-
PDF
- Split View
-
Views
-
Cite
Cite
Priscilla Kim, Steven M Gordon, Megan M Sheehan, Michael B Rothberg, Duration of Severe Acute Respiratory Syndrome Coronavirus 2 Natural Immunity and Protection Against the Delta Variant: A Retrospective Cohort Study, Clinical Infectious Diseases, Volume 75, Issue 1, 1 July 2022, Pages e185–e190, https://doi.org/10.1093/cid/ciab999
Close - Share Icon Share
Abstract
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been shown to be highly protective against reinfection and symptomatic disease. However, effectiveness against the Delta variant and duration of natural immunity remain unknown.
This retrospective cohort study included 325 157 patients tested for SARS-CoV-2 via polymerase chain reaction (PCR) from 9 March 2020 to 31 December 2020 (Delta variant analysis) and 152 656 patients tested from 9 March 2020 to 30 August 2020 (long-term effectiveness analysis) with subsequent testing through 9 September 2021. The primary outcome was reinfection, defined as a positive PCR test >90 days after the initial positive test.
Among 325 157 patients tested before 31 December 2020, 50 327 (15.5%) tested positive. After 1 July 2021 (Delta dominant period), 40 (0.08%) initially positive and 1494 (0.5%) initially negative patients tested positive. Protection of prior infection against reinfection with Delta was 85.4% (95% confidence interval [CI], 80.0–89.3). For the long-term effectiveness analysis, among 152 656 patients tested before 30 August 2020, 11 186 (7.3%) tested positive. After at least 90 days, 81 (0.7%) initially positive and 7167 (5.1%) initially negative patients tested positive. Overall protection of previous infection was 85.7% (95% CI, 82.2–88.5) and lasted up to 13 months. Patients aged >65 years had slightly lower protection.
SARS-CoV-2 infection is highly protective against reinfection with Delta. Immunity from prior infection lasts at least 13 months. Countries facing vaccine shortages should consider delaying vaccinations for previously infected patients to increase access.
Nearly 2 years after the identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus disease 2019 (COVID-19) pandemic remains a major global health challenge due to the emergence of SARS-CoV-2 variants that have been associated with increased transmissibility and potential immune escape. In the United States, these variants of concern have included B.1.1.7 (Alpha), B.1.351 (Beta), B.1.1.28.1 (Gamma), and B.1.617.2 (Delta) [1, 2]. The Delta variant, first detected in India in December 2020, has become the predominant variant in the United States, United Kingdom, and several other countries [1]. By 24 July 2021, the Delta variant represented 93.7% of sequenced viruses circulating in the United States [1]. Characterized by multiple spike protein mutations, the Delta variant appears to lead to higher viral loads and increased transmission [3, 4].
Previous studies have demonstrated that infection with SARS-CoV-2 is highly protective against reinfection and symptomatic disease [5–9]. Studies from the United Kingdom [7, 8], United States [5], and Denmark [6] all describe approximately 85% reduced risk of reinfection for 5–7 months. Duration of immunity beyond this period is unknown, but recent findings suggest that vaccine-induced immunological responses (eg, neutralizing antibodies) wane after 6 months [10–13].
Additionally, the protection afforded by prior COVID-19 infection against reinfection with variants of concern is not well understood. In vitro neutralization assays have shown that the Delta variant has reduced sensitivity to antibodies present in the sera of individuals who have recovered from COVID-19, as well as the sera of vaccinated individuals [2, 4]. These findings suggest that natural infection may not offer the same protection against the Delta variant. Understanding the effectiveness of prior infection against Delta is important, as it can inform vaccination strategies in countries with vaccine constraints and SARS-CoV-2 transmission models. Our aim in this study was to determine whether prior infection protects against reinfection with the Delta variant and to estimate duration of immunity following COVID-19 infection.
METHODS
Patients and Outcomes
Individuals tested for COVID-19 via polymerase chain reaction (PCR) within the Cleveland Clinic Health System in Ohio and Florida between 9 March 2020 and 9 September 2021 were included. PCR testing was performed on patients who were symptomatic, hospitalized for any reason, required preprocedural screening, or sought international travel clearance. We conducted 2 analyses following natural infection: one to assess effectiveness against the Delta variant and one to assess long-term effectiveness. For the Delta analysis, initial status was based on tests performed before 31 December 2020. For the long-term effectiveness analysis, initial infection status was based on tests performed before 30 August 2020. Patients with at least 1 positive test during these periods were considered initially positive. The primary outcome was a positive PCR retest. According to Centers for Disease Control and Prevention criteria, reinfection is defined as occurring >90 days after initial testing [14]. Therefore, for initially positive patients, any positive test >90 days after initial infection was defined as a reinfection. To avoid bias, initially negative patients who retested positive within 90 days of their initial test were excluded.
Data Analyses
For the Delta variant analysis, we selected patients with a positive or negative PCR test before 31 December 2020 (Figure 1). For each group, we then determined retesting and retest positivity rates specifically during the period of Delta predominance, that is, from 1 July 2021 to 9 September 2021. Patients who retested positive were reviewed for hospitalizations, intensive care unit (ICU) admission, need for mechanical ventilation, and death within 30 days of the test. Patients who retested positive between 1 January 2021 and 30 June 2021 were excluded from this analysis. For each group of patients, the infection rate was determined by dividing the number of patients who retested positive by the total number of patients in that group. Protection offered by prior infection against repeat infections was calculated as 1 minus the ratio of infection rate for the initially positive patients to the infection rate for the initially negative patients. Patients who had no subsequent tests were assumed to be negative. To estimate the effectiveness of prior infection against symptomatic infection, we repeated the analysis including only symptomatic infections. For most tests, the ordering physician recorded the presence of symptoms in a questionnaire. Initially positive patients with missing questionnaires were chart-reviewed to determine symptoms. For initially negative patients who had missing questionnaires, we imputed the results by chart reviewing a random sample of patients with missing questionnaires to determine the proportion of patients with symptomatic infection and then applied that proportion to the remaining patients with missing symptomatic data.
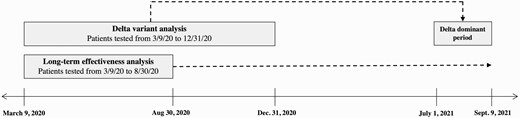
Schematic depicting 2 distinct cohorts of the Delta variant analysis and long-term effectiveness analysis.
To assess whether protection offered by prior infection differs by age, we calculated a protective effect estimate separately for different age groups (0–17 years, 18–34 years, 35–50 years, 51–64 years, 65–79 years, and >80 years). To increase our power to detect a difference, we compared rates in patients aged 0–64 years with those aged ≥65 years. Because many patients were vaccinated during this period, and vaccination might modify the risk of contracting Delta, we also estimated the protection of prior infection restricted to patients who were documented to have received 2 doses of the COVID-19 vaccine before 1 July 2021. We limited the vaccination analysis to patients who received 2 doses of the COVID-19 vaccine because we were unable to distinguish between patients who were unvaccinated and those who were missing vaccine data, and the type of vaccine that patients received was unknown.
For the long-term effectiveness analysis, patients with a positive or negative PCR test before 30 August 2020 were followed through 9 September 2021 to determine retesting and retest positivity rates during this period. Infection rates and protection offered by prior infection were calculated for distinct time periods using the equations previously described. Protection estimates were also calculated for different age groups. All analyses were conducted using R v4.1.0 (R Core Team, Vienna). The Cleveland Clinic Institutional Review Board approved this work.
RESULTS
During the study period, 878 830 PCR tests were collected from 527 134 individuals (average age, 48.8 ± 23.2 years; 55.1% female), with a 10.3% overall positivity rate.
Delta Analysis
After excluding 781 patients who tested positive between 1 January 2021 and 30 June 2021, there were 471 499 tests performed before 31 December 2020 on 325 157 individuals. Of these, 50 327 (15.5%) individuals tested positive (Table 1). After 1 July 2021, 2712 (5.4%) of the initially positive patients were retested, and 41 (1.5%) of those patients retested positive; 1 patient had immediate subsequent negative test results and was thus excluded due to a presumed false-positive test. Of the 40 reinfections, 28 (70%) were symptomatic; the rest had an asymptomatic indication (eg, preprocedural screening, preadmission screening for non-COVID–related diagnosis). Of the 274 830 initially negative patients, 19 157 (7.0%) were retested after 1 July 2021, and 1494 (7.8%) of those patients retested positive. Of these, 1298 (86.9%) were symptomatic. Retested patients who had previously been infected were slightly more likely to be symptomatic at the time of retesting than those not previously infected (57.1% vs 54.0%, P = .008). The overall protection of prior infection against reinfection during the period of Delta dominance was 85.4% (95% confidence interval [CI], 80.0–89.3). Protection was higher against symptomatic infection (88.2%; 95% CI, 82.9–91.9) than asymptomatic infection (66.6%; 95% CI, 40.6–81.2).
Characteristics of Patients With Initial Positive vs Negative Test in the Delta Analysis
| Characteristic . | Initial Positive Test . | Initial Negative Test . |
|---|---|---|
| Number of patients | 50 327 | 274 830 |
| Age ± SD, years | 48.9 ± 21.1 | 50.3 ± 22.1 |
| Sex female (%) | 26 252 (52.2) | 155 100 (56.4) |
| Number retested after 1 July 2021 (%) | 2712 (5.4) | 19 157 (7.0) |
| Age ± SD, years | 50.6 ± 23.4 | 50.0 ± 24.3 |
| Sex female (%) | 1575 (58.1) | 11 372 (59.4) |
| Retest positive, any (%) | 40 (0.08) | 1494 (0.5) |
| Time to positive retest ± SD, days | 300.4 ± 75.7 | 349.1 ± 83.0 |
| Retest positive, symptomatic (%) | 28 (0.06) | 1298 (0.5) |
| Effectiveness | ||
| Any infection | 85.4% (95% CI, 80.0–89.3) | |
| Symptomatic infection | 88.2% (95% CI, 82.9–91.9) | |
| Asymptomatic infection | 66.6% (95% CI, 40.6–81.2) | |
| Characteristic . | Initial Positive Test . | Initial Negative Test . |
|---|---|---|
| Number of patients | 50 327 | 274 830 |
| Age ± SD, years | 48.9 ± 21.1 | 50.3 ± 22.1 |
| Sex female (%) | 26 252 (52.2) | 155 100 (56.4) |
| Number retested after 1 July 2021 (%) | 2712 (5.4) | 19 157 (7.0) |
| Age ± SD, years | 50.6 ± 23.4 | 50.0 ± 24.3 |
| Sex female (%) | 1575 (58.1) | 11 372 (59.4) |
| Retest positive, any (%) | 40 (0.08) | 1494 (0.5) |
| Time to positive retest ± SD, days | 300.4 ± 75.7 | 349.1 ± 83.0 |
| Retest positive, symptomatic (%) | 28 (0.06) | 1298 (0.5) |
| Effectiveness | ||
| Any infection | 85.4% (95% CI, 80.0–89.3) | |
| Symptomatic infection | 88.2% (95% CI, 82.9–91.9) | |
| Asymptomatic infection | 66.6% (95% CI, 40.6–81.2) | |
Abbreviations: CI, confidence interval; SD, standard deviation.
Characteristics of Patients With Initial Positive vs Negative Test in the Delta Analysis
| Characteristic . | Initial Positive Test . | Initial Negative Test . |
|---|---|---|
| Number of patients | 50 327 | 274 830 |
| Age ± SD, years | 48.9 ± 21.1 | 50.3 ± 22.1 |
| Sex female (%) | 26 252 (52.2) | 155 100 (56.4) |
| Number retested after 1 July 2021 (%) | 2712 (5.4) | 19 157 (7.0) |
| Age ± SD, years | 50.6 ± 23.4 | 50.0 ± 24.3 |
| Sex female (%) | 1575 (58.1) | 11 372 (59.4) |
| Retest positive, any (%) | 40 (0.08) | 1494 (0.5) |
| Time to positive retest ± SD, days | 300.4 ± 75.7 | 349.1 ± 83.0 |
| Retest positive, symptomatic (%) | 28 (0.06) | 1298 (0.5) |
| Effectiveness | ||
| Any infection | 85.4% (95% CI, 80.0–89.3) | |
| Symptomatic infection | 88.2% (95% CI, 82.9–91.9) | |
| Asymptomatic infection | 66.6% (95% CI, 40.6–81.2) | |
| Characteristic . | Initial Positive Test . | Initial Negative Test . |
|---|---|---|
| Number of patients | 50 327 | 274 830 |
| Age ± SD, years | 48.9 ± 21.1 | 50.3 ± 22.1 |
| Sex female (%) | 26 252 (52.2) | 155 100 (56.4) |
| Number retested after 1 July 2021 (%) | 2712 (5.4) | 19 157 (7.0) |
| Age ± SD, years | 50.6 ± 23.4 | 50.0 ± 24.3 |
| Sex female (%) | 1575 (58.1) | 11 372 (59.4) |
| Retest positive, any (%) | 40 (0.08) | 1494 (0.5) |
| Time to positive retest ± SD, days | 300.4 ± 75.7 | 349.1 ± 83.0 |
| Retest positive, symptomatic (%) | 28 (0.06) | 1298 (0.5) |
| Effectiveness | ||
| Any infection | 85.4% (95% CI, 80.0–89.3) | |
| Symptomatic infection | 88.2% (95% CI, 82.9–91.9) | |
| Asymptomatic infection | 66.6% (95% CI, 40.6–81.2) | |
Abbreviations: CI, confidence interval; SD, standard deviation.
Protection by age is shown in Table 2. There were relatively few patients aged >80 years, but the protective effect estimate for patients aged >80 years was higher than, but not statistically different from, that of patients aged 65–79 years; the 2 categories are thus reported together. Compared with patients aged 0–64 years, those aged ≥65 years had a lower rate of protection (75.1% vs 87.9%, P = .06). The proportion of patients who received 2 doses of COVID-19 vaccine before 1 July 2021 was similar for previously infected and noninfected patients (25.4% vs 26.6%, P < .001). Among the vaccinated patients, previous infection had an estimated effectiveness of 86.8% (95% CI, 74.5–93.2) against reinfection with Delta.
| Age Group, Years . | Number of Patients . | Retest Positive . | Infection Rate, % . | Estimated Protection, % . | |||
|---|---|---|---|---|---|---|---|
| Initial + . | Initial – . | Initial + . | Initial – . | Initial + . | Initial – . | ||
| Overall | 50 327 | 274 830 | 40 | 1494 | 0.08 | 0.5 | 85.4 |
| 0–17 | 3156 | 20 507 | 4 | 153 | 0.1 | 0.7 | 83.0 |
| 18–34 | 11 614 | 56 304 | 6 | 395 | 0.05 | 0.7 | 92.6 |
| 35–50 | 11 618 | 54 592 | 11 | 341 | 0.09 | 0.6 | 84.8 |
| 51–64 | 11 785 | 62 711 | 7 | 285 | 0.06 | 0.5 | 86.9 |
| 0–64 | 38 173 | 194 115 | 28 | 1174 | 0.07 | 0.6 | 87.9 |
| ≥65 | 12 154 | 80 715 | 12 | 320 | 0.1 | 0.4 | 75.1 |
| Age Group, Years . | Number of Patients . | Retest Positive . | Infection Rate, % . | Estimated Protection, % . | |||
|---|---|---|---|---|---|---|---|
| Initial + . | Initial – . | Initial + . | Initial – . | Initial + . | Initial – . | ||
| Overall | 50 327 | 274 830 | 40 | 1494 | 0.08 | 0.5 | 85.4 |
| 0–17 | 3156 | 20 507 | 4 | 153 | 0.1 | 0.7 | 83.0 |
| 18–34 | 11 614 | 56 304 | 6 | 395 | 0.05 | 0.7 | 92.6 |
| 35–50 | 11 618 | 54 592 | 11 | 341 | 0.09 | 0.6 | 84.8 |
| 51–64 | 11 785 | 62 711 | 7 | 285 | 0.06 | 0.5 | 86.9 |
| 0–64 | 38 173 | 194 115 | 28 | 1174 | 0.07 | 0.6 | 87.9 |
| ≥65 | 12 154 | 80 715 | 12 | 320 | 0.1 | 0.4 | 75.1 |
P value comparing age group ≥65 years vs all patients aged <65 years = .06.
| Age Group, Years . | Number of Patients . | Retest Positive . | Infection Rate, % . | Estimated Protection, % . | |||
|---|---|---|---|---|---|---|---|
| Initial + . | Initial – . | Initial + . | Initial – . | Initial + . | Initial – . | ||
| Overall | 50 327 | 274 830 | 40 | 1494 | 0.08 | 0.5 | 85.4 |
| 0–17 | 3156 | 20 507 | 4 | 153 | 0.1 | 0.7 | 83.0 |
| 18–34 | 11 614 | 56 304 | 6 | 395 | 0.05 | 0.7 | 92.6 |
| 35–50 | 11 618 | 54 592 | 11 | 341 | 0.09 | 0.6 | 84.8 |
| 51–64 | 11 785 | 62 711 | 7 | 285 | 0.06 | 0.5 | 86.9 |
| 0–64 | 38 173 | 194 115 | 28 | 1174 | 0.07 | 0.6 | 87.9 |
| ≥65 | 12 154 | 80 715 | 12 | 320 | 0.1 | 0.4 | 75.1 |
| Age Group, Years . | Number of Patients . | Retest Positive . | Infection Rate, % . | Estimated Protection, % . | |||
|---|---|---|---|---|---|---|---|
| Initial + . | Initial – . | Initial + . | Initial – . | Initial + . | Initial – . | ||
| Overall | 50 327 | 274 830 | 40 | 1494 | 0.08 | 0.5 | 85.4 |
| 0–17 | 3156 | 20 507 | 4 | 153 | 0.1 | 0.7 | 83.0 |
| 18–34 | 11 614 | 56 304 | 6 | 395 | 0.05 | 0.7 | 92.6 |
| 35–50 | 11 618 | 54 592 | 11 | 341 | 0.09 | 0.6 | 84.8 |
| 51–64 | 11 785 | 62 711 | 7 | 285 | 0.06 | 0.5 | 86.9 |
| 0–64 | 38 173 | 194 115 | 28 | 1174 | 0.07 | 0.6 | 87.9 |
| ≥65 | 12 154 | 80 715 | 12 | 320 | 0.1 | 0.4 | 75.1 |
P value comparing age group ≥65 years vs all patients aged <65 years = .06.
Severity of illness within 30 days was similar between those who had and had not been previously infected, including rates of hospitalization (14 of 40, 35.0% vs 587 of 1494, 39.3%; P = .58), admission to the ICU (3 of 14, 21.4% vs 94 of 587, 16.0%; P = .59), need for mechanical ventilation (2 of 14, 14.3% vs 38 of 587, 6.5%; P = .25), and death (1 of 14, 7.1% vs 25 of 587, 4.3%; P = .60).
Long-term Effectiveness Analysis
There were 199 406 tests performed before 30 August 2020 on 152 656 individuals. Of these, 11 186 (7.3%) tested positive (Table 3). After at least 90 days, 2187 (19.6%) of the initially positive patients were retested, and 84 (3.8%) of these patients retested positive; 3 patients had immediate subsequent negative test results and were thus excluded due to presumed false-positive tests. Of the 81 reinfections, 38 (46.9%) were symptomatic. Of the 141 470 initially negative patients, 47 539 (33.6%) individuals were retested, and 7167 (15.1%) retested positive. Of these, 6030 (84.1%) were symptomatic. The estimated overall protection against reinfection was 85.7% (95% CI, 82.2–88.5). Again, protection was greater against symptomatic infection (92.0%; 95% CI, 89.1–94.2) than asymptomatic infection (52.2%; 95% CI, 35.3–64.7), particularly during the first 90–150 days when effectiveness against asymptomatic infection was actually negative. After the first 5 months, protection against reinfection exceeded 90% for up to 13 months from initial infection. Protection by age is shown in Table 4. Again, there were very few cases among patients aged >80 years, and their results were combined with patients aged 65–79 years. Protection among those aged ≥65 years was lower than that of individuals aged <65 years (76.3% vs 88.9%, P < .001).
Characteristics of Patients With Initial Positive vs Negative Test in the Long-term Effectiveness Analysis
| Characteristic . | Initial Positive Test . | Initial Negative Test . | |
|---|---|---|---|
| Number of patients | 11 186 | 141 470 | |
| Age ± SD, years | 50.5 ± 21.0 | 52.8 ± 21.4 | |
| Sex female (%) | 5900 (52.7) | 79 975 (56.5) | |
| Number who were retested (%) | 2187 (19.6) | 47 539 (33.6) | |
| Age ± SD, years | 52.9 ± 21.2 | 54.0 ± 21.3 | |
| Sex female (%) | 1252 (57.2) | 27 471 (57.8) | |
| Retest positive, any (%) | 81 (0.7) | 7167 (5.1) | |
| Time to positive retest ± SD, days | 181.6 ± 106.8 | 223.3 ± 93.3 | |
| Retest positive, symptomatic (%) | 38 (0.3) | 6030 (4.3) | |
| Any Infection, % | Symptomatic Infection, % | Asymptomatic Infection, % | |
| Effectiveness, days | 85.7 | 92.0 | 52.2 |
| 90–150 | 63.9 | 82.9 | –34.6 |
| 151–210 | 93.2 | 96.0 | 77.9 |
| 211–270 | 93.9 | 96.3 | 79.8 |
| 271–330 | 91.3 | 91.6 | 89.7 |
| 331–390 | 90.8 | 88.9 | 100 |
| After 390 | 87.3 | 95.0 | 44.0 |
| Characteristic . | Initial Positive Test . | Initial Negative Test . | |
|---|---|---|---|
| Number of patients | 11 186 | 141 470 | |
| Age ± SD, years | 50.5 ± 21.0 | 52.8 ± 21.4 | |
| Sex female (%) | 5900 (52.7) | 79 975 (56.5) | |
| Number who were retested (%) | 2187 (19.6) | 47 539 (33.6) | |
| Age ± SD, years | 52.9 ± 21.2 | 54.0 ± 21.3 | |
| Sex female (%) | 1252 (57.2) | 27 471 (57.8) | |
| Retest positive, any (%) | 81 (0.7) | 7167 (5.1) | |
| Time to positive retest ± SD, days | 181.6 ± 106.8 | 223.3 ± 93.3 | |
| Retest positive, symptomatic (%) | 38 (0.3) | 6030 (4.3) | |
| Any Infection, % | Symptomatic Infection, % | Asymptomatic Infection, % | |
| Effectiveness, days | 85.7 | 92.0 | 52.2 |
| 90–150 | 63.9 | 82.9 | –34.6 |
| 151–210 | 93.2 | 96.0 | 77.9 |
| 211–270 | 93.9 | 96.3 | 79.8 |
| 271–330 | 91.3 | 91.6 | 89.7 |
| 331–390 | 90.8 | 88.9 | 100 |
| After 390 | 87.3 | 95.0 | 44.0 |
Abbreviation: SD, standard deviation.
Characteristics of Patients With Initial Positive vs Negative Test in the Long-term Effectiveness Analysis
| Characteristic . | Initial Positive Test . | Initial Negative Test . | |
|---|---|---|---|
| Number of patients | 11 186 | 141 470 | |
| Age ± SD, years | 50.5 ± 21.0 | 52.8 ± 21.4 | |
| Sex female (%) | 5900 (52.7) | 79 975 (56.5) | |
| Number who were retested (%) | 2187 (19.6) | 47 539 (33.6) | |
| Age ± SD, years | 52.9 ± 21.2 | 54.0 ± 21.3 | |
| Sex female (%) | 1252 (57.2) | 27 471 (57.8) | |
| Retest positive, any (%) | 81 (0.7) | 7167 (5.1) | |
| Time to positive retest ± SD, days | 181.6 ± 106.8 | 223.3 ± 93.3 | |
| Retest positive, symptomatic (%) | 38 (0.3) | 6030 (4.3) | |
| Any Infection, % | Symptomatic Infection, % | Asymptomatic Infection, % | |
| Effectiveness, days | 85.7 | 92.0 | 52.2 |
| 90–150 | 63.9 | 82.9 | –34.6 |
| 151–210 | 93.2 | 96.0 | 77.9 |
| 211–270 | 93.9 | 96.3 | 79.8 |
| 271–330 | 91.3 | 91.6 | 89.7 |
| 331–390 | 90.8 | 88.9 | 100 |
| After 390 | 87.3 | 95.0 | 44.0 |
| Characteristic . | Initial Positive Test . | Initial Negative Test . | |
|---|---|---|---|
| Number of patients | 11 186 | 141 470 | |
| Age ± SD, years | 50.5 ± 21.0 | 52.8 ± 21.4 | |
| Sex female (%) | 5900 (52.7) | 79 975 (56.5) | |
| Number who were retested (%) | 2187 (19.6) | 47 539 (33.6) | |
| Age ± SD, years | 52.9 ± 21.2 | 54.0 ± 21.3 | |
| Sex female (%) | 1252 (57.2) | 27 471 (57.8) | |
| Retest positive, any (%) | 81 (0.7) | 7167 (5.1) | |
| Time to positive retest ± SD, days | 181.6 ± 106.8 | 223.3 ± 93.3 | |
| Retest positive, symptomatic (%) | 38 (0.3) | 6030 (4.3) | |
| Any Infection, % | Symptomatic Infection, % | Asymptomatic Infection, % | |
| Effectiveness, days | 85.7 | 92.0 | 52.2 |
| 90–150 | 63.9 | 82.9 | –34.6 |
| 151–210 | 93.2 | 96.0 | 77.9 |
| 211–270 | 93.9 | 96.3 | 79.8 |
| 271–330 | 91.3 | 91.6 | 89.7 |
| 331–390 | 90.8 | 88.9 | 100 |
| After 390 | 87.3 | 95.0 | 44.0 |
Abbreviation: SD, standard deviation.
| Age Group, Years . | Number of Patients . | Retest Positive . | Infection Rate, % . | Estimated Protection, % . | P Valuea . | |||
|---|---|---|---|---|---|---|---|---|
| Initial + . | Initial – . | Initial + . | Initial – . | Initial + . | Initial – . | |||
| Overall | 11 186 | 141 470 | 81 | 7167 | 0.7 | 5.1 | 85.7 | -- |
| 0–17 | 368 | 6650 | 1 | 230 | 0.3 | 3.5 | 92.1 | .26 |
| 18–34 | 2735 | 26 832 | 14 | 1459 | 0.5 | 5.4 | 90.6 | .03 |
| 35–50 | 2542 | 27 702 | 15 | 1531 | 0.6 | 5.5 | 89.3 | .01 |
| 51–64 | 2630 | 34 119 | 17 | 1671 | 0.6 | 4.9 | 86.8 | .004 |
| 0–64 | 8275 | 95 304 | 47 | 4891 | 0.6 | 5.1 | 88.9 | <.001 |
| ≥65 | 2911 | 46 166 | 34 | 2276 | 1.2 | 4.9 | 76.3 | ref |
| Age Group, Years . | Number of Patients . | Retest Positive . | Infection Rate, % . | Estimated Protection, % . | P Valuea . | |||
|---|---|---|---|---|---|---|---|---|
| Initial + . | Initial – . | Initial + . | Initial – . | Initial + . | Initial – . | |||
| Overall | 11 186 | 141 470 | 81 | 7167 | 0.7 | 5.1 | 85.7 | -- |
| 0–17 | 368 | 6650 | 1 | 230 | 0.3 | 3.5 | 92.1 | .26 |
| 18–34 | 2735 | 26 832 | 14 | 1459 | 0.5 | 5.4 | 90.6 | .03 |
| 35–50 | 2542 | 27 702 | 15 | 1531 | 0.6 | 5.5 | 89.3 | .01 |
| 51–64 | 2630 | 34 119 | 17 | 1671 | 0.6 | 4.9 | 86.8 | .004 |
| 0–64 | 8275 | 95 304 | 47 | 4891 | 0.6 | 5.1 | 88.9 | <.001 |
| ≥65 | 2911 | 46 166 | 34 | 2276 | 1.2 | 4.9 | 76.3 | ref |
P values were calculated using age group ≥65 years as reference group.
| Age Group, Years . | Number of Patients . | Retest Positive . | Infection Rate, % . | Estimated Protection, % . | P Valuea . | |||
|---|---|---|---|---|---|---|---|---|
| Initial + . | Initial – . | Initial + . | Initial – . | Initial + . | Initial – . | |||
| Overall | 11 186 | 141 470 | 81 | 7167 | 0.7 | 5.1 | 85.7 | -- |
| 0–17 | 368 | 6650 | 1 | 230 | 0.3 | 3.5 | 92.1 | .26 |
| 18–34 | 2735 | 26 832 | 14 | 1459 | 0.5 | 5.4 | 90.6 | .03 |
| 35–50 | 2542 | 27 702 | 15 | 1531 | 0.6 | 5.5 | 89.3 | .01 |
| 51–64 | 2630 | 34 119 | 17 | 1671 | 0.6 | 4.9 | 86.8 | .004 |
| 0–64 | 8275 | 95 304 | 47 | 4891 | 0.6 | 5.1 | 88.9 | <.001 |
| ≥65 | 2911 | 46 166 | 34 | 2276 | 1.2 | 4.9 | 76.3 | ref |
| Age Group, Years . | Number of Patients . | Retest Positive . | Infection Rate, % . | Estimated Protection, % . | P Valuea . | |||
|---|---|---|---|---|---|---|---|---|
| Initial + . | Initial – . | Initial + . | Initial – . | Initial + . | Initial – . | |||
| Overall | 11 186 | 141 470 | 81 | 7167 | 0.7 | 5.1 | 85.7 | -- |
| 0–17 | 368 | 6650 | 1 | 230 | 0.3 | 3.5 | 92.1 | .26 |
| 18–34 | 2735 | 26 832 | 14 | 1459 | 0.5 | 5.4 | 90.6 | .03 |
| 35–50 | 2542 | 27 702 | 15 | 1531 | 0.6 | 5.5 | 89.3 | .01 |
| 51–64 | 2630 | 34 119 | 17 | 1671 | 0.6 | 4.9 | 86.8 | .004 |
| 0–64 | 8275 | 95 304 | 47 | 4891 | 0.6 | 5.1 | 88.9 | <.001 |
| ≥65 | 2911 | 46 166 | 34 | 2276 | 1.2 | 4.9 | 76.3 | ref |
P values were calculated using age group ≥65 years as reference group.
DISCUSSION
We conducted a large retrospective cohort study of patients tested for COVID-19 at 1 health system to estimate protection of previous infection against the Delta variant and to assess the duration of immunity conferred by SARS-CoV-2 infection. We found that patients with prior infection were less likely to be retested or retest positive compared with individuals who initially tested negative during the same period. Effectiveness of previous infection against symptomatic infection with Delta was 88.2%, which was similar to the protection against symptomatic infection throughout the entire study period (92.0%). Even when asymptomatic cases were included, protection offered against reinfection with Delta and throughout the entire study period was 85.4% and 85.7%, respectively. After the first 5 months, protection against reinfection exceeded 90% for up to 13 months after primary infection, suggesting that natural immunity lasts longer than has been previously reported [5, 7, 8]. Effectiveness against repeat infection was slightly reduced in patients aged ≥65 years. Among those who were infected with the Delta variant, severity of illness, as measured by 30-day rates of hospitalization and ICU admission, appeared to be similar between those who had and had not been previously infected. Thus, while prior infection appears to be highly protective against infection with the Delta variant, in contrast to vaccination, it does not appear to reduce severity of disease. Finally, among patients who were fully vaccinated against COVID-19, prior SARS-CoV-2 infection offered an additional 87% protection against infection with the Delta variant.
Several large cohort studies from the United Kingdom, Austria, and Qatar have reported similar estimates of short-term protection against non-Delta strains [5, 7, 8, 15, 16]. Two studies of healthcare workers in the United Kingdom demonstrated that immunity from natural infection lasts at least 5–6 months [7, 8], and a study of 43 044 antibody-positive individuals in Qatar estimated a 95.2% efficacy of prior infection that can last for at least 7 months [15]. Our study adds to these by assessing the long-term immunity conferred by SARS-CoV-2 infection. We found no evidence of waning protection for up to 13 months. This is consistent with in vitro studies that have demonstrated the persistence of antibodies with neutralizing activity for at least 12 months [17]. Interestingly, we found that protection against reinfection, in particular asymptomatic reinfection, was lowest 3–5 months after initial infection, increasing thereafter. This counterintuitive finding might be explained by persistent viral shedding, as asymptomatic patients can continue to shed virus for many months following initial infection [18]; this would have caused us to underestimate protection during this time period and overall.
While in vitro neutralization studies have shown that antibodies present in the sera of previously infected individuals are less potent against the Delta variant [2, 4], we found that natural infection offers high levels of protection against Delta. In fact, natural infection appears to offer more sustained protection against Delta than vaccine-mediated immunity. Recent studies have shown that vaccine-mediated immunity wanes after 6 months, with a rapid decline in efficacy against Delta after as little as 90 days [10, 11, 13, 19]. This finding is further supported by a large observational study in Israel that reported that SARS-CoV-2–naive individuals who received 2 doses of the BioNTech/Pfizer mRNA vaccine were 6–13 times more likely to become infected with Delta than patients who had experienced infection previously [20]. Similarly, we found that among vaccinated patients, those who were not previously infected were almost 8 times as likely to be subsequently infected with Delta.
Our study has several limitations. Chief among them is that we did not have access to testing results outside of the Cleveland Clinic Health System. However, unless there was a differential in patients seeking testing at the Cleveland Clinic based on their previous infection status, our findings should still be valid. Similarly, some patients may have been infected but did not seek testing, especially if they were asymptomatic or mildly so. Patients with previous infection may be less likely to seek testing because they think that they are immune, which would result in an overestimation of the protective effect of prior infection. It is also possible that patients with previous infection are more or less likely to forgo vaccination or engage in high-risk behaviors, both of which would be associated with reinfection risk apart from immunity. However, when we analyzed our results stratified by age, patients aged 18–34 years did not show reduced effectiveness against Delta, even though such patients are more likely to engage in risky behavior [21] and less likely to be vaccinated [22]. Moreover, when analyzing our results among vaccinated patients, the findings were the same. Additionally, because sequencing was not routinely performed on clinical specimens during our study period, it is possible that some positive tests after 1 July 2021 do not represent infection with Delta. However, given that Delta represented 87% of the sequenced viruses in the United States by mid-July 2021, with this percentage reaching 96% by 31 July 2021, the vast majority of SARS-CoV-2 infections after 1 July can be assumed to have been caused by Delta [1]. It is also possible that there are specific Delta strains that can escape natural immunity and to which our protective estimates would not apply. Last, we were unable to assess the impact of vaccinations because many took place outside of our health system; this constrained our ability to distinguish between missing data and zero vaccine doses. The type of vaccines that patients received was also unknown, so we could not distinguish between patients who received single-dose or 2-dose vaccines. Therefore, we limited our vaccination analysis to those patients who had 2 confirmed date entries for their COVID-19 vaccines.
Our study has several important implications. Most low- and middle-income countries face significant challenges in obtaining adequate supplies of COVID-19 vaccines [23]. Based on our findings, countries with vaccine shortages should consider delaying vaccinations of previously infected individuals to prioritize vaccinating nonimmune individuals, beginning with the most vulnerable populations (eg, elderly patients, those with multiple comorbidities, front-line healthcare workers). Our findings may also have implications for the recent US emergency temporary standard, which would require large employers to enforce mandatory COVID-19 vaccination, a policy under which all employees must either be vaccinated against COVID-19 or undergo weekly COVID-19 testing [24]. Patients who have recovered from COVID-19 infection are notably not exempt. This stands in contrast to the policies of several other countries that acknowledge the immunity provided by prior COVID-19 infection. Israel [25], the European Union [26], and the United Kingdom [27] all provide some version of a COVID pass, which allows access to public spaces following either vaccination or recovery from COVID-19. Given that previous infection appears to offer sustained protection against the Delta variant, our study suggests that natural immunity should be considered in the US emergency temporary standard.
Our findings also suggest that some countries may approach herd immunity against future variants due to the protection offered by the combination of previous SARS-CoV-2 infections and COVID-19 vaccinations. With an R0 of 6, the Delta variant would require a herd immunity threshold of at least 85% [28, 29]. Researchers estimate that about one-third of the US population had been infected with COVID-19 by the end of 2020, and 58.8% of the US population had been fully vaccinated against COVID-19 as of 15 November 2021 [20, 30]. Given the many additional cases of COVID-19 in 2021, the United States may be approaching herd immunity [31]. However, large-scale vaccination programs are critical for continued progress. A recent phylogenetic analysis of evolutionarily close coronavirus relatives estimated reinfection by SARS-CoV-2 to occur at a median of 16 months following peak antibody response, suggesting that immunity does not last forever [32].
Although our findings highlight the effectiveness of natural immunity, intentional exposure to COVID-19 in order to gain such immunity is not recommended. Recent studies from Canada [33] and Singapore [34] showed that the Delta variant was associated with an increased risk for hospitalization, ICU admission, oxygen requirement, and death. Therefore, achieving immunity through vaccination is preferred. Unlike natural immunity, vaccination substantially reduces the risk of developing severe disease [35], as well as potential long-term complications of COVID-19 infection [36].
In summary, we found that previous infection with SARS-CoV-2 offers strong protection against reinfection with the highly transmissible Delta variant. We also found that such natural immunity appears to persist for at least 13 months. Together, these results support vaccination efforts that prioritize patients who have no known history of prior infection and suggest that herd immunity may soon be within reach.
Note
Potential conflicts of interest. S. M. G. has served on the clinical endpoint committee for the Abbott Heartmate 3 M3PAS study. M. B. R. has owned stock in Moderna. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




