-
PDF
- Split View
-
Views
-
Cite
Cite
Eric P F Chow, Christopher K Fairley, Huachun Zou, Rebecca Wigan, Suzanne M Garland, Alyssa M Cornall, Steph Atchison, Sepehr N Tabrizi, Marcus Y Chen, Human Papillomavirus Antibody Levels Following Vaccination or Natural Infection Among Young Men Who Have Sex With Men, Clinical Infectious Diseases, Volume 75, Issue 2, 15 July 2022, Pages 323–329, https://doi.org/10.1093/cid/ciab1052
Close - Share Icon Share
Abstract
Australia introduced a school-based gender-neutral human papillomavirus (HPV) vaccination program for girls and boys aged 12–13 years in 2013. We examined HPV type–specific antibody levels in unvaccinated young men who have sex with men (MSM) with natural infection and compared these with levels in those vaccinated against HPV.
Serum specimens at baseline were collected from MSM aged 16–20 years in the HYPER1 (Human Papillomavirus in Young People Epidemiological Research) and HYPER2 studies, conducted in 2010–2013 and 2017–2019, respectively. Merck’s 4-plex HPV competitive Luminex Immunoassay was used to quantify HPV6-, HPV11-, HPV16-, and HPV18-specific antibodies. We compared antibody levels for each HPV genotype between unvaccinated men (HYPER1) and vaccinated men (HYPER2) using the Mann-Whitney U test.
There were 200 unvaccinated men and 127 vaccinated men included in the analysis. Median antibody levels among vaccinated men were significantly higher than levels among unvaccinated men for HPV6 (223 milli-Merck units per milliliter [mMU/mL] vs 48 mMU/mL, P < .0001), HPV11 (163 mMU/mL vs 21 mMU/mL, P < .0001), HPV16 (888 mMU/mL vs 72 mMU/mL, P < .0001), and HPV18 (161 mMU/mL vs 20 mMU/mL, P < .0001). Antibody levels did not change over time for up to 66 months for all 4 genotypes among vaccinated men.
Among young MSM vaccinated with the quadrivalent HPV vaccine, antibody levels for HPV6, HPV11, HPV16, and HPV18 were significantly higher than those in unvaccinated MSM following natural infection. Antibody levels following vaccination appeared to remain stable over time.
NCT01422356 for HYPER1 and NCT03000933 for HYPER2.
Gay, bisexual, and other men who have sex with men (MSM) have a high prevalence and incidence of human papillomavirus HPV infection and anal cancer, particularly those living with human immunodeficiency virus [1]. Several countries, including the United Kingdom and Australia, have implemented gender-neutral HPV school-based vaccination programs that include male vaccination [2, 3]. In Australia, a gender-neutral HPV school-based vaccination program was introduced in 2013 for both girls and boys aged 12–13 years. There was also a catch-up program for boys aged up to 15 years in 2013–2014. A 3-dose schedule of quadrivalent HPV vaccines (covering genotypes 6, 11, 16, and 18) was used in the national program until 2017, which was replaced by an ongoing 2-dose schedule of the nonavalent HPV vaccine (also covering genotypes 31, 33, 45, 52, and 58) in 2018.
Serum antibody responses following natural infection with HPV have varied in different populations. Antibody levels following natural infection with HPV genotypes 6, 11, 16, and 18 have been found in 7% of heterosexual men [4], 50%–70% of women [5–8], and 18%–73% of MSM [9, 10]. A previous study showed that HPV antibodies were more likely to be detected following anorectal infection than penile infection in MSM [11]. Thus, there may be differences in the likelihood of antibody responses between heterosexual men and MSM. In contrast, HPV vaccines are highly immunogenic, inducing HPV antibodies rapidly and in more than 97% of vaccine recipients, with rates of seroconversion similar between different gender and sexual orientation groups [12, 13].
The antibody levels are substantially higher after vaccination compared with natural infection [12–14]. Hence, surveillance of HPV antibodies can be used as a serological marker of population levels of vaccine uptake [15] as self-reported vaccination status can be inaccurate, particularly when the vaccine is administered in adolescence [16, 17]. Very few studies have examined serum antibody responses following vaccination among young MSM. Serum antibody responses have been measured in clinical vaccine trials among young adult MSM [4, 13] but not specifically among MSM who received the HPV vaccine when they were at the age of 12–13 years. Furthermore, studies examining cutoffs for vaccine-induced seropositivity have mainly been conducted among women, with no data for MSM [15]. In this study, our aim was to measure serum HPV antibody levels among young MSM aged 16–20 years following quadrivalent HPV vaccination between the age of 12–13 years, compare vaccine-induced antibody levels with levels following natural infection among unvaccinated men, and estimate cutoff values for probable quadrivalent vaccine-induced seropositivity following vaccination.
METHODS
Study Setting and Population
The HYPER1 (Human Papillomavirus in Young People Epidemiological Research) and HYPER2 studies were repeated cross-sectional studies aimed at examining the prevalence of oral, penile, and anal HPV in 2 birth cohorts of young MSM separated in time: one before and one after, the introduction of a gender-neutral HPV vaccination program in Australia in 2013. The methodology and main findings of HYPER1 and HYPER2 have been previously reported [9, 18, 19]. In brief, men aged 16–20 years who self-identified as same-sex attracted were recruited through sexual health clinics and the community in Melbourne, Australia. Men in the HYPER1 study were recruited between October 2010 and September 2012 [9]; men in the HYPER2 study were recruited between January 2017 and March 2019 [18]. The study procedures and specimen collection for both studies were conducted at the Melbourne Sexual Health Centre. Written consent was obtained from all men. The Alfred Hospital Research Ethics Committee, Melbourne, approved the HYPER1 and HYPER2 studies (174/10 and 429/16, respectively).
Men were asked to self-report their HPV vaccination status in both studies. At baseline, all men in the HYPER1 study were unvaccinated. Given that men in the HYPER2 study were recruited after the introduction of the school-based gender-neutral HPV vaccination program, we also obtained additional written consent from these men to verify their vaccination status including number and date of vaccine doses via the National HPV Vaccination Program Register (NHVPR) and the Australian Immunisation Register (AIR).
In this analysis, we included all unvaccinated men from the HYPER1 study and all men from the HYPER2 study who had their HPV vaccination verified. In the HYPER2 study, a small number of men received their first dose of the vaccine during the time-limited MSM catch-up program in 2017–2019 instead of from the school-based program [20, 21], and those men were excluded.
HPV Serology
A 10-mL specimen of blood was collected in a serum collection tube (Greiner Bio-One, Kremsmünster, Austria) from participants in the HYPER1 and HYPER2 studies at baseline for HPV serum antibodies. Blood specimens were centrifuged at 4800 × g for 10 minutes to separate the clotted blood from serum. An aliquot of 1 mL of serum was placed in screw-cap tubes and stored at –80°C until testing. Serum specimens were tested at the end of each study. The HYPER1 specimens were tested at PPD Vaccines and Biologics Lab (Wayne, PA); the HYPER2 specimens were tested at Q2 Solutions (San Juan Capistrano, CA). In addition to blood specimens, a clinician-collected anal swab, self-collected penile swab, and oral rinse were obtained for HPV DNA detection, as previously published [18].
At both laboratories, Merck’s 4-plex HPV virus-like particle–based competitive Luminex Immunoassay was used to simultaneously quantify HPV6-, HPV11-, HPV16-, and HPV18-specific antibodies from each single serum specimen. This assay was validated in the clinical development of the qHPV vaccine (Gardasil, Merck & Co., Inc) [22, 23]. Antibody levels were expressed in milli-Merck units per milliliter (mMU/mL). The seropositivity cutoffs following natural infection with HPV6, HPV11, HPV16, and HPV18 were ≥20, ≥16, ≥20, and ≥24 mMU/mL, respectively, as previously determined [23] Given that all men in the HYPER1 study were unvaccinated, any antibodies detected among men in the HYPER1 study were presumably to be from natural infection.
Statistical Analyses
We calculated the median and interquartile range of antibody levels detected in sera for each HPV genotype. The Mann-Whitney U test was used to determine whether there was a significant difference in antibody levels for each HPV genotype between unvaccinated men (HYPER1) following natural infection and vaccinated men (HYPER2) following vaccination. The antibody levels of HPV6 and HPV11 among men were illustrated using scatter plots, and we repeated this for HPV16 and HPV18. Scatter plots were used to examine patterns and clustering of antibody levels between vaccinated men and unvaccinated men. It was expected that vaccinated men would have high antibody levels for all 4 quadrivalent HPV vaccine genotypes and that unvaccinated men with natural infection would have lower antibody levels and unlikely to be infected with all 4 types [9, 24]. The Spearmen rank correlation (Rs) was used to examine the correlation between HPV6 and HPV11 and between HPV16 and HPV18 among vaccinated men in HYPER2.
There was no recognized seropositivity cutoff following HPV vaccination [23]. In this study, the 97.5th percentiles of the antibody levels for each HPV genotype were calculated and used as the estimated cutoff values for “probable vaccine-induced seropositivity” [25]. This method is recommended in the Clinical Laboratory Standards Institute guideline EP28-A3 [26].
The dates of the HPV vaccine doses administered at school or via general practices were obtained from the NHVPR and AIR vaccine registers. We also extracted the dates of HPV vaccine administrated at the Melbourne Sexual Health Centre from the electronic clinical database. The time between antibody level measured and the last HPV vaccine received was calculated and presented in months. The Rs was used to examine the correlation between antibody level measured and time since the last HPV vaccine received for each HPV genotype. All analyses were performed in R (version 4.0.5).
RESULTS
Two hundred men were recruited in the HYPER1 study and an additional 200 in the HYPER2 study. In the HYPER1 study, all 200 men provided serum specimens for HPV serology and were included in this analysis. In HYPER1, all 200 men self-reported not being vaccinated against HPV. In HYPER2, 73 of the 200 men were excluded from this analysis; this included 63 men who did not have any HPV vaccination records in the vaccine registry but might have received the vaccine elsewhere, 8 men who received their first dose of HPV vaccine from the time-limited HPV catch-up program for MSM in 2017 instead of from the school-based vaccination program, and 2 men who did not provide a blood specimen. The remaining 127 men in HYPER2 had received at least 1 dose of HPV vaccine and had this verified by the vaccine registry. Of the 127 vaccinated men in HYPER2, 5 had an HPV DNA positive result for any quadrivalent HPV genotype (2 had penile HPV6, 1 had anal HPV11, 1 had anal HPV16, and 1 had oral HPV16).
Of the 327 men (200 in HYPER1 and 127 in HYPER2) included in this analysis, the mean age was 18.6 years (standard deviation, 1.1 years), with no difference between the 2 studies (P = .069). Overall, 228 men (58.5%) were seropositive for HPV6, HPV11, HPV16, and/or HPV18. In HYPER1, seropositivity was 12.5% (25 of 200) for HPV6, 7.0% (14 of 200) for HPV11, 3.5% (7 of 200) for HPV16, and 3.0% (6 of 200) for HPV18. In HYPER2, seropositivity was 100% (127 of 127) for HPV6, 99.2% (126 of 127) for HPV11, 99.2% (126 of 127) for HPV16, and 99.2% (126 of 127) for HPV18.
Antibody levels for HPV6, HPV11, HPV16, and HPV18 were significantly higher among vaccinated men in HYPER2 compared with unvaccinated men in HYPER1 (Figure 1). The median antibody level for HPV6 in vaccinated men was 4-fold higher than in unvaccinated men (223; interquartile range [IQR], 132–554 vs 48; IQR, 22–149; P < .0001). The median antibody level for HPV11 in vaccinated men was almost 8-fold higher than in unvaccinated men (163; IQR, 92–328 vs 21; IQR, 14–100; P < .0001). The median antibody level for HPV16 in vaccinated men was 12-fold higher than in unvaccinated men (888; IQR, 383–1779 vs 72; IQR, 51–169; P < .0001). The median antibody level for HPV18 in vaccinated men was 8-fold higher than in unvaccinated men (161; IQR, 97–426 vs 20; IQR, 13–57; P < .0001).
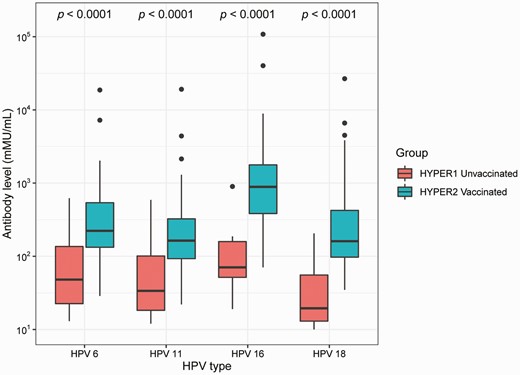
Serum antibody levels for HPV6, HPV11, HPV16, and HPV18 among unvaccinated and vaccinated men. Antibody levels are expressed in mMU/mL and presented as a box plot where the middle line represents the median, the bottom margin of the box represents the 25th quartile, the top margin of the box represents the 75th quartile, and the whiskers represent the lowest and highest extreme values. The dots represent the outliers. The P value from the Mann-Whitney U test was used to compare the antibody levels between unvaccinated men and vaccinated men. Abbreviations: HYPER, Human Papillomavirus in Young People Epidemiological Research study; HPV, human papillomavirus; mMU/mL, milli-Merck units per milliliter.
Figure 2A illustrates the correlation between antibody levels for low-risk genotypes HPV6 and HPV11. The antibody levels for both HPV6 and HPV11 were higher among vaccinated men compared with unvaccinated men. A similar pattern was also observed for high-risk genotypes HPV16 and HPV18 (Figure 2B). There was a strong correlation between vaccine-induced antibodies against HPV6 and HPV11 (Rs = 0.856, P < .0001) and a strong correlation between vaccine-induced antibodies against HPV16 and HPV18 (Rs = 0.761, P < .0001). One man had extremely high antibody levels to all 4 types: 18 701 mMU/mL for HPV6, 19 154 mMU/mL for HPV11, 108 255 mMU/mL for HPV16, and 26 722 mMU/mL for HPV18. He had received 3 doses of vaccine at school in 2013–2015 and also received an additional vaccine dose from the catch-up program 1.5 months before enrollment.
![Log-log scatter plots of antibody level for low-risk genotypes (HPV6 and HPV11) (A) and high-risk genotypes (HPV16 and HPV18) (B), stratified by vaccination status. Antibody levels are expressed in milli-Merck units per milliliter (mMU/mL) and plotted on a logarithmic scale. Each symbol represents an individual man. A, Each triangle represents a vaccinated man and his levels of antibodies against HPV6 and HPV11, and each circle represents the antibody levels for unvaccinated men. B, Each triangle represents a vaccinated man and his levels of antibodies against HPV16 and HPV18, and each circle represents the antibody levels for unvaccinated men. The vertical and horizontal lines represent previously published cutoff values for seropositivity for HPV6 (20 mMU/mL), HPV11 (16 mMU/mL), HPV16 (20 mMU/mL), and HPV18 (24 mMU/mL) [23]. Abbreviation: HPV, human papillomavirus.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/75/2/10.1093_cid_ciab1052/1/m_ciab1052_fig2.jpeg?Expires=1750209607&Signature=yQHYZ2mhbchKU-q6TnRqm2-tLaQ1Ke8c-x7QCBjUXw1SP9UkbBpIExQ~vJZmE7QkTvRbDjGho2-lICZlFiSuuAyAImUxcK-a1aJtKY~fCAUrnXx7co-L9T3nO4jcANaBIbdHHHOf3PxH9u1okCZ3YLfCt0OqTczNXWEpKiMRRA9SVr7Lrm6-~yodhnGWT7HKdxAKczsufBjQ6UdvjXbODK3F9h2R671K~QGjnbRw-BLFqWRNVTSDnAYDf3-kOnL7QZDGK32yShdXnlFMi3VVZqnWdCxJ55InLrZrkNWa0tRngBNkKoQZUWYYxRZ3wf-HvPSU362NIDsVFOkfnvmGiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Log-log scatter plots of antibody level for low-risk genotypes (HPV6 and HPV11) (A) and high-risk genotypes (HPV16 and HPV18) (B), stratified by vaccination status. Antibody levels are expressed in milli-Merck units per milliliter (mMU/mL) and plotted on a logarithmic scale. Each symbol represents an individual man. A, Each triangle represents a vaccinated man and his levels of antibodies against HPV6 and HPV11, and each circle represents the antibody levels for unvaccinated men. B, Each triangle represents a vaccinated man and his levels of antibodies against HPV16 and HPV18, and each circle represents the antibody levels for unvaccinated men. The vertical and horizontal lines represent previously published cutoff values for seropositivity for HPV6 (20 mMU/mL), HPV11 (16 mMU/mL), HPV16 (20 mMU/mL), and HPV18 (24 mMU/mL) [23]. Abbreviation: HPV, human papillomavirus.
Using the 97.5th percentile of antibody level concentrations as probable vaccine-induced seropositivity, we determined the cutoff values for seropositivity to be 1862 mMU/mL for HPV6, 1298 mMU/mL for HPV11, 8239 mMU/mL for HPV16, and 3842 mMU/mL for HPV18.
There were 122 men who were HPV vaccinated through the school-based program in 2013–2015 and who did not receive any further HPV vaccine before enrollment into the HYPER2 study. Among these 122 men, the median time between their last HPV vaccine and sampling for antibodies was 47.7 months (IQR, 40.4–54.1). There was no significant correlation between antibody level and time since the last HPV vaccine (i.e. up to 66 months following vaccination) for all 4 HPV genotypes (Figure 3). Five men were HPV vaccinated through the school-based vaccine program in 2013–2015 but were unsure about their vaccination status and received the vaccine again in 2017–2019 before enrollment into the HYPER2 study. Among these 5 men, the median time between their last HPV vaccine and sampling for antibodies was 3.5 months (IQR, 2.0–4.8).
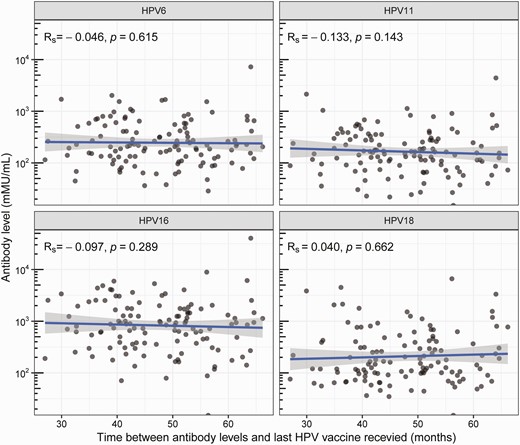
Correlation between antibody level and the time since last HPV vaccine for HPV6, HPV11, HPV16, and HPV18 among 122 vaccinated men excluding 5 men who were recently vaccinated. Antibody levels are expressed in mMU/mL and plotted on a logarithmic scale. Rs represents the Spearmen rank correlation coefficient. Each circle represents an individual. The straight line represents the line of best fit, and the gray shaded region represents the 95% confidence intervals of the line of best fit. Abbreviations: HPV, human papillomavirus; mMU/mL, milli-Merck units per milliliter.
DISCUSSION
We found that serum antibody levels in vaccinated men are substantially higher than those in unvaccinated men with antibodies from presumed natural infection for all quadrivalent HPV genotypes, providing further evidence of substantially higher serum antibody levels from vaccination compared with natural infection in MSM. Additionally, our results suggest that antibody levels did not change significantly over time up to 66 months following vaccination.
Previously, we found that a proportion of young MSM in the HYPER1 study seroconverted following natural HPV infection during a prospective follow-up period of 12 months [11]. The likelihood of seroconversion from natural infection varied by anatomical site of infection and HPV type. Men with anal low-risk HPV infection (HPV6, 73% or HPV11, 71%) were more likely to seroconvert than men with anal high-risk infection (HPV16, 13% or HPV18, 21%), with a similar pattern observed among men with penile infection (HPV6, 50%; HPV11, 33%; HPV16, 25%; and HPV18, 0%) [11]. In contrast, clinical vaccine trials have demonstrated that almost all (>97%) vaccinated men aged 16–26 years seroconvert to all 4 HPV genotypes by month 7 [13].
Consistent with previous studies among men and women vaccinated against the quadrivalent vaccine [13, 14], we found that vaccinated men had substantially higher antibody levels against HPV16 compared with HPV6, HPV11, and HPV18. Our findings show that among vaccinated men with a median of 48 months since the last dose of HPV vaccine, median antibody levels were 223 mMU/mL for HPV6, 163 mMU/mL for HPV11, 888 mMU/mL for HPV16, and 161 mMU/mL for HPV18. In comparison, Hillman et al reported the geometric mean titers of antibody levels among 299 vaccinated MSM aged 16–26 years recruited from 18 countries were 73 mMU/mL for HPV6, 84 mMU/mL for HPV11, 309 mMU/mL for HPV16, and 34 mMU/mL for HPV18 at month 36 [13]. Men received the quadrivalent vaccine and the same assay quantifying antibody levels were used in our study and in Hillman et al\'s study [13]. However, caution must be taken when comparing both studies as the study population was different. Additionally, men in Hillman et al’s study included only men aged 16–26 years who had 1–5 lifetime sexual partners, and these men were vaccinated at the age of 16–26 years [13]. In our study, all men were vaccinated at school at the age of 12–13 years, before sexual debut. Furthermore, Hillman et al reported that there was a significant reduction in antibody levels for all 4 HPV genotypes from month 7 to month 36 [13]. However, this was not seen in our study where the last dose of vaccine ranged between 27 and 66 months prior to measurement. We postulate that the higher antibody levels seen in our study may be due to men being vaccinated at a younger age when there is greater immunogenicity [13]. Furthermore, antibody responses may be different when individuals who receive the HPV vaccine are also exposed to HPV infection.
There are several limitations to this study. First, we assumed that the antibodies detected among unvaccinated men in HYPER1 were from natural infection. Second, serum specimens were taken from 2 cross-sectional studies among MSM aged 16–20 years recruited from sexual health clinics and communities in Melbourne, Australia; thus, our findings may not be representative of the wider MSM population or other settings. Third, most vaccinated men in our study received 3 doses of vaccine; thus, we were unable to compare the antibody level by the number of doses received. Fourth, the immune response against HPV varies across demographic characteristics such as age and ethnicity [13]. We were unable to compare immune response by these factors due to the limited sample size. Fifth, vaccination status was self-reported by men in HYPER1 and not verified via a vaccine registry. However, these men were unlikely to have been vaccinated as they were recruited before the introduction of the school-based gender-neutral HPV vaccination program.
In summary, high antibody levels are achieved among MSM who receive their HPV vaccination during early adolescence and persist up to at least 5.5 years. We have estimated the cutoff values for probable vaccine-induced seropositivity, which can be used as a serological marker of previous HPV vaccination status and could be used for vaccine surveillance purposes. However, the use of these probable vaccine-induced seropositivity cutoffs may have limited practical benefits as these immunoassay are usually not available in clinical practice.
Notes
Author Contributions. E. P. F. C. and M. Y. C. conceived and designed the study, and C. K. F. provided input. H. Z. was involved in recruitment and data collection and oversaw the recruitment for the HYPER1 (Human Papillomavirus in Young People Epidemiological Research) study. R. W. was involved in recruitment and data collection and oversaw the recruitment for the HYPER2 study. S. N. T., A. M. C., and S. M. G. provided advice on study design in relation to laboratory testing and specimen collection. R. W., A. M. C., and S. A. W. coordinated collection of serum specimens for human papillomavirus (HPV) serology testing. E. P. F. C. performed data analyses and wrote the first draft of the manuscript. All authors provided data interpretation, revised the manuscript for intellectual content, and approved the final version of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments. The authors thank the clinic staff at the Melbourne Sexual Health Centre (MSHC) for referring men to the HYPER2 study. The authors acknowledge Mark Chung from MSHC for designing graphics and David Samson from MSHC for study recruitment. They also thank staff at the National HPV Vaccination Program Register and the Department of Health and Human Services Immunisation Registers for their assistance with validating vaccination status.
Disclaimer. The funder had no role in study design, data collection, data analysis, data interpretation, preparation of the manuscript, or decision to publish.
Financial support. The HYPER2 study was funded by Merck & Co. (54860); funding was provided to the Melbourne Sexual Health Centre to conduct this investigator-initiated study.
References
Author notes
Potential conflict of interest. E. P. F. C. is supported by an Australian National Health and Medical Research Council (NHMRC) Emerging Leadership Investigator grant (GNT1172873); reports grant payments to their institution from the World Health Organization and Gilead Sciences; and reports personal payments for educational activities from Roche, Merck & Co., and Gilead Sciences SL. C. K. F. and S. M. G. are supported by an Australian NHMRC Leadership Investigator grant (GNT1172900 and GNT1197951, respectively). E. P. F. C. and A. M. C. have received educational grants from Seqirus Australia and bioCSL to assist with education, training, and academic purposes in the area of HPV outside the submitted work. E. P. F. C. has received an honorarium from Merck Sharp & Dohme and Roche outside the submitted work. C. K. F. has received research funding from CSL Biotherapies and owns shares in CSL Biotherapies. S. M. G. has received advisory board fees and lecture fees from Merck & Co. for work in private time; through her institution (Royal Women’s Hospital), has received funding for an investigator-initiated grant from Merck & Co. for a young women’s study on HPV; is a member of the Merck Global Advisory Board for HPV vaccines; and serves as president for the International Papillomavirus Society. H. Z. is supported by the Natural Science Foundation of China Excellent Young Scientists Fund (82022064), Natural Science Foundation of China International/Regional Research Collaboration Project (72061137001), and the Shenzhen Science and Technology Innovation Commission Basic Research Program (JCYJ20190807155409373). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




