenem | 132 | 42.4 | 28 | 17.4 | 160 | 33.9 |
| Moxifloxacin | 54 | 17.4 | 102 | 63.4 | 156 | 33.1 |
| Cycloserine | 78 | 25.1 | 77 | 47.8 | 155 | 32.8 |
| Ethionamide or Prothionamide | 36 | 11.6 | 69 | 42.9 | 105 | 22.2 |
| Injectable medicatione | 26 | 8.4 | 56 | 34.8 | 82 | 17.4 |
| P-Aminosalicylic acid | 27 | 8.7 | 46 | 28.6 | 73 | 15.5 |
Abbreviations: Bdq, bedaquiline; Dlm, delamanid; HIV, human immunodeficiency virus; MDR/RR-TB, multi-drug/rifampicin resistant tuberculosis.
HIV-positive patients: 54 on antiretroviral treatment (4 HIV-positive with missing antiretroviral therapy [ART] data).
Hepatitis C positive antibody patients: 2 on direct-acting antivirals (DAAs).
Other non-communicable diseases: renal insufficiency, cirrhosis, COPD, cancer, heart disease, depression.
Extensive disease: positive baseline sputum smear of 3+ and cavitary disease on the chest X-ray.
Injectable medication: 42 capreomycin, 19 amikacin, 21 kanamycin.
Baseline Characteristics of 472 MDR-TB Patients Receiving Concomitant Bedaquiline and Delamanid Therapy in 14 Countries.
| . | Concomitant Bdq and Dlm at MDR/RR-TB Treatment Initiation N = 311 . | Concomitant Bdq and Dlm during MDR/RR-TB Treatment N = 161 . | Total N = 472 . | |||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . |
| Demographics (N = 472) | ||||||
| Men | 200 | 64.3 | 89 | 55.3 | 289 | 61.2 |
| Median age [25th, 75th percentile] | 36 [2946] | 36 [29–46] | 36 [29–46] | |||
| Comorbidities and clinical status | ||||||
| Low body mass index (<18.5 Kg/m2) (N = 438) | 102 | 35.4 | 69 | 46.0 | 171 | 39.0 |
| HIV positive (N = 471)a | 33 | 10.6 | 40 | 24.8 | 73 | 15.5 |
| Hepatitis C positive antibody (N = 470)b | 54 | 17.4 | 15 | 9.4 | 69 | 14.7 |
| Hepatitis B positive surface antigen (N = 469) | 22 | 7.1 | 2 | 1.3 | 24 | 5.1 |
| Diabetes (N = 467) | 48 | 15.5 | 27 | 17.1 | 75 | 16.1 |
| Anemia (Hemoglobin <8 g/dl) (N = 462) | 5 | 1.6 | 7 | 4.5 | 12 | 2.6 |
| Other non-communicable diseasesc (N = 472) | 34 | 10.9 | 16 | 9.9 | 50 | 10.6 |
| Hospitalized at treatment initiation (N = 472) | 252 | 81.0 | 92 | 57.1 | 344 | 72.9 |
| Previous TB treatment (N = 472) | ||||||
| No previous TB treatment | 16 | 5.1 | 21 | 13.0 | 37 | 7.8 |
| Previously treated only with first line TB drugs | 6 | 1.9 | 26 | 16.1 | 32 | 6.8 |
| Previously treated with second line TB drugs | 289 | 92.9 | 114 | 70.8 | 403 | 85.4 |
| Disease site and severity (N = 472) | ||||||
| Extrapulmonary | 1 | 0.3 | 1 | 0.6 | 2 | 0.4 |
| Pulmonary | 310 | 99.7 | 160 | 99.4 | 470 | 99.6 |
| Extensive diseased (N = 432) | 270 | 90.6 | 120 | 89.6 | 390 | 90.3 |
| Resistance profiles (N = 472) | ||||||
| MDR/RR-TB with fluoroquinolone and injectable resistance | 193 | 62.1 | 58 | 36.0 | 251 | 53.2 |
| MDR/RR-TB with fluoroquinolone resistance | 54 | 17.4 | 45 | 28.0 | 99 | 21.0 |
| MDR/RR-TB with injectable medication resistance | 14 | 4.5 | 17 | 10.6 | 31 | 6.6 |
| MDR/RR-TB without fluoroquinolone or injectable resistance | 29 | 9.3 | 23 | 14.3 | 52 | 11.0 |
| No resistance test results | 21 | 6.7 | 18 | 11.2 | 39 | 8.3 |
| Anti-TB drugs received at treatment initiation (N = 472) | ||||||
| Linezolid | 302 | 97.1 | 121 | 75.2 | 423 | 89.6 |
| Clofazimine | 280 | 90.0 | 119 | 73.9 | 399 | 84.5 |
| Pyrazinamide | 107 | 34.4 | 101 | 62.7 | 208 | 44.1 |
| Carbapenem | 132 | 42.4 | 28 | 17.4 | 160 | 33.9 |
| Moxifloxacin | 54 | 17.4 | 102 | 63.4 | 156 | 33.1 |
| Cycloserine | 78 | 25.1 | 77 | 47.8 | 155 | 32.8 |
| Ethionamide or Prothionamide | 36 | 11.6 | 69 | 42.9 | 105 | 22.2 |
| Injectable medicatione | 26 | 8.4 | 56 | 34.8 | 82 | 17.4 |
| P-Aminosalicylic acid | 27 | 8.7 | 46 | 28.6 | 73 | 15.5 |
| . | Concomitant Bdq and Dlm at MDR/RR-TB Treatment Initiation N = 311 . | Concomitant Bdq and Dlm during MDR/RR-TB Treatment N = 161 . | Total N = 472 . | |||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . |
| Demographics (N = 472) | ||||||
| Men | 200 | 64.3 | 89 | 55.3 | 289 | 61.2 |
| Median age [25th, 75th percentile] | 36 [2946] | 36 [29–46] | 36 [29–46] | |||
| Comorbidities and clinical status | ||||||
| Low body mass index (<18.5 Kg/m2) (N = 438) | 102 | 35.4 | 69 | 46.0 | 171 | 39.0 |
| HIV positive (N = 471)a | 33 | 10.6 | 40 | 24.8 | 73 | 15.5 |
| Hepatitis C positive antibody (N = 470)b | 54 | 17.4 | 15 | 9.4 | 69 | 14.7 |
| Hepatitis B positive surface antigen (N = 469) | 22 | 7.1 | 2 | 1.3 | 24 | 5.1 |
| Diabetes (N = 467) | 48 | 15.5 | 27 | 17.1 | 75 | 16.1 |
| Anemia (Hemoglobin <8 g/dl) (N = 462) | 5 | 1.6 | 7 | 4.5 | 12 | 2.6 |
| Other non-communicable diseasesc (N = 472) | 34 | 10.9 | 16 | 9.9 | 50 | 10.6 |
| Hospitalized at treatment initiation (N = 472) | 252 | 81.0 | 92 | 57.1 | 344 | 72.9 |
| Previous TB treatment (N = 472) | ||||||
| No previous TB treatment | 16 | 5.1 | 21 | 13.0 | 37 | 7.8 |
| Previously treated only with first line TB drugs | 6 | 1.9 | 26 | 16.1 | 32 | 6.8 |
| Previously treated with second line TB drugs | 289 | 92.9 | 114 | 70.8 | 403 | 85.4 |
| Disease site and severity (N = 472) | ||||||
| Extrapulmonary | 1 | 0.3 | 1 | 0.6 | 2 | 0.4 |
| Pulmonary | 310 | 99.7 | 160 | 99.4 | 470 | 99.6 |
| Extensive diseased (N = 432) | 270 | 90.6 | 120 | 89.6 | 390 | 90.3 |
| Resistance profiles (N = 472) | ||||||
| MDR/RR-TB with fluoroquinolone and injectable resistance | 193 | 62.1 | 58 | 36.0 | 251 | 53.2 |
| MDR/RR-TB with fluoroquinolone resistance | 54 | 17.4 | 45 | 28.0 | 99 | 21.0 |
| MDR/RR-TB with injectable medication resistance | 14 | 4.5 | 17 | 10.6 | 31 | 6.6 |
| MDR/RR-TB without fluoroquinolone or injectable resistance | 29 | 9.3 | 23 | 14.3 | 52 | 11.0 |
| No resistance test results | 21 | 6.7 | 18 | 11.2 | 39 | 8.3 |
| Anti-TB drugs received at treatment initiation (N = 472) | ||||||
| Linezolid | 302 | 97.1 | 121 | 75.2 | 423 | 89.6 |
| Clofazimine | 280 | 90.0 | 119 | 73.9 | 399 | 84.5 |
| Pyrazinamide | 107 | 34.4 | 101 | 62.7 | 208 | 44.1 |
| Carbapenem | 132 | 42.4 | 28 | 17.4 | 160 | 33.9 |
| Moxifloxacin | 54 | 17.4 | 102 | 63.4 | 156 | 33.1 |
| Cycloserine | 78 | 25.1 | 77 | 47.8 | 155 | 32.8 |
| Ethionamide or Prothionamide | 36 | 11.6 | 69 | 42.9 | 105 | 22.2 |
| Injectable medicatione | 26 | 8.4 | 56 | 34.8 | 82 | 17.4 |
| P-Aminosalicylic acid | 27 | 8.7 | 46 | 28.6 | 73 | 15.5 |
Abbreviations: Bdq, bedaquiline; Dlm, delamanid; HIV, human immunodeficiency virus; MDR/RR-TB, multi-drug/rifampicin resistant tuberculosis.
HIV-positive patients: 54 on antiretroviral treatment (4 HIV-positive with missing antiretroviral therapy [ART] data).
Hepatitis C positive antibody patients: 2 on direct-acting antivirals (DAAs).
Other non-communicable diseases: renal insufficiency, cirrhosis, COPD, cancer, heart disease, depression.
Extensive disease: positive baseline sputum smear of 3+ and cavitary disease on the chest X-ray.
Injectable medication: 42 capreomycin, 19 amikacin, 21 kanamycin.
A majority (59.1%; 279) of patients received concomitant treatment for more than 6 months. Very few patients (1.1%; 5) received the 2 drugs for <2 weeks. The median duration of concomitant treatment with Bdq and Dlm was 8 months (IQR: 4–13): 10 months (IQR: 5–14) for patients who received the 2 drugs at MDR/RR-TB treatment initiation and 6 months (IQR: 3–11) for those who received the 2 drugs concomitantly later in treatment. For the latter group, concomitant treatment began a median of 4 months (IQR: 1–7) after treatment initiation.
Safety Profiles
The most common clinically relevant AESIs found in this cohort were peripheral neuropathy (134, 28.4%), often associated with linezolid, and electrolyte depletion (94, 19.9%), often associated with injectable agents (Table 2). Although the severity grade was low for most cases of peripheral neuropathy, only one third (30.0%) of them recovered without sequelae (Figure 1, Supplementary Table 3). Other AESIs commonly associated with linezolid use, such as myelosuppression and optic neuritis, were seen in 5.1% and 2.5% of patients, respectively. Most of the myelosuppression and two-thirds of the optic neuritis events resolved without sequelae. Acute renal failure and hearing loss, commonly associated with use of injectable agents were detected in 8.5% and 3.4% of patients, respectively. Less than half (46.2%) of patients with hearing loss recovered without sequelae. Prolongation of the QT interval (often associated with clofazimine, bedaquiline, fluoroquinolones, and delamanid) was infrequent, occurring in 1.5% of patients, and all episodes resolved. Most clinically relevant AESIs occurred in the first 2–4 months of treatment except optic neuritis, which was detected after a median of 7 months. The frequency of serious adverse events reported during treatment is shown in Table 3. Further information on the 2 patients for whom death was reported as a serious adverse event during Bdq and Dlm treatment (1 sudden death and 1 death of undetermined cause) can be found in Supplementary Table 4. Patients who received concomitant Bdq and Dlm at treatment initiation (almost all also with linezolid or clofazimine) had similar safety profiles to those who received the two drugs during treatment (along with linezolid and clofazimine in lower proportions, and injectables in a higher proportion). Notable exceptions were peripheral neuropathy and acute renal failure, which were more frequent in patients who received Bdq and Dlm at treatment initiation (31.8% vs 21.7% and 10.9% vs 3.7%, respectively), and hearing loss, which was more common in patients in whom concomitant use started later (2.6% vs 5.0%) (Supplementary Table 5). Among patients who received Bdq, Dlm at treatment initiation, the frequency of temporary and permanent drug discontinuation due to AEs during the period of concomitant use was respectively: 21.5% (65/302) and 10.6% (32/302) for linezolid, 16.7% (47/280) and 6.4% (18/280) for clofazimine, 13.2% (41/311) and 4.5% (14/311) for delamanid, 12.9% (40/311), and 3.9% (12/311) for bedaquiline.
Adverse Events of Special Interest (AESI) Clinically Relevant or Reported as Serious Adverse Events (SAE), Occurring During Concomitant Treatment With Bedaquiline and Delamanid Among 472 MDR/RR-TB Patients in 14 Countries
| . | Frequency . | Time to First Event . | Incidence/1000 person-month (95% CI) . |
|---|---|---|---|
| . | n (%) . | Median [IQR] . | |
| Peripheral neuropathy | 134 (28.4) | 3.0 [1.0–5.3] | 39.0 (33.0–46.9) |
| Electrolyte depletion | 94 (19.9) | 4.0 [1.4–7.6] | 23.8 (19.4–29.1) |
| Acute renal failure | 40 (8.5) | 3.7 [1.7–8.4] | 9.4 (6.9–12.9) |
| Myelosuppression | 24 (5.1) | 2.8 [1.3–7.8] | 5.6 (3.7–8.4) |
| Hearing loss | 16 (3.4) | 2.3 [1.0–6.4] | 3.6 (2.2–5.9) |
| Hepatotoxicity | 13 (2.7) | 2.1 [1.8–10.0] | 2.9 (1.6–4.9) |
| Optic neuritis | 12 (2.5) | 6.9 [5.2–9.3] | 2.7 (1.5–4.8) |
| Hypothyroidism | 7 (1.5) | 2.2 [0.4–2.8] | 1.6 (0.7–3.3) |
| QT prolongation | 7 (1.5) | 3.2 [2.0–5.5] | 1.5 (0.7–3.2) |
| . | Frequency . | Time to First Event . | Incidence/1000 person-month (95% CI) . |
|---|---|---|---|
| . | n (%) . | Median [IQR] . | |
| Peripheral neuropathy | 134 (28.4) | 3.0 [1.0–5.3] | 39.0 (33.0–46.9) |
| Electrolyte depletion | 94 (19.9) | 4.0 [1.4–7.6] | 23.8 (19.4–29.1) |
| Acute renal failure | 40 (8.5) | 3.7 [1.7–8.4] | 9.4 (6.9–12.9) |
| Myelosuppression | 24 (5.1) | 2.8 [1.3–7.8] | 5.6 (3.7–8.4) |
| Hearing loss | 16 (3.4) | 2.3 [1.0–6.4] | 3.6 (2.2–5.9) |
| Hepatotoxicity | 13 (2.7) | 2.1 [1.8–10.0] | 2.9 (1.6–4.9) |
| Optic neuritis | 12 (2.5) | 6.9 [5.2–9.3] | 2.7 (1.5–4.8) |
| Hypothyroidism | 7 (1.5) | 2.2 [0.4–2.8] | 1.6 (0.7–3.3) |
| QT prolongation | 7 (1.5) | 3.2 [2.0–5.5] | 1.5 (0.7–3.2) |
Frequencies include SAEs also reported in Table 3.
Abbreviations: CI, confidence interval; IQR, interquartile range; MDR/RR-TB, multi-drug/rifampicin resistant tuberculosis.
Adverse Events of Special Interest (AESI) Clinically Relevant or Reported as Serious Adverse Events (SAE), Occurring During Concomitant Treatment With Bedaquiline and Delamanid Among 472 MDR/RR-TB Patients in 14 Countries
| . | Frequency . | Time to First Event . | Incidence/1000 person-month (95% CI) . |
|---|---|---|---|
| . | n (%) . | Median [IQR] . | |
| Peripheral neuropathy | 134 (28.4) | 3.0 [1.0–5.3] | 39.0 (33.0–46.9) |
| Electrolyte depletion | 94 (19.9) | 4.0 [1.4–7.6] | 23.8 (19.4–29.1) |
| Acute renal failure | 40 (8.5) | 3.7 [1.7–8.4] | 9.4 (6.9–12.9) |
| Myelosuppression | 24 (5.1) | 2.8 [1.3–7.8] | 5.6 (3.7–8.4) |
| Hearing loss | 16 (3.4) | 2.3 [1.0–6.4] | 3.6 (2.2–5.9) |
| Hepatotoxicity | 13 (2.7) | 2.1 [1.8–10.0] | 2.9 (1.6–4.9) |
| Optic neuritis | 12 (2.5) | 6.9 [5.2–9.3] | 2.7 (1.5–4.8) |
| Hypothyroidism | 7 (1.5) | 2.2 [0.4–2.8] | 1.6 (0.7–3.3) |
| QT prolongation | 7 (1.5) | 3.2 [2.0–5.5] | 1.5 (0.7–3.2) |
| . | Frequency . | Time to First Event . | Incidence/1000 person-month (95% CI) . |
|---|---|---|---|
| . | n (%) . | Median [IQR] . | |
| Peripheral neuropathy | 134 (28.4) | 3.0 [1.0–5.3] | 39.0 (33.0–46.9) |
| Electrolyte depletion | 94 (19.9) | 4.0 [1.4–7.6] | 23.8 (19.4–29.1) |
| Acute renal failure | 40 (8.5) | 3.7 [1.7–8.4] | 9.4 (6.9–12.9) |
| Myelosuppression | 24 (5.1) | 2.8 [1.3–7.8] | 5.6 (3.7–8.4) |
| Hearing loss | 16 (3.4) | 2.3 [1.0–6.4] | 3.6 (2.2–5.9) |
| Hepatotoxicity | 13 (2.7) | 2.1 [1.8–10.0] | 2.9 (1.6–4.9) |
| Optic neuritis | 12 (2.5) | 6.9 [5.2–9.3] | 2.7 (1.5–4.8) |
| Hypothyroidism | 7 (1.5) | 2.2 [0.4–2.8] | 1.6 (0.7–3.3) |
| QT prolongation | 7 (1.5) | 3.2 [2.0–5.5] | 1.5 (0.7–3.2) |
Frequencies include SAEs also reported in Table 3.
Abbreviations: CI, confidence interval; IQR, interquartile range; MDR/RR-TB, multi-drug/rifampicin resistant tuberculosis.
Serious Adverse Events (SAE) Occurring During Concomitant Treatment With Bedaquiline and Delamanid Among 472 MDR/RR-TB Patients in 14 Countries
| . | n . | % . |
|---|---|---|
| Respiratory failure/Respiratory distress | 11 | 2.3 |
| Anemia/Platelet decrease | 7 | 1.5 |
| Increased liver enzymes/Hepatotoxicity | 7 | 1.5 |
| Peripheral neuropathy | 7 | 1.5 |
| QT interval prolongation | 6 | 1.3 |
| Vomiting/Diarrhea | 5 | 1.1 |
| Acute/Chronic kidney Injury | 4 | 0.8 |
| Hemoptysis/Hemorrhage | 4 | 0.8 |
| Optic nerve disorder | 3 | 0.6 |
| Acute coronary/ischemic disease | 3 | 0.6 |
| Hip fracture/Chest injury/Ligament injury | 3 | 0.6 |
| Psychosis | 2 | 0.4 |
| Heart failure | 2 | 0.4 |
| Othera | 8 | 2.1 |
| . | n . | % . |
|---|---|---|
| Respiratory failure/Respiratory distress | 11 | 2.3 |
| Anemia/Platelet decrease | 7 | 1.5 |
| Increased liver enzymes/Hepatotoxicity | 7 | 1.5 |
| Peripheral neuropathy | 7 | 1.5 |
| QT interval prolongation | 6 | 1.3 |
| Vomiting/Diarrhea | 5 | 1.1 |
| Acute/Chronic kidney Injury | 4 | 0.8 |
| Hemoptysis/Hemorrhage | 4 | 0.8 |
| Optic nerve disorder | 3 | 0.6 |
| Acute coronary/ischemic disease | 3 | 0.6 |
| Hip fracture/Chest injury/Ligament injury | 3 | 0.6 |
| Psychosis | 2 | 0.4 |
| Heart failure | 2 | 0.4 |
| Othera | 8 | 2.1 |
All SAE occurring during concomitant Bdq and Dlm are included (whether treatment related or not). Part of the SAE reported in Table 3 are also included in Table 2.
Abbreviation: MDR/RR-TB, multi-drug/rifampicin resistant tuberculosis.
One instance (0.2%) of each of the following SAEs were seen in the cohort: death, hearing impairment, hyperkalemia, hypovolemic shock, hemiparesis, severe muscle spam, pneumonia, premature new-born, pulmonary embolism, sudden death.
Serious Adverse Events (SAE) Occurring During Concomitant Treatment With Bedaquiline and Delamanid Among 472 MDR/RR-TB Patients in 14 Countries
| . | n . | % . |
|---|---|---|
| Respiratory failure/Respiratory distress | 11 | 2.3 |
| Anemia/Platelet decrease | 7 | 1.5 |
| Increased liver enzymes/Hepatotoxicity | 7 | 1.5 |
| Peripheral neuropathy | 7 | 1.5 |
| QT interval prolongation | 6 | 1.3 |
| Vomiting/Diarrhea | 5 | 1.1 |
| Acute/Chronic kidney Injury | 4 | 0.8 |
| Hemoptysis/Hemorrhage | 4 | 0.8 |
| Optic nerve disorder | 3 | 0.6 |
| Acute coronary/ischemic disease | 3 | 0.6 |
| Hip fracture/Chest injury/Ligament injury | 3 | 0.6 |
| Psychosis | 2 | 0.4 |
| Heart failure | 2 | 0.4 |
| Othera | 8 | 2.1 |
| . | n . | % . |
|---|---|---|
| Respiratory failure/Respiratory distress | 11 | 2.3 |
| Anemia/Platelet decrease | 7 | 1.5 |
| Increased liver enzymes/Hepatotoxicity | 7 | 1.5 |
| Peripheral neuropathy | 7 | 1.5 |
| QT interval prolongation | 6 | 1.3 |
| Vomiting/Diarrhea | 5 | 1.1 |
| Acute/Chronic kidney Injury | 4 | 0.8 |
| Hemoptysis/Hemorrhage | 4 | 0.8 |
| Optic nerve disorder | 3 | 0.6 |
| Acute coronary/ischemic disease | 3 | 0.6 |
| Hip fracture/Chest injury/Ligament injury | 3 | 0.6 |
| Psychosis | 2 | 0.4 |
| Heart failure | 2 | 0.4 |
| Othera | 8 | 2.1 |
All SAE occurring during concomitant Bdq and Dlm are included (whether treatment related or not). Part of the SAE reported in Table 3 are also included in Table 2.
Abbreviation: MDR/RR-TB, multi-drug/rifampicin resistant tuberculosis.
One instance (0.2%) of each of the following SAEs were seen in the cohort: death, hearing impairment, hyperkalemia, hypovolemic shock, hemiparesis, severe muscle spam, pneumonia, premature new-born, pulmonary embolism, sudden death.
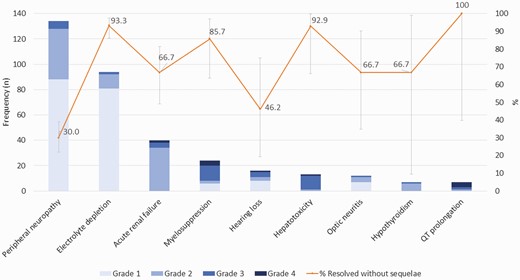
Maximum severity grade and outcome of AESI clinically relevant or reported as SAE) among 472 MDR/RR-TB patients receiving concomitant Bdq and Dlm therapy in 14 countries. Abbreviations: AESI, adverse events of special interest; MDR/RR-TB, multi-drug/rifampicin resistant tuberculosis; SAE, serious adverse events.
Treatment Outcomes
Overall, 78.0% (358/458) patients—239 (79.1%) who received concomitant Bdq and Dlm at treatment initiation and 119 (76.3%) among those in whom it was started later—had a favorable treatment outcome (Table 4). Frequencies of death and treatment failure were 8.9% (41/458) and 7.2% (33/458), respectively. Causes of death were reported as: TB disease in 20 patients, cause other than TB disease in 16 patients, unknown in 2 patients, surgery-related in 1 patient, and possibly related to TB treatment in 1 patient. The information was missing for 1 patient. Treatment failure was based on bacteriological findings in 28/33 (81.8%) patients and based on change of 2 or more drugs due to adverse events in 4/33 (12.1%). For 1 patient the reason of failure was unknown. Sensitivity analyses using algorithm-derived end-of-treatment outcomes showed similar percentages of treatment success (81.3%) (Supplementary Table 6).
Treatment Outcomes Among 458 MDR/RR-TB Patients Receiving Concomitant Bedaquiline and Delamanid Therapy in 14 Countries
| . | Concomitant Bdq and Dlm at MDR/RR-TB Treatment Initiation (N = 302) . | Concomitant Bdq and Dlm During MDR/RR-TB Treatment (N = 156) . | Total Patients (N = 458) . | |||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . |
| Favorable | 239 | 79.1 | 119 | 76.3 | 358 | 78.2 |
| Cured | 229 | 75.8 | 113 | 72.4 | 342 | 74.5 |
| Treatment completed | 10 | 3.3 | 6 | 3.8 | 16 | 3.5 |
| Unfavorable | 63 | 20.9 | 37 | 23.7 | 100 | 21.8 |
| Died | 23 | 7.6 | 18 | 11.5 | 41 | 8.9 |
| Treatment failed | 21 | 6.9 | 12 | 7.7 | 33 | 7.2 |
| Lost to follow-up | 19 | 6.3 | 7 | 4.5 | 26 | 5.7 |
| . | Concomitant Bdq and Dlm at MDR/RR-TB Treatment Initiation (N = 302) . | Concomitant Bdq and Dlm During MDR/RR-TB Treatment (N = 156) . | Total Patients (N = 458) . | |||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . |
| Favorable | 239 | 79.1 | 119 | 76.3 | 358 | 78.2 |
| Cured | 229 | 75.8 | 113 | 72.4 | 342 | 74.5 |
| Treatment completed | 10 | 3.3 | 6 | 3.8 | 16 | 3.5 |
| Unfavorable | 63 | 20.9 | 37 | 23.7 | 100 | 21.8 |
| Died | 23 | 7.6 | 18 | 11.5 | 41 | 8.9 |
| Treatment failed | 21 | 6.9 | 12 | 7.7 | 33 | 7.2 |
| Lost to follow-up | 19 | 6.3 | 7 | 4.5 | 26 | 5.7 |
Among 472 patients included, treatment outcome was “not evaluated” in 14 patients.
Abbreviations: Bdq, bedaquiline; Dlm, delamanid; MDR/RR-TB, multi-drug/rifampicin resistant tuberculosis.
Treatment Outcomes Among 458 MDR/RR-TB Patients Receiving Concomitant Bedaquiline and Delamanid Therapy in 14 Countries
| . | Concomitant Bdq and Dlm at MDR/RR-TB Treatment Initiation (N = 302) . | Concomitant Bdq and Dlm During MDR/RR-TB Treatment (N = 156) . | Total Patients (N = 458) . | |||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . |
| Favorable | 239 | 79.1 | 119 | 76.3 | 358 | 78.2 |
| Cured | 229 | 75.8 | 113 | 72.4 | 342 | 74.5 |
| Treatment completed | 10 | 3.3 | 6 | 3.8 | 16 | 3.5 |
| Unfavorable | 63 | 20.9 | 37 | 23.7 | 100 | 21.8 |
| Died | 23 | 7.6 | 18 | 11.5 | 41 | 8.9 |
| Treatment failed | 21 | 6.9 | 12 | 7.7 | 33 | 7.2 |
| Lost to follow-up | 19 | 6.3 | 7 | 4.5 | 26 | 5.7 |
| . | Concomitant Bdq and Dlm at MDR/RR-TB Treatment Initiation (N = 302) . | Concomitant Bdq and Dlm During MDR/RR-TB Treatment (N = 156) . | Total Patients (N = 458) . | |||
|---|---|---|---|---|---|---|
| . | n . | % . | n . | % . | n . | % . |
| Favorable | 239 | 79.1 | 119 | 76.3 | 358 | 78.2 |
| Cured | 229 | 75.8 | 113 | 72.4 | 342 | 74.5 |
| Treatment completed | 10 | 3.3 | 6 | 3.8 | 16 | 3.5 |
| Unfavorable | 63 | 20.9 | 37 | 23.7 | 100 | 21.8 |
| Died | 23 | 7.6 | 18 | 11.5 | 41 | 8.9 |
| Treatment failed | 21 | 6.9 | 12 | 7.7 | 33 | 7.2 |
| Lost to follow-up | 19 | 6.3 | 7 | 4.5 | 26 | 5.7 |
Among 472 patients included, treatment outcome was “not evaluated” in 14 patients.
Abbreviations: Bdq, bedaquiline; Dlm, delamanid; MDR/RR-TB, multi-drug/rifampicin resistant tuberculosis.
Treatment Outcomes
Overall, 78.0% (358/458) patients—239 (79.1%) who received concomitant Bdq and Dlm at treatment initiation and 119 (76.3%) among those in whom it was started later—had a favorable treatment outcome (Table 4). Frequencies of death and treatment failure were 8.9% (41/458) and 7.2% (33/458), respectively. Causes of death were reported as: TB disease in 20 patients, cause other than TB disease in 16 patients, unknown in 2 patients, surgery-related in 1 patient, and possibly related to TB treatment in 1 patient. The information was missing for 1 patient. Treatment failure was based on bacteriological findings in 28/33 (81.8%) patients and based on change of 2 or more drugs due to adverse events in 4/33 (12.1%). For 1 patient the reason of failure was unknown. Sensitivity analyses using algorithm-derived end-of-treatment outcomes showed similar percentages of treatment success (81.3%) (Supplementary Table 6).
DISCUSSION
This study presents results from the largest cohort of MDR/RR-TB patients to receive regimens containing concomitant Bdq and Dlm at either treatment initiation or at any time during their care. Our encouraging findings, from a diverse cohort from 14 countries, offers reassurance to clinicians and health policy makers that concomitant Bdq-Dlm therapy, delivered frequently in combination with linezolid or clofazimine or both, is a safe and effective choice for patients.
The most common clinically relevant AESI observed in these highly treatment experienced patients was peripheral neuropathy. Its occurrence is likely associated with the use of linezolid [25], a drug that nearly all patients received (usually at 600 mg daily) in addition to Bdq and Dlm. However, in our study the frequency of peripheral neuropathy was much lower (28%) than that found in Nix-TB (81%), a single-arm clinical trial where patients received linezolid (1200 mg daily with dose adjustment depending on toxic effect) with bedaquiline and pretonamid [25]. Although the majority of patients in our study had mild to moderate symptoms, we found that only one-third of the patients recovered without sequelae. This finding has important implications for the clinical management of patients treated with linezolid. In our study, similar to findings in Nix-TB, other AESIs commonly associated with linezolid such as optic neuritis and myelosuppression were less frequent. In endTB, linezolid was also the drug most frequently permanently discontinued due to AEs in patients treated with Bdq, Dlm, linezolid, and clofazimine. In a meta-analysis of individual data from MDR/RR-TB patients [26], linezolid and para-aminosalicylic acid had the highest incidences of adverse events leading to permanent drug discontinuation while fluoroquinolones, bedaquiline, and clofazimine were the drugs associated with the lowest incidences of adverse events leading to permanent drug discontinuation.
Confirming results from smaller studies, we did not see substantial QT prolongation or arrythmias [10, 13, 15, 16]. This was despite overlapping use of clofazimine and fluoroquinolones, agents known to prolong the QT-interval, in 84.5% and 33.1% of patients, respectively, and the use of concomitant Bdq and Dlm for more than 6 months in more than half of the patients. These findings are reassuring for patients with complex MDR/RR-TB disease (e.g., prior 2nd line treatment, resistance, intolerance) who may need concomitant Bdq and Dlm (along with other drugs) for more than 6 months. In a recent report, among 26 countries that had indicated the possibility of using combined Bdq and Dlm in their countries’ policies, only 6 allowed their combined use beyond 6 months without special approval [27]. We note that 1 sudden death was reported, and we cannot exclude a cardiac origin.
We found a high frequency of treatment success relative to global reported rates in patients with MDR/RR-TB (59%) [28]. The observed percentage of treatment success fell between that reported in the Nix-TB clinical trial (92%) [25] and those reported in smaller observational studies of patients receiving concomitant Bdq-Dlm (46%–70%) [12, 13, 15]. Our study demonstrates that high treatment success rates can be achieved in patients with complex medical conditions treated in programmatic conditions.
Taken together, the findings on effectiveness and toxicity suggest that the emphasis on reducing perceived risk of cardiotoxicity by avoiding Bdq and Dlm use in combination may be disproportionate to the real risk and benefits of this combination. Rather, efforts should be redoubled to optimize monitoring for other drug-related events in addition to cardiotoxicity, especially peripheral neuropathy, renal failure, and electrolyte depletion (the latter can lead to hypokalaemia that may itself lead to QT prolongation and arrythmia) that can have a life-threatening and/or irreversible impact.
Many guidelines currently encourage comprehensive toxicity monitoring for all MDR-TB drugs, yet the clinical reality is that this guidance is sometimes not widely applied, especially in the low resource settings that harbor important burdens of DR-TB. Encouraging the wider use of concomitant Bdq-Dlm therapy, increasing clinicians’ awareness about the low cardiotoxicity risk of co-administering Bdq and Dlm, and urging vigilance and increased resources for monitoring other drug-related adverse events will be important next steps in the fight against MDR-TB.
This study has some limitations. First, we may have overestimated the risk of adverse events because baseline data on preexisting medical conditions was sometimes incomplete. In these cases, a pre-existing condition may have been interpreted as an adverse event. For example, renal failure was more frequent in patients who received Bdq and Dlm at treatment initiation compared to those who received concomitantly the drugs later during their treatment, despite the higher proportion of injectable drugs used in the latter group. The higher frequency of patients with resistance to injectable drugs in the first group may reflect more frequent prior use of injectable drugs which may have led to preexisting renal impairment. Second, we limited systematic reporting to selected AEs of interest and SAEs. Although these result in a possibly incomplete list of toxicities experienced, it provides confidence that the selected AESIs were comprehensively and consistently monitored, and their relative occurrence can therefore be compared. Third, despite the programmatic conditions in which the study was conducted, patients’ participation in a prospective study that included systematic data collection on safety and efficacy indicators may have had a positive impact on treatment outcomes, which may not be observed in all treatment settings.
CONCLUSION
We found that the concomitant use of Bdq and Dlm, along with other second-line anti-TB drugs notably linezolid or clofazimine, was safe and effective in MDR/RR-TB patients with extensive disease. Using these drugs concomitantly is a good therapeutic option for patients with resistance to many anti-TB drugs. Systematic monitoring for common toxicities such as peripheral neuropathy and electrolytes depletion is important.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge and thank our funders, Unitaid, for financing the endTB project, including the observational study, and the Harvard Medical School Center for Global Health Delivery for supporting the present analysis. They are indebted to the patients who participated in the endTB observational study and to the clinicians and program staff of participating national tuberculosis programs. They thank endTB staff at Partners In Health, Médecins Sans Frontières, Epicentre, and Interactive Research and Development and Janet Ousley for editing the manuscript.
Author contributions. Conceived and designed the activity: H. H., C. H., M. B., C. D. M., U. K., M. R., M. F. F., P.Y. K., K. J. S., and F. V.
Acquired data: M. A., L. L., M. N., A. K., G. J. L., S. I., N. D., O. K., B. K., H. K., P. T., M. K. K., S. A., S. M. , A. J., A. K., S. P., N. M., N. L., S. C., E. O., and S. A.
Analyzed: M. B. and S. A.
Interpreted the data: H. H., C. H., M. B., C. M., U. K., M. R., M. F., P. K., K. S., and F. V.
Wrote first draft of the manuscript: H. H.
Approved final version of the manuscript: All authors.
Financial Support. This work was supported by grants from UNITAID (endTB project), the Harvard Medical School Center for Global Health Delivery (present analysis) as well as by Médecins Sans Frontières, Partners in Health, and Interactive Research & Development.
References
Author notes
H. H. and U. K. contributed equally to this work.
M. F. F. and C. H. contributed equally to this work.
Potential conflicts of interest. Bedaquiline donations made from Janssen to the Global Drug Facility were used for patients in the endTB observational study. Donations of delamanid from Otsuka were used for initial patients enrolled in the endTB Observational Study. The companies from which drug donations were received did not have any role on the study design, data analyses, data interpretation or manuscript writing. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
- peripheral neuropathy
- rifampin
- prolonged qt interval
- clofazimine
- drug resistance
- renal failure, acute
- fluoroquinolones
- safety
- treatment failure
- tuberculosis
- treatment outcome
- multidrug-resistant tuberculosis
- linezolid
- myelosuppression
- electrolyte depletion
- generalized illness
- bedaquiline
- adverse event
- delamanid




