-
PDF
- Split View
-
Views
-
Cite
Cite
Patricia J Kissinger, Charlotte A Gaydos, Arlene C Seña, R Scott McClelland, David Soper, W Evan Secor, Davey Legendre, Kimberly A Workowski, Christina A Muzny, Diagnosis and Management of Trichomonas vaginalis: Summary of Evidence Reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines, Clinical Infectious Diseases, Volume 74, Issue Supplement_2, 15 April 2022, Pages S152–S161, https://doi.org/10.1093/cid/ciac030
Close - Share Icon Share
Abstract
Trichomonas vaginalis is likely the most prevalent nonviral sexually transmitted infection, affecting an estimated 3.7 million women and men in the United States. Health disparities are prominent in the epidemiology of trichomoniasis, as African Americans are >4 times more likely to be infected than persons of other races. Since publication of the 2015 Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines, additional data have bolstered the importance of T. vaginalis infection sequelae in women, including increased risk of human immunodeficiency virus (HIV) acquisition, cervical cancer, preterm birth, and other adverse pregnancy outcomes. Less is known about the clinical significance of infection in men. Newly available diagnostic methods, including point-of-care assays and multiple nucleic acid amplification tests, can be performed on a variety of genital specimens in women and men, including urine, allowing more accurate and convenient testing and screening of those at risk for infection. Repeat and persistent infections are common in women; thus, rescreening at 3 months after treatment is recommended. In vitro antibiotic resistance to 5-nitroimidazole in T. vaginalis remains low (4.3%) but should be monitored. High rates of T. vaginalis among sexual partners of infected persons suggest a role for expedited partner treatment. A randomized controlled trial in HIV-uninfected women demonstrated that multidose metronidazole 500 mg twice daily for 7 days reduced the proportion of women with Trichomonas infection at 1 month test of cure compared with women receiving single-dose therapy (2 g). The 2-g single-dose oral metronidazole regimen remains the preferred treatment in men.
Trichomonas vaginalis is a highly prevalent parasitic infection that causes the sexually transmitted infection (STI) trichomoniasis. Since 2013 when the medical literature was systematically reviewed for development of the 2015 Centers for Disease Control and Prevention (CDC) sexually transmitted diseases (STD) treatment guidelines, additional data have been published regarding the epidemiology, clinical significance, clinical presentation, diagnosis, and treatment of T. vaginalis infections [1]. This article is a review of the current evidence in each of these key topic areas used for development of the 2021 CDC STI treatment guidelines’ updated Trichomonas section.
METHODS
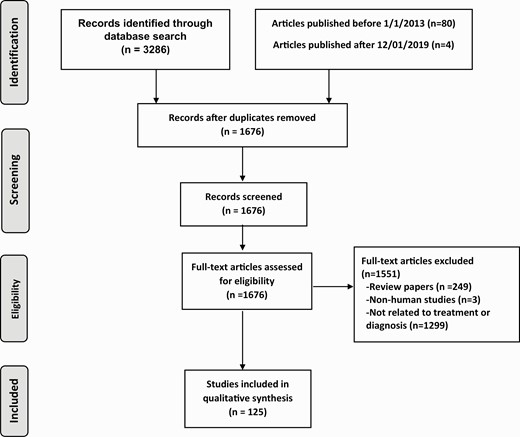
Specific key questions and a table of evidence assembled by our expert review committee related to updates on the epidemiology, diagnosis, and management of trichomoniasis for the 2021 CDC STI treatment guidelines can be found online at https://www.cdc.gov/std/treatment-guidelines/evidence.htm. To answer these questions, a PubMed (United States [US] National Library of Medicine and National Institutes of Health) search was conducted of all English-language human subjects research published on T. vaginalis between 1 January 2013 and 1 December 2019. The search terms “trichomonas” (1590 articles), “Trichomonas vaginalis” (1102 articles), and “trichomoniasis” (594 articles) were reviewed. Articles that were relevant but were published before 1 January 2013 (n=80) or after 1 December 2019 (n=4) were also included. Abstracts in hard copy and online from relevant STI conferences were reviewed using the dates and search terms above. The total search contained 1676 nonduplicated papers. All abstracts were reviewed, as was the full text of pertinent articles, to determine whether each contained data relevant to the 2021 CDC STI treatment guidelines. Additional unpublished data of which our expert committee was aware were added to the tables, with the permission of the authors (Figure 1). The quality of the evidence was discussed within our expert review committee and during the CDC STI Treatment Guidelines Meeting in Atlanta, Georgia, on 11–13 June 2019, to inform updates to the T. vaginalis section of the 2021 CDC STI treatment guidelines.
RESULTS
Epidemiology and Clinical Significance of T. vaginalis
Trichomonas vaginalis is likely the most common nonviral STI in the world. While not a reportable disease, global estimates indicate that among women there are 156 million new cases per year [2]. The global prevalence of T. vaginalis is higher than that of Chlamydia trachomatis, Neisseria gonorrhoeae, and syphilis combined [2]. In the US, the prevalence of T. vaginalis in a recent population-based study utilizing urine nucleic acid amplification tests (NAATs) was 1.8% among women and 0.5% for men [3]. An estimated 3.1 million new infections occur each year in the US [4]. African Americans are at particular risk with a >4-fold higher prevalence of infection than other racial groups, constituting a dramatic health disparity [3]. Other risk factors for T. vaginalis include female sex, older age, 2 or more sexual partners in the past year, having less than a high school education, living below the poverty level, and history of incarceration [3]. The prevalence of T. vaginalis in men who have sex with men is very low [5]. In addition, extragenital (oral, rectal) T. vaginalis occurs occasionally but is much less common than genital T. vaginalis [5, 6]. Because of this, oral and rectal testing or screening is not currently recommended in women or men.
Trichomonas vaginalis is an extracellular parasite that primarily infects the squamous epithelium of the genital tract. It commonly infects the female lower genital tract (vagina, urethra, and endocervix) and the male urethra and prostate. Trichomonas vaginalis is transmitted among humans, its only known host, primarily by sexual intercourse. Infection in women may persist for long periods of time, possibly months or even years [7] but may be shorter (eg, less than a month) for men [8].
The T. vaginalis parasite does not appear to have a cyst form and does not survive well in the external environment, but can survive outside the human body in a wet and warm environment for >3 hours [9]. There may be, however, a pseudocyst form [10], which has been found to be more virulent in animals and could have relevance for humans, particularly in the case of cervical neoplasia [10, 11]. While thought to be rare [12], evidence of nonsexual transmission via fomites and possibly water has been described [13–15]. Trichomonas vaginalis can be infected with 1 of 4 double-stranded RNA (dsRNA) viruses (T. vaginalis virus [TVV]), of which TVV2 has been previously linked to more severe genital symptoms [16]. However, since the 2015 guidelines, a study of 355 T. vaginalis isolates found that 40% were positive for TVV, and there was no association between TVV positivity and genital symptoms [17].
Clinical Presentation of T. vaginalis
The majority of women (85%) [18] and men (77%) [19] with T. vaginalis are asymptomatic. Half of the asymptomatic women may become symptomatic within 6 months [12]. Symptomatic women can have vaginal erythema, dyspareunia, dysuria, and vaginal discharge (which is often diffuse, malodorous, and yellow-green in color), as well as pruritus in the genital region. The normal vaginal pH is 4.5, but with T. vaginalis infection this may increase markedly, often to >5 [12]. However, T. vaginalis may still be present in the setting of a normal vaginal pH. Colpitis macularis, or “strawberry cervix,” is seen in about 5% of women on pelvic examination, though with colposcopy this rises to nearly 50% [20]. Complications of T. vaginalis among women include infection of the Skene and Bartholin glands, and increased risk of pelvic inflammatory disease among women with human immunodeficiency virus (HIV) infection [21]. In men, it can cause urethritis, epididymitis, prostatitis, and decreased sperm motility [22].
T. vaginalis and Perinatal Outcomes
Since the 2015 guidelines, 2 meta-analyses have been published providing greater evidence that T. vaginalis is associated with poor birth outcomes such as low birth weight, preterm delivery, and premature rupture of membranes [23, 24]. One study also showed an association between maternal T. vaginalis infection and intellectual disability in children born to infected mothers, but there have been no additional studies published on this topic [25]. Although rare, T. vaginalis infection can be transmitted perinatally [26] and cause vaginal and respiratory infections in neonates [27, 28].
T. vaginalis and HIV Transmission
Previous investigations show that T. vaginalis infections are associated with increased risk of HIV acquisition [29]. Since the 2015 guidelines, a meta-analysis of 19 peer-reviewed studies found that persons with T. vaginalis were 1.5 times more likely to acquire HIV infection [30]. This greater susceptibility is biologically plausible for 3 reasons: (1) the inflammatory response to T. vaginalis infection results in an increased appearance of HIV target cells in the genital tract mucosa [31]; (2) T. vaginalis infection can impair the mechanical barrier to HIV via punctate mucosal hemorrhages [32]; and (3) T. vaginalis infection may change the normal vaginal microbiota, rendering it more permissive for the development of bacterial vaginosis (BV) [33], which, in turn, can increase the risk of HIV acquisition [34]. The influence of T. vaginalis on HIV transmission in the era of HIV preexposure prophylaxis needs further study.
There is less direct evidence that persons with HIV and T. vaginalis are more likely to transmit HIV. A review article found that 7 of 14 studies demonstrated higher likelihood of shedding of HIV in genital fluids if a person had T. vaginalis compared to persons with HIV without Trichomonas infection [29]. HIV vaginal shedding was decreased after T. vaginalis treatment in a cohort of women from Kenya, diagnosed by microscopy and culture [35], and in another cohort in New Orleans, Louisiana, diagnosed by culture [36].
A study by Sorvillo and Kerndt estimated that in a community with a high prevalence of T. vaginalis, as many as 20% of HIV infections could be attributed to T. vaginalis infection [37]. Chesson et al estimated that 6.2% of all HIV infections among US women may be attributable to T. vaginalis infection [38]. There have been no newer models published since the 2015 guidelines. Control of T. vaginalis, therefore, may provide a cost-effective strategy for reducing HIV transmission, especially in settings where T. vaginalis is common [39, 40] or among subgroups that have higher rates of T. vaginalis, such as African Americans [41]. While these studies were done outside the context of HIV treatment as prevention, they underscore the importance of screening and treatment among women at risk for trichomoniasis.
T. vaginalis and Other STIs
Trichomonas vaginalis appears to have a similar bidirectional epidemiologic association with herpes simplex virus type 2 (HSV-2). Concomitant infection with T. vaginalis has been associated with HSV-2 shedding [42] and women with T. vaginalis have a higher incidence of HSV-2 [43]. Trichomonas vaginalis has been associated with the presence of other STIs including C. trachomatis, N. gonorrhoeae, and human papillomavirus (HPV) [44, 45].
T. vaginalis and Bacterial Vaginosis
Up to 40%–60% of women with T. vaginalis also have BV [46–48], and women with BV are at higher risk for acquiring T. vaginalis [49]. While vaginal dysbiosis has been associated with increased pathogenicity of T. vaginalis, it is not clear if BV interferes with T. vaginalis treatment. In randomized trials, BV was found to increase metronidazole treatment failure among women with HIV [47] but not among women without HIV [48]. This difference may be due to impaired immunity among women with HIV, altered pharmacokinetics and pharmacodynamics of metronidazole, or inadequate power in the studies conducted [50].
T. vaginalis and Neoplasia
Since the 2015 guidelines, a study of women in Tanzania found that women with T. vaginalis were 6.5 times more likely to have high-risk HPV, suggesting an indirect link between T. vaginalis and cervical neoplasia [45]. A meta-analysis also found that T. vaginalis was associated with a 1.9-fold risk of cervical neoplasia [51]. Studies of Finnish, Dutch, Belgian, and Chinese women have all found elevated odds (1.4–2.0) of cervical neoplasia among women who have T. vaginalis or vice versa [52–56]. The association between T. vaginalis and prostate cancer has been inconclusive to date, and additional data are needed [57, 58].
Diagnostics
Diagnostic testing for T. vaginalis should be performed in women seeking care for vaginal discharge. Annual screening can be considered for women receiving care in high-prevalence settings (eg, STD clinics and correctional facilities) and for asymptomatic persons at high risk for infection (eg, persons with multiple sexual partners, exchanging sex for payment, illicit drug use, or a history of STD or incarceration). However, data are lacking on whether screening and treatment for asymptomatic trichomoniasis in high-prevalence settings or persons at high risk can reduce adverse health events, health disparities, or community burden of infection. Population-based rates of T. vaginalis in men are low (0.5%) [3], though testing can be considered among men with urethritis [59]. Decisions about screening should be informed by local epidemiology of T. vaginalis infection.
Since the 2015 guidelines, the diagnosis of T. vaginalis is becoming more precise and additional tests, including point-of-care tests, have become available in the last decade. Wet mount microscopy has been the primary method to diagnose T. vaginalis in women, as it is inexpensive and can be performed in clinical settings as a point-of-care test. However, wet mount microscopy has low sensitivity (44%–68%) compared to NAATs [60–62], depending upon the expertise of the reader, and is not recommended for use in men [19]. The wet mount slide should be read within 10 minutes of collection, as sensitivity declines quickly over time, decreasing to 20% within 1 hour after collection [63, 64]. While T. vaginalis culture has better sensitivity than wet mount relative to polymerase chain reaction (66% vs 48%) [65], it is more expensive, more time consuming, and has poor sensitivity in men [66]. Longitudinal studies of T. vaginalis treatment also demonstrate that culture misses some infections. One study of women without HIV and 1 study of women with HIV found that that after single-dose metronidazole treatment, T. vaginalis infection was nondetectable for months via culture and then reappeared in the absence of reported sexual exposure [67, 68], underscoring the need for more sensitive testing. Trichomonas vaginalis can be found incidentally on liquid Pap test but is unreliable as a diagnostic test and should not be used for screening [69]. The BD Affirm VPIII (Becton Dickinson, Sparks, Maryland) is a synthetic oligonucleotide DNA probe test that has been approved by the US Food and Drug Administration (FDA) for use in vaginal fluids to detect T. vaginalis, Gardnerella, and Candida species. It has a sensitivity of 91%–100% and a specificity of 93%–96%, with test results available in <1 hour [70].
NAATs for T. vaginalis are the most sensitive tests and are moderately priced, but high-complexity assays. The Aptima T. vaginalis assay (Hologic Gen-Probe, San Diego, California) was FDA-cleared in 2011 for use with urine, endocervical and vaginal swabs, and endocervical specimens collected in the Hologic PreserveCyt solution (ThinPrep) from asymptomatic and symptomatic women. Sensitivity and specificity are both 95%–100% [65]. This assay has not been FDA-cleared for use in men and must be internally validated per Clinical Laboratory Improvement Amendments (CLIA) regulations prior to use. The BD Probe Tec TV Qx Amplified DNA Assay (Becton Dickinson, Franklin Lakes, New Jersey) is FDA-cleared for detection of T. vaginalis from vaginal (self-collected and clinician-collected) swabs, endocervical swabs, or urine specimens from women, with a sensitivity and specificity of 98.3% and 99.6%, respectively [71], compared to wet mount and culture. Similar to the Aptima T. vaginalis assay, this test is only FDA-cleared for use in women and must be internally validated per CLIA regulations prior to use in men [65]. Although it is feasible to perform these NAAT tests on the same specimen used for chlamydia and gonorrhea screening, testing for the latter 2 STIs is primarily recommended for sexually active women aged 24 years and younger. By contrast, trichomoniasis is more common in women >24 years, and this demographic should not be overlooked. The BD Max CTGCTV2 assay is also FDA-cleared for detection of T. vaginalis in patient or clinician-collected vaginal swab specimens (in a clinical setting) and male and female urine specimens, with sensitivity and specificity ranging from 81.1% to 100% and 98.7% to 100%, respectively, depending on the specimen type [72]. The Roche Cobas TV/MG test can be used on self-collected vaginal swab specimens (collected in a clinical setting), clinician-collected vaginal swab specimens, and endocervical specimens in women and urine and meatal swabs in men, with sensitivity of 77.2%–100% and specificity of 96.1%–99.9% (Table 1) [73, 74].
| Test [Reference] . | Sample Type . | Sensitivity/Specificity Compared to NAAT . | Complexity/Time . | Costa . |
|---|---|---|---|---|
| Culture [65, 66] | Women: Vaginal swab Men: Urethral or penile-meatal swabs or semen | Women: Sensitivity: 75%–96% Specificity: 100% Men: Sensitivity: 50%–80% Specificity: 100% | Incubator and microscope with ×40 objective. Results in 5–7 days. | $$ |
| Hologic Aptima T. vaginalis assay [65] | Women: Vaginal or endocervical swab or urine Men: Urethral or urine specimens (if test is internally validated) | Women: Sensitivity: 88%–100% Specificity: 98%–100% | Panther, Viper, or Tigris instrumentation needed. Results in <8 hours. | $$ |
| BD Probe Tec TV NAAT [71] | Women: Vaginal or endocervical swab or urine Men: Urethral or urine specimens (if test is internally validated) | Sensitivity: 98%–100% Specificity: 98%–100% | CLIA high complexity. Requires Viper system. Results available in <8 hours. | $$ |
| BD Max CT/GC/TV2 assay [72] | Women: Vaginal or endocervical swab or urine Men: Urine | Women: Sensitivity: 89.8%–99.7% Specificity: 98.1%–99.5% Men: Sensitivity: 81.1%–100% Specificity: 98.7%–100% | Requires BD MAX system equipment. | $$ |
| Roche Cobas TV/MG assay [73, 74] | Women: Vaginal or endocervical swab Men: Urine, meatal swab | Women: Sensitivity: 96.4%–100% Specificity: 96.5%– 98.8% Men: Sensitivity: 77.2%–100% Specificity: 97.2%–99.9% | For use on Cobas 6800/8800 systems. | $$ |
| BD Affirm VPIII [70] | Women: Vaginal specimen | Women: Sensitivity: 91%–100% Specificity: 93%–96% | Uses direct hybridization technology. | $$ |
| Test [Reference] . | Sample Type . | Sensitivity/Specificity Compared to NAAT . | Complexity/Time . | Costa . |
|---|---|---|---|---|
| Culture [65, 66] | Women: Vaginal swab Men: Urethral or penile-meatal swabs or semen | Women: Sensitivity: 75%–96% Specificity: 100% Men: Sensitivity: 50%–80% Specificity: 100% | Incubator and microscope with ×40 objective. Results in 5–7 days. | $$ |
| Hologic Aptima T. vaginalis assay [65] | Women: Vaginal or endocervical swab or urine Men: Urethral or urine specimens (if test is internally validated) | Women: Sensitivity: 88%–100% Specificity: 98%–100% | Panther, Viper, or Tigris instrumentation needed. Results in <8 hours. | $$ |
| BD Probe Tec TV NAAT [71] | Women: Vaginal or endocervical swab or urine Men: Urethral or urine specimens (if test is internally validated) | Sensitivity: 98%–100% Specificity: 98%–100% | CLIA high complexity. Requires Viper system. Results available in <8 hours. | $$ |
| BD Max CT/GC/TV2 assay [72] | Women: Vaginal or endocervical swab or urine Men: Urine | Women: Sensitivity: 89.8%–99.7% Specificity: 98.1%–99.5% Men: Sensitivity: 81.1%–100% Specificity: 98.7%–100% | Requires BD MAX system equipment. | $$ |
| Roche Cobas TV/MG assay [73, 74] | Women: Vaginal or endocervical swab Men: Urine, meatal swab | Women: Sensitivity: 96.4%–100% Specificity: 96.5%– 98.8% Men: Sensitivity: 77.2%–100% Specificity: 97.2%–99.9% | For use on Cobas 6800/8800 systems. | $$ |
| BD Affirm VPIII [70] | Women: Vaginal specimen | Women: Sensitivity: 91%–100% Specificity: 93%–96% | Uses direct hybridization technology. | $$ |
Abbreviations: CLIA, Clinical Laboratory Improvement Amendments; CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae; MG, Mycoplasma genitalium; NAAT, nucleic acid amplification test; TV, Trichomonas vaginalis.
$, lower cost; $$, midrange; $$$, expensive. Cost estimates may vary depending on availability of equipment and volume of testing.
| Test [Reference] . | Sample Type . | Sensitivity/Specificity Compared to NAAT . | Complexity/Time . | Costa . |
|---|---|---|---|---|
| Culture [65, 66] | Women: Vaginal swab Men: Urethral or penile-meatal swabs or semen | Women: Sensitivity: 75%–96% Specificity: 100% Men: Sensitivity: 50%–80% Specificity: 100% | Incubator and microscope with ×40 objective. Results in 5–7 days. | $$ |
| Hologic Aptima T. vaginalis assay [65] | Women: Vaginal or endocervical swab or urine Men: Urethral or urine specimens (if test is internally validated) | Women: Sensitivity: 88%–100% Specificity: 98%–100% | Panther, Viper, or Tigris instrumentation needed. Results in <8 hours. | $$ |
| BD Probe Tec TV NAAT [71] | Women: Vaginal or endocervical swab or urine Men: Urethral or urine specimens (if test is internally validated) | Sensitivity: 98%–100% Specificity: 98%–100% | CLIA high complexity. Requires Viper system. Results available in <8 hours. | $$ |
| BD Max CT/GC/TV2 assay [72] | Women: Vaginal or endocervical swab or urine Men: Urine | Women: Sensitivity: 89.8%–99.7% Specificity: 98.1%–99.5% Men: Sensitivity: 81.1%–100% Specificity: 98.7%–100% | Requires BD MAX system equipment. | $$ |
| Roche Cobas TV/MG assay [73, 74] | Women: Vaginal or endocervical swab Men: Urine, meatal swab | Women: Sensitivity: 96.4%–100% Specificity: 96.5%– 98.8% Men: Sensitivity: 77.2%–100% Specificity: 97.2%–99.9% | For use on Cobas 6800/8800 systems. | $$ |
| BD Affirm VPIII [70] | Women: Vaginal specimen | Women: Sensitivity: 91%–100% Specificity: 93%–96% | Uses direct hybridization technology. | $$ |
| Test [Reference] . | Sample Type . | Sensitivity/Specificity Compared to NAAT . | Complexity/Time . | Costa . |
|---|---|---|---|---|
| Culture [65, 66] | Women: Vaginal swab Men: Urethral or penile-meatal swabs or semen | Women: Sensitivity: 75%–96% Specificity: 100% Men: Sensitivity: 50%–80% Specificity: 100% | Incubator and microscope with ×40 objective. Results in 5–7 days. | $$ |
| Hologic Aptima T. vaginalis assay [65] | Women: Vaginal or endocervical swab or urine Men: Urethral or urine specimens (if test is internally validated) | Women: Sensitivity: 88%–100% Specificity: 98%–100% | Panther, Viper, or Tigris instrumentation needed. Results in <8 hours. | $$ |
| BD Probe Tec TV NAAT [71] | Women: Vaginal or endocervical swab or urine Men: Urethral or urine specimens (if test is internally validated) | Sensitivity: 98%–100% Specificity: 98%–100% | CLIA high complexity. Requires Viper system. Results available in <8 hours. | $$ |
| BD Max CT/GC/TV2 assay [72] | Women: Vaginal or endocervical swab or urine Men: Urine | Women: Sensitivity: 89.8%–99.7% Specificity: 98.1%–99.5% Men: Sensitivity: 81.1%–100% Specificity: 98.7%–100% | Requires BD MAX system equipment. | $$ |
| Roche Cobas TV/MG assay [73, 74] | Women: Vaginal or endocervical swab Men: Urine, meatal swab | Women: Sensitivity: 96.4%–100% Specificity: 96.5%– 98.8% Men: Sensitivity: 77.2%–100% Specificity: 97.2%–99.9% | For use on Cobas 6800/8800 systems. | $$ |
| BD Affirm VPIII [70] | Women: Vaginal specimen | Women: Sensitivity: 91%–100% Specificity: 93%–96% | Uses direct hybridization technology. | $$ |
Abbreviations: CLIA, Clinical Laboratory Improvement Amendments; CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae; MG, Mycoplasma genitalium; NAAT, nucleic acid amplification test; TV, Trichomonas vaginalis.
$, lower cost; $$, midrange; $$$, expensive. Cost estimates may vary depending on availability of equipment and volume of testing.
In addition to wet mount microscopy, additional point-of-care diagnostic tests for T. vaginalis that are FDA-cleared among women are the OSOM lateral flow test (Sekisui Diagnostics, Bedford, Massachusetts), the Isothermal Helicase-Dependent AmpliVue test (Quidel, San Diego, California), and the Solana TV assay (Quidel) [75]. The Cepheid GeneXpert TV assay (Cepheid, Sunnyvale, California) is a moderately complex rapid test that can be performed in <1 hour and has been FDA-cleared for use on female urine [76], endocervical swabs, and patient- and clinician-collected vaginal specimens as well as male urine specimens, with sensitivities and specificities ranging from 99.5% to 100% and 99.4% to 99.9%, compared to wet mount and culture, respectively. In women, these tests have sensitivities and specificities >97%; however, these tests tend to be more expensive than standard NAATs run on high-volume platforms [77]. OSOM should not be used with male urine specimens because of low sensitivity (37.5%) [78]. Solana has not been evaluated in men (Table 2 ).
| Test . | Sample Type . | Sensitivity/Specificity Compared to NAAT . | Complexity/Time . | Costa . |
|---|---|---|---|---|
| Wet mount microscopy [60–62] | Women: Vaginal swab | Women only: Sensitivity: 44%–68% Specificity: 100% | CLIA waived. Microscope with ×40 objective Results in 5 minutes. | $ |
| OSOM [75] | Women: Vaginal swab | Symptomatic women only: Sensitivity: 83%–92% Specificity: 99%–100% | CLIA waived. Detects antigen. No instrumentation needed. Results in 10 minutes. | $$ |
| Solana [75] | Women: Vaginal swab or urine | Asymptomatic or symptomatic women: Sensitivity: 90%–99% Specificity: 97%–99% | Not CLIA waived. Requires some instrumentation. Results in <1 hour. | $$ |
| Cepheid GeneXpert [76] | Women: Endocervical or vaginal swab and urine Men: Urine | In women: Sensitivity: 95%–100% Specificity: 98%–99% | CLIA waived. Requires instrumentation. Results in approximately 40 minutes. | $$$ |
| AmpliVue [75] | Women: Vaginal swab | Asymptomatic or symptomatic women: Sensitivity: 97%–100% Specificity: 97%–99% | Not CLIA waived. Requires some instrumentation. Results in approximately 45 minutes. | $$ |
| Test . | Sample Type . | Sensitivity/Specificity Compared to NAAT . | Complexity/Time . | Costa . |
|---|---|---|---|---|
| Wet mount microscopy [60–62] | Women: Vaginal swab | Women only: Sensitivity: 44%–68% Specificity: 100% | CLIA waived. Microscope with ×40 objective Results in 5 minutes. | $ |
| OSOM [75] | Women: Vaginal swab | Symptomatic women only: Sensitivity: 83%–92% Specificity: 99%–100% | CLIA waived. Detects antigen. No instrumentation needed. Results in 10 minutes. | $$ |
| Solana [75] | Women: Vaginal swab or urine | Asymptomatic or symptomatic women: Sensitivity: 90%–99% Specificity: 97%–99% | Not CLIA waived. Requires some instrumentation. Results in <1 hour. | $$ |
| Cepheid GeneXpert [76] | Women: Endocervical or vaginal swab and urine Men: Urine | In women: Sensitivity: 95%–100% Specificity: 98%–99% | CLIA waived. Requires instrumentation. Results in approximately 40 minutes. | $$$ |
| AmpliVue [75] | Women: Vaginal swab | Asymptomatic or symptomatic women: Sensitivity: 97%–100% Specificity: 97%–99% | Not CLIA waived. Requires some instrumentation. Results in approximately 45 minutes. | $$ |
Abbreviations: CLIA, Clinical Laboratory Improvement Amendments; NAAT, nucleic acid amplification test.
$, lower cost; $$, midrange; $$$, expensive. Cost estimates may vary depending on availability of equipment and volume of testing.
| Test . | Sample Type . | Sensitivity/Specificity Compared to NAAT . | Complexity/Time . | Costa . |
|---|---|---|---|---|
| Wet mount microscopy [60–62] | Women: Vaginal swab | Women only: Sensitivity: 44%–68% Specificity: 100% | CLIA waived. Microscope with ×40 objective Results in 5 minutes. | $ |
| OSOM [75] | Women: Vaginal swab | Symptomatic women only: Sensitivity: 83%–92% Specificity: 99%–100% | CLIA waived. Detects antigen. No instrumentation needed. Results in 10 minutes. | $$ |
| Solana [75] | Women: Vaginal swab or urine | Asymptomatic or symptomatic women: Sensitivity: 90%–99% Specificity: 97%–99% | Not CLIA waived. Requires some instrumentation. Results in <1 hour. | $$ |
| Cepheid GeneXpert [76] | Women: Endocervical or vaginal swab and urine Men: Urine | In women: Sensitivity: 95%–100% Specificity: 98%–99% | CLIA waived. Requires instrumentation. Results in approximately 40 minutes. | $$$ |
| AmpliVue [75] | Women: Vaginal swab | Asymptomatic or symptomatic women: Sensitivity: 97%–100% Specificity: 97%–99% | Not CLIA waived. Requires some instrumentation. Results in approximately 45 minutes. | $$ |
| Test . | Sample Type . | Sensitivity/Specificity Compared to NAAT . | Complexity/Time . | Costa . |
|---|---|---|---|---|
| Wet mount microscopy [60–62] | Women: Vaginal swab | Women only: Sensitivity: 44%–68% Specificity: 100% | CLIA waived. Microscope with ×40 objective Results in 5 minutes. | $ |
| OSOM [75] | Women: Vaginal swab | Symptomatic women only: Sensitivity: 83%–92% Specificity: 99%–100% | CLIA waived. Detects antigen. No instrumentation needed. Results in 10 minutes. | $$ |
| Solana [75] | Women: Vaginal swab or urine | Asymptomatic or symptomatic women: Sensitivity: 90%–99% Specificity: 97%–99% | Not CLIA waived. Requires some instrumentation. Results in <1 hour. | $$ |
| Cepheid GeneXpert [76] | Women: Endocervical or vaginal swab and urine Men: Urine | In women: Sensitivity: 95%–100% Specificity: 98%–99% | CLIA waived. Requires instrumentation. Results in approximately 40 minutes. | $$$ |
| AmpliVue [75] | Women: Vaginal swab | Asymptomatic or symptomatic women: Sensitivity: 97%–100% Specificity: 97%–99% | Not CLIA waived. Requires some instrumentation. Results in approximately 45 minutes. | $$ |
Abbreviations: CLIA, Clinical Laboratory Improvement Amendments; NAAT, nucleic acid amplification test.
$, lower cost; $$, midrange; $$$, expensive. Cost estimates may vary depending on availability of equipment and volume of testing.
Treatment of T. vaginalis Infection
Previously, the CDC and the World Health Organization recommended single-dose (2 g) oral metronidazole as the preferred treatment for T. vaginalis infection, with metronidazole 400–500 mg orally twice daily for 7 days, or oral tinidazole (2 g) as alternative therapies [79]. Both medications belong to the 5-nitroimidazole drug class. In the 2015 guidelines, the preferred line of treatment was changed to the 7-day oral metronidazole dose for women with HIV infection in response to a multicenter randomized clinical trial (RCT) demonstrating superiority of 7-day over single-dose metronidazole [80], with 2 g tinidazole as an alternative. It was not changed for HIV-uninfected woman because the evidence was only among HIV-infected women. Since the 2015 guidelines, a meta-analysis [81] and multicenter RCT [82] found similar results in women without HIV infection. In these studies, repeat infections were half as likely in women taking the 7-day regimen compared to the 2-g dose. Thus, the 7-day oral metronidazole regimen is now the preferred treatment regimen for all women, with 2 g tinidazole as the alternative. The 2-g metronidazole regimen is no longer recommended in women. Given the lack of a comparable clinical trial in men, 2 g of metronidazole will remain the recommended therapy for men with or exposed to T. vaginalis with the 2-g tinidazole regimen as an alternate, until additional studies are conducted.
Oral secnidazole, a next-generation 5-nitroimidazole with a longer half-life (~17 hours) than metronidazole (~7–8 hours) or tinidazole (~12–13 hours) [83], currently FDA-approved for the treatment of BV, is an emerging treatment option for trichomoniasis in women [84, 85]. A phase 3 multicenter, randomized, placebo-controlled, delayed-treatment, double-blind study to evaluate the effectiveness and safety of a single 2-g oral dose of secnidazole for trichomoniasis found it to be superior to placebo [86]. If approved by the FDA, it will be the only oral single-dose medication for both BV and T. vaginalis.
Treatment for Pregnant and Lactating Women
Metronidazole is a class B drug and several meta-analyses have found it to be safe in pregnant women in all stages of pregnancy [87, 88]. Tinidazole has not been evaluated in pregnant women and remains a class C drug.
In lactating women who are administered metronidazole, interrupting breastfeeding during treatment and for 12–24 hours after the last dose will reduce the exposure of the infant to metronidazole. For lactating women treated with tinidazole, interruption of breastfeeding is recommended during treatment and for 3 days after the last dose [89].
Approach to Determining if Infection Posttreatment Is a Repeat or Persistent Infection
Repeat infections with T. vaginalis are common, ranging from 5% to 37% [90–96], and share similar sequelae to primary infections. Possible sources of retest positives after treatment include treatment failure, reinfection from an untreated/infected baseline partner, or infection from a new partner. Whether treatment failure or reinfection, each of these sources of retest positives requires a different approach to prevent ongoing infection (Figure 2). For example, if the cause is reinfection, then assuring the original partners are treated (ie, expedited partner treatment) is needed. In instances of treatment failure, additional testing is needed, followed by appropriate therapy.
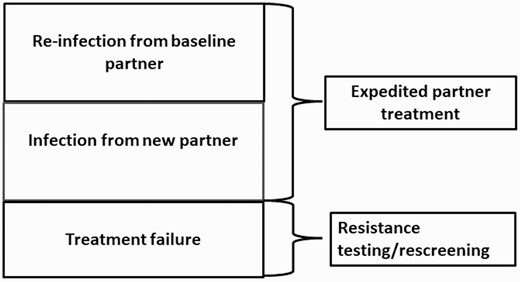
Approaches to repeat Trichomonas vaginalis infections posttreatment.
In vitro resistance testing among T. vaginalis isolates in the US has consistently demonstrated low rates of resistance to metronidazole. Reported rates of metronidazole resistance among mostly women without HIV range from 2.2% to 9.6% [91, 97–99] and were usually resolved with repeat metronidazole treatment at the same or higher dosage [99]. The majority of recurrent infections likely result from reinfection. When using a single 2-g metronidazole dose, treatment failure was the most likely cause of retesting positive in women [90]; treatment failures rates may diminish as the 7-day metronidazole regimen becomes more widely used.
Rescreening
Recommendations that all persons with T. vaginalis should be retested 3 months after treatment because of high repeat infection rates remain the same. However, the earliest possible retest time has changed. When using NAATs for T. vaginalis, 2 prior studies have shown that retesting too soon after diagnosis could lead to false-positive results [7, 100]. There was an additional study since 2015 that confirmed that the optimal time for retesting for T. vaginalis using NAATs is 3 weeks after completion of treatment [101].
Treatment for Persons With 5-Nitroimidazole Allergies or Persistent T. vaginalis
The most common reactions reported from metronidazole are urticaria and facial edema, while other adverse reactions include flushing, fever, and anaphylactic shock from immediate-type immunoglobulin E–mediated hypersensitivity [102]. Referral to an allergist for desensitization should be made in these instances. In situations where desensitization cannot be performed or tolerated, treatment with alternative agents outside of the 5-nitroimidazole class should be considered. There are several regimens (eg, intravaginal boric acid 600 mg twice daily for 60 days, intravaginal paromomycin 6.25% cream nightly for 14 days) [103–105], which have occasionally been successful in treatment in women; however, they may not reach all sites infected with T. vaginalis (ie, Bartholin or Skene glands). In addition, they must be made at a compounding pharmacy, are more costly, and may be associated with adverse effects (ie, vaginal ulceration with use of paromomycin cream; this ulceration can spontaneously regress when therapy is stopped) [105]. Consultation with an infectious disease expert may be useful in these situations.
If the woman has persistent T. vaginalis infection after treatment with the 7-day multidose metronidazole regimen and reinfection from an untreated sexual partner or medication noncompliance have been ruled out, the isolate should be sent to the CDC for 5-nitroimidazole susceptibility testing. (A kit may be requested from the CDC; see https://www.cdc.gov/laboratory/specimen-submission/detail.html?CDCTestCode=CDC-10239.) While waiting on these test results, treatment with 2 g metronidazole or tinidazole daily for 7 days should be given. If the patient fails this 7-day regimen of high-dose oral metronidazole or tinidazole, 2 additional treatment options have been found to be successful: (1) high-dose oral tinidazole 2–3 g daily in divided doses plus intravaginal tinidazole 500 mg twice daily, both for 14 days [106]; or (2) high-dose oral tinidazole 2–3 g daily in divided doses in combination with paromomycin 4 g of 6.25% intravaginal cream nightly, both for 14 days [107]. It is important to note that single-dose 5-nitroimidazole therapy should be avoided in the setting of persistent T. vaginalis infection [108]. Other intravaginal treatments have been studied or are under investigation for resistant T. vaginalis infection including acetarsol [109], boric acid [110], and furazolidone [110], but these are not commonly used at the present time. Nitazoxanide has been examined as an alternative oral agent for metronidazole-resistant T. vaginalis but was not found to be effective [111, 112]. Some plant extracts have shown anti–T. vaginalis activity, but these have not yet been tested in clinical trials [113].
If a man is still infected with T. vaginalis after a single dose of 2 g oral metronidazole and has been reexposed to an untreated partner, he should be redosed with another single dose of 2 g metronidazole. If he has not been reexposed, he should be given a course of metronidazole 500 mg twice daily for 7 days. There are currently no data regarding optimal treatment of male sexual partners of women with metronidazole-resistant trichomoniasis.
Treatment for Women With HIV Infection
In a randomized trial among women with HIV infection with T. vaginalis, 7-day metronidazole was superior to single-dose treatment [114]. Further analysis revealed that the superiority is only in the presence of BV [47]. It is possible that some of the organisms associated with BV could be serving as “sponge organisms” of metronidazole [50]. Studies have also found that protease inhibitors for the treatment of HIV may interfere with the efficacy of metronidazole among women with HIV infection [46, 115].
Given the high rates of T. vaginalis in women with HIV infection and that treatment can reduce vaginal viral shedding of HIV [29], the CDC recommends T. vaginalis screening among asymptomatic women with HIV. It has been estimated that if the CDC recommendation for T. vaginalis screening and treatment among women with HIV is followed, the lifetime cost of new HIV infections averted would approximate US$159264000 via prevention of new HIV cases secondary to female-to-male transmissions [116].
Partner Management
Sex partners of patients with T. vaginalis should be treated. Commonly, patients are told by their providers to tell their partners to seek testing and treatment. Providers should consider treating partners of positive patients presumptively. Expedited partner therapy might have a role in partner management; however, no partner management intervention has been demonstrated to be superior in reducing reinfection rates [1].
One randomized clinical trial demonstrated that partner treatment with 2 g tinidazole resulted in a >4-fold reduction in repeat infections among women with T. vaginalis [117]. Two other studies using 2 g metronidazole for male partners of women with T. vaginalis found no effect of expedited partner treatment [92] or a borderline effect [118]. While it is possible that the 2 studies that used metronidazole were either underpowered or did not use the correct controls, it is also possible that tinidazole is a better treatment for men [119, 120]. More treatment studies in men are needed.
Antimicrobial Resistance
Currently approved drugs are from the 5-nitroimidazole class (ie, metronidazole and tinidazole). In vitro resistance rates can range from 4.3% to 10%, and no new resistance studies were found in this review [121]. In vitro resistance may not exactly correlate with clinical treatment failure [97], especially in pregnant women [122], but use of recommended alternative treatment regimens following in vitro drug resistance testing results in >80% cure of resistant infections, suggesting that there is benefit to in vitro resistance testing [123].
CONCLUSIONS
Trichomonas vaginalis is highly prevalent, causes important reproductive morbidity among women, and contributes to HIV transmission. There is mounting evidence that T. vaginalis can cause poor obstetrical outcomes. Treatment recommendations have changed. Recent randomized clinical trials have shown that a single dose of 2 g oral metronidazole, preferred for many decades, is not as effective as the 7-day twice-daily dose in women and should not be used in women. There has been a dramatic increase in diagnostic tests, including point-of-care tests that are highly sensitive and specific for the detection of T. vaginalis, primarily in women but also some tests for men. The significance of T. vaginalis in asymptomatic women and in men needs more research.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) or the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the NIAID/NIH (1R01AI097080-01A1 to P. J. K. and C. A. M.). C. A. G. was supported by the National Institute of Biomedical Imaging and Bioengineering (U54007958) and NIAID (U01068613).
Supplement sponsorship. This supplement is sponsored by The Centers for Disease Control and Prevention.
Potential conflicts of interest. C. A. M. is a consultant for Lupin Pharmaceuticals, Abbott Molecular, and BioFire Diagnostics; has received research funding support from Lupin Pharmaceuticals, Gilead Sciences, Abbott Molecular, and NIH/NIAID; and has received honoraria from Lupin Pharmaceuticals, Cepheid, Roche Diagnostics, Abbott Molecular, Becton Dickinson, DynaMed, PhagoMed, and Jesperson & Associates. A. C. S. is a consultant for Hologic and has received royalties from Hologic and UpToDate. R. S. M. receives research funding, paid to the University of Washington, from Hologic, and has received honoraria for consulting from Lupin Pharmaceuticals. C. A. G. has received research funding from Cepheid, Abbott Molecular, Hologic, SpeeDX, Quidel, and Becton Dickinson. P. J. K. has received travel fees from the CDC. All other authors report no potential conflicts of interest..
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- hiv
- epidemiology
- trichomoniasis
- centers for disease control and prevention (u.s.)
- diagnostic techniques and procedures
- metronidazole
- nitroimidazoles
- sexual partners
- sexually transmitted diseases
- trichomonas vaginalis
- infections
- diagnosis
- urine
- genital system
- nucleic acid amplification tests
- treatment guidelines
- single-dose regimen
- rescreening
- persistent infection




