-
PDF
- Split View
-
Views
-
Cite
Cite
Grace E Marx, Brad J Biggerstaff, Courtney C Nawrocki, Sarah E Totten, Emily A Travanty, Alexis W Burakoff, Tracy Scott, Jesse Chavez-Van De Hey, Jesse J Carlson, Karen A Wendel, Jennifer L Harcourt, Azaibi Tamin, Jennifer D Thomas, Sarah E Rowan, Colorado Department of Public Health and Environment COVID-19 Laboratory Response Team , Centers for Disease Control and Prevention COVID-19 Laboratory Response Team , Detection of Severe Acute Respiratory Syndrome Coronavirus 2 on Self-Collected Saliva or Anterior Nasal Specimens Compared With Healthcare Personnel–Collected Nasopharyngeal Specimens, Clinical Infectious Diseases, Volume 73, Issue Supplement_1, 15 July 2021, Pages S65–S73, https://doi.org/10.1093/cid/ciab330
Close - Share Icon Share
Abstract
Nasopharyngeal specimens (NPS) are commonly used for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing but can be uncomfortable for patients. Self-collected saliva specimens (SS) or anterior nasal specimens (ANS) for SARS-CoV-2 detection are less invasive, but the sensitivity of these specimen types has not been thoroughly evaluated.
During September–November 2020, 730 adults undergoing SARS-CoV-2 testing at community testing events and homeless shelters in Denver provided self-collected SS and ANS before NPS collection and answered a short survey about symptoms and specimen preference. Specimens were tested for SARS-CoV-2 by means of real-time reverse-transcription polymerase chain reaction (rRT-PCR); viral culture was performed on a subset of specimens positive by rRT-PCR. The sensitivity of SS and ANS for SARS-CoV-2 detection by rRT-PCR was measured against that of NPS. Subgroup analyses included test outcomes by symptom status and culture results.
Sensitivity for SARS-CoV-2 detection by rRT-PCR appeared higher for SS than for ANS (85% vs 80%) and higher among symptomatic participants than among those without symptoms (94% vs 29% for SS; 87% vs 50% for ANS). Among participants with culture-positive SARS-CoV-2 by any specimen type, the sensitivities of SS and ANS by rRT-PCR were 94% and 100%, respectively. SS and ANS were equally preferred by participants; most would undergo NPS collection again despite this method’s being the least preferred.
SS were slightly more sensitive than ANS for SARS-CoV-2 detection with rRT-PCR. With both SS and ANS, SARS-CoV-2 was reliably detected among participants with symptoms. Self-collected SS and ANS offer practical advantages, are preferred by patients, and might be most useful for testing people with coronavirus disease 2019 symptoms.
Testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), was first authorized to be performed on multiple specimen types as part of the Centers for Disease Control and Prevention (CDC) 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel [1], with nasopharyngeal specimens (NPS) the most widely used specimen type [2]. NPS collection can be uncomfortable for the patient and should be performed by a trained healthcare professional wearing appropriate personal protective equipment (PPE) [3], as the procedure can generate infectious aerosols from sneezing and coughing caused by irritation of the nasopharynx [4]. Self-collected anterior nasal specimens (ANS) or saliva specimens (SS) for SARS-CoV-2 detection could decrease patient discomfort during specimen collection, decrease the need for trained healthcare professionals to collect specimens, conserve PPE, reduce testing-associated transmission risk, and improve testing uptake [4–6].
Prior studies have suggested that real-time reverse-transcription polymerase chain reaction (rRT-PCR) testing of ANS and SS can reliably detect SARS-CoV-2 in patients with COVID-19 [7–9], although these specimen types have not been systematically evaluated as screening tools in high-volume testing events or in congregate settings such as homeless shelters. To evaluate whether testing of self-collected ANS or SS might accurately and reliably detect SARS-CoV-2 in real-life settings, participants were enrolled during testing events in communities in Denver, Colorado, that were disproportionately affected by the COVID-19 pandemic. Participants answered questions about symptoms and specimen preference after providing ANS, SS, and NPS. Test performances for SARS-CoV-2 detection by rRT-PCR for self-collected ANS and SS were compared with that of standard healthcare personnel–obtained NPS. To understand the relationship between test sensitivity by specimen type and presence of culturable virus, a subset of paired NPS and ANS from participants who tested positive for SARS-CoV-2 by rRT-PCR was sent for viral culture. This multilayered evaluation was designed to inform programmatic decisions about whether the less invasive of SS and ANS might be suitable alternatives to the conventional NPS for testing and screening programs in community and congregate living settings.
METHODS
Project Design, Setting, and Population
We performed a cross-sectional evaluation of adults seeking SARS-CoV-2 testing at testing events held by Denver Public Health in Denver, Colorado, during September–November 2020. Testing events took place at walk-up or drive-up sites in communities disproportionately affected by the pandemic and at shelters for people experiencing homelessness. We enrolled both symptomatic and asymptomatic participants. Participant inclusion criteria were (1) attending a SARS-CoV-2 testing event; (2) age ≥18 years at date of testing; (3) ability and willingness to provide informed consent; and (4) willingness to comply with study procedures. We excluded participants who reported receiving a previous positive SARS-CoV-2 test result. This project was determined not to be human subject research by the Colorado Multiple Institutional Review Board and by the Colorado Department of Public Health and Environment (CDPHE) Institutional Review Board as a public health surveillance activity. This activity was reviewed by the CDC and was conducted consistent with applicable federal law and CDC policy. All participants provided verbal informed consent before enrollment.
Specimen Collection and Transport
Trained healthcare personnel observed and coached participants to self-collect an ANS (inserting a polyester foam swab 1.0–1.5 cm and rotating it for 10–15 seconds in each nostril) and to provide an SS (1–5 mL) by spitting several times into a sterile container [3]. Healthcare personnel then collected NPS (using a minitip flocked polyester swab inserted through 1 naris to the nasopharynx and rotated for 5 seconds) [3]. ANS were immediately placed into a sterile transport tube containing 2–3 mL of sterile saline; NPS were immediately placed into a sterile transport tube containing 2–3 mL of viral transport medium (AccuViral Collection Kit). All specimens were placed immediately in a cooler with ice packs. On the same day of collection, after each testing event, coolers were transported to the CDPHE laboratory and moved to 4°C refrigerators until processing within 72 hours of collection [3].
Participant Survey
After specimen collection, trained interviewers asked structured survey questions from participants in English or Spanish. Questions included demographic characteristics, detailed assessment for COVID-19 symptoms, whether participants had had close contact with a known COVID-19 case in the past 2 weeks, which specimen type they preferred, and whether they would agree to be tested again by specimen type (Supplementary Figure 1). COVID-19 symptoms in this investigation were defined as reports of new or worsening fever (measured or subjective) or chills, cough, shortness of breath or difficulty breathing, fatigue, myalgia, headache, anosmia or ageusia, sore throat, congestion or nasal discharge, nausea or vomiting, or diarrhea [10]. Data were entered into Research Electronic Data Capture (REDCap) software (Vanderbilt).
Testing for SARS-CoV-2 by rRT-PCR
All specimens were processed and tested for SARS-CoV-2 at the CDPHE laboratory using the protocol for the CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel [1]. Nucleic acid extraction was performed according to the instructions for use authorized by the US Food and Drug Administration for the assay. SS were vortexed for 1 minute and then incubated at room temperature for 30 seconds before removal of the slightly separated supernatant from a viscous bottom layer; nucleic acid extraction was performed on the supernatant. Results were considered positive for SARS-CoV-2 when cycle threshold (Ct) values for the viral nucleocapsid protein genes N1 and N2 were <40.00 [11]. Ct values from rRT-PCR tests are inversely correlated with the amount of viral genetic material present in the specimen [12].
Testing for SARS-CoV-2 by Viral Culture
A subset of paired ANS and NPS from participants who tested positive for SARS-CoV-2 by rRT-PCR with ≥1 specimen type were placed in cryovial boxes on ice packs and shipped to the CDC for viral culture. Given limited viral culture testing capacity (maximum, 100 specimens) and because of the practical challenges of processing SS for culture [13], only ANS and NPS were submitted for viral culture. Specimens prioritized for this subset included (1) specimens from participants with discrepant results by rRT-PCR (eg, a participant who tested positive by NPS but negative by ANS or SS, or a participant who tested negative by NPS but positive by ANS or SS) and (2) specimens with the lowest Ct values. These criteria were systematically applied until the allowed quantity of 100 specimens was identified. Viral culture was performed using Vero-CCL-81 cells, as described elsewhere [14]. rRT-PCR was performed on specimens in which a cytopathic effect developed to confirm isolation of infectious virus in culture. Recovery of infectious virus was confirmed if the Ct of the recovered isolate was ≥2 Cts lower than that of the clinical specimen [15].
Statistical Analyses
Analyses were performed to evaluate and compare test performance of self-collected ANS and SS with that of NPS. Sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were computed for ANS and SS, using NPS as the reference standard; corresponding 95% score confidence intervals (CIs) were calculated for each measure. The sensitivity and 95% CI of ANS or SS for detection of SARS-CoV-2 by rRT-PCR were also computed, using culturable virus on any specimen as the reference standard. Not considering NPS as the reference standard, Cohen kappa statistics (with 95% bootstrap CIs) were computed as measures of agreement between ANS and NPS, and between SS and NPS. Pearson correlations and 95% CIs were calculated between rRT-PCR genetic targets N1 and N2 on ANS and NPS. Receiver operating characteristic analysis of rRT-PCR Ct values was used to identify the cutoff Ct value that yielded maximum equal sensitivity and specificity. All statistical analyses were conducted using R software (version 4.0.2).
RESULTS
Of 730 total participants enrolled, 452 (61.9%) were enrolled at 10 community testing events and 278 (38.0%) at 7 homeless shelter testing events. Age, sex, and race and ethnicity distributions differed between participants enrolled at community sites and those enrolled at homeless shelters (Table 1). Participants enrolled at community sites tended to be younger than those enrolled at homeless shelters (54.2% vs 30.6%, respectively, aged ≤40 years) and were more often female (57.3% vs 16.2%). Hispanic ethnicity was more commonly reported by participants at community sites (44.0% vs 20.9% for homeless shelters), and white, non-Hispanic race/ethnicity was more commonly reported by those at homeless shelters (51.8% vs 39.2% for community sites). Overall, 36.8% of participants reported ≥1 symptom consistent with COVID-19 [10] at the time of testing, with a much higher proportion of symptomatic participants enrolled at community sites (47.8%) than at homeless shelters (19.1%). Close contact with a person with confirmed COVID-19 was reported by 33.0% of participants at community sites, compared with 3.6% of participants at homeless shelters. In total, 84 participants (11.5%) were positive for SARS-CoV-2 by rRT-PCR for ≥1 specimen type; 82 (97.6%) were enrolled at community sites.
Self-Reported Demographic, Clinical, and Epidemiologic Characteristics, Specimen Preference, and Overall Severe Acute Respiratory Syndrome Coronavirus 2 Positivity With Real-Time Reverse-Transcription Polymerase Chain Reaction by Testing Site
| Participant Characteristic . | Participants by Testing Site, No. (%) . | . | . | ||
|---|---|---|---|---|---|
| . | All Sites (N = 730) . | Community Sites (n = 452) . | Homeless Service Sites (n = 278 ) . | Difference of Proportionsa (95% CI) . | P Value . |
| Age group, y | |||||
| 18–40 | 330 (45.2) | 245 (54.2) | 85 (30.6) | 0.24 (.16–.31) | <.001 |
| 41–65 | 334 (45.8) | 167 (36.9) | 167 (60.1) | −0.23 (−.30 to −.16) | |
| >65 | 66 (9.0) | 40 (8.8) | 26 (9.4) | −0.01 (−.05 to .04) | |
| Sex | |||||
| Male | 420 (57.5) | 190 (42.0) | 230 (82.7) | −0.41 (−.47 to −.34) | <.001 |
| Female | 304 (41.6) | 259 (57.3) | 45 (16.2) | 0.41 (.35–.47) | |
| Other | 5 (0.7) | 3 (0.7) | 2 (0.7) | −0.0006 (−.02 to .01) | |
| Race/ethnicity | |||||
| Black, non-Hispanic | 85 (11.6) | 44 (9.7) | 41 (14.7) | −0.05 (−.103 to −.002) | <.001 |
| White, non-Hispanic | 321 (44.0) | 177 (39.2) | 144 (51.8) | 0.13 (−.20 to −.05) | |
| Asian, non-Hispanic | 17 (2.3) | 12 (2.7) | 5 (1.8) | 0.01 (−.02 to .03) | |
| Hispanic | 257 (35.2) | 199 (44.0) | 58 (20.9) | 0.23 (.16–.30) | |
| Native American/Alaska Native, non-Hispanic | 14 (1.9) | 4 (0.9) | 10 (3.6) | −0.03 (−.06 to −.01) | |
| Other, non-Hispanic | 33 (4.5) | 15 (3.3) | 18 (6.5) | -0.03 (−.07 to −.0005) | |
| New or worsening symptoms reported at testinga | |||||
| Any symptom | 269 (36.8) | 216 (47.8) | 53 (19.1) | 0.29 (.22–.35) | <.001 |
| Cough, shortness of breath or difficulty breathing, anosmia, or ageusia | 168 (23.0) | 139 (30.8) | 29 (10.4) | 0.20 (.15–.26) | <.001 |
| ≥2 Symptoms, including fever (measured or subjective) or chills, rigors, myalgia, headache, sore throat, nausea or vomiting, diarrhea, fatigue, and congestion or nasal discharge | 187 (25.6) | 172 (38.1) | 15 (5.4) | 0.33 (.27–.38) | <.001 |
| Flulike symptoms (fever plus either cough or sore throat) | 66 (9.0) | 62 (13.7) | 4 (1.4) | 0.12 (.09–.16) | <.001 |
| Fever (measured or subjective) or chills | 90 (12.3) | 84 (18.6) | 6 (2.2) | 0.16 (.12–.21) | <.001 |
| Cough | 79 (10.8) | 61 (13.5) | 18 (6.5) | 0.07 (.03–.11) | .004 |
| Shortness of breath or difficulty breathing | 86 (11.8) | 69 (15.3) | 17 (6.1) | 0.09 (.05–.13) | <.001 |
| Fatigue | 87 (11.9) | 76 (16.8) | 11 (4.0) | 0.13 (.09–.17) | <.001 |
| Myalgia | 108 (14.8) | 97 (21.5) | 11 (4.0) | 0.18 (.13–.22) | <.001 |
| Headache | 66 (9.0) | 54 (11.9) | 12 (4.3) | 0.08 (.04–.11) | .001 |
| Anosmia or ageusia | 76 (10.4) | 73 (16.2) | 3 (1.1) | 0.15 (.12–.19) | <.001 |
| Sore throat | 116 (15.9) | 109 (24.1) | 7 (2.5) | 0.22 (.39–.49) | <.001 |
| Congestion or nasal discharge | 66 (9.0) | 42 (9.3) | 24 (8.6) | 0.01 (−.04 to .05) | .87 |
| Nausea or vomiting | 30 (4.1) | 21 (4.6) | 9 (3.2) | 0.01 (−.02 to .04) | .46 |
| Diarrhea | 165 (22.6) | 156 (34.5) | 9 (3.2) | 0.31 (.26–.36) | <.001 |
| Close contact with known COVID-19 case within prior 2 wk | 159 (21.8) | 149 (33.0) | 10 (3.6) | 0.29 (.24–.34) | <.001 |
| Preferred specimen type | |||||
| Self-collected ANS | 272 (37.3) | 174 (38.5) | 98 (35.3) | 0.03 (−.04 to .10) | .67 |
| Self-collected SS | 335 (45.9) | 205 (45.4) | 130 (46.8) | −0.01 (−.09 to .06) | |
| Healthcare provider–collected NPS | 98 (13.4) | 64 (14.2) | 34 (12.2) | 0.02 (−.03 to .07) | |
| Willingness to be tested again by specimen type, if it were the only type being collected | |||||
| Self-collected ANS | 704 (96.4) | 445 (98.5) | 259 (93.2) | 0.05 (.02–.09) | <.001 |
| Self-collected SS | 707 (96.8) | 444 (98.2) | 263 (94.6) | 0.04 (.01–.07) | .07 |
| Healthcare provider–collected NPS | 641 (87.8) | 403 (89.2) | 238 (85.6) | 0.04 (−.01 to .09) | .44 |
| Positive for SARS-COV-2 by ≥1 specimen typeb | 84 (11.5) | 82 (18.1) | 2 (0.7) | 0.17 (.14–.21) | <.001 |
| Participant Characteristic . | Participants by Testing Site, No. (%) . | . | . | ||
|---|---|---|---|---|---|
| . | All Sites (N = 730) . | Community Sites (n = 452) . | Homeless Service Sites (n = 278 ) . | Difference of Proportionsa (95% CI) . | P Value . |
| Age group, y | |||||
| 18–40 | 330 (45.2) | 245 (54.2) | 85 (30.6) | 0.24 (.16–.31) | <.001 |
| 41–65 | 334 (45.8) | 167 (36.9) | 167 (60.1) | −0.23 (−.30 to −.16) | |
| >65 | 66 (9.0) | 40 (8.8) | 26 (9.4) | −0.01 (−.05 to .04) | |
| Sex | |||||
| Male | 420 (57.5) | 190 (42.0) | 230 (82.7) | −0.41 (−.47 to −.34) | <.001 |
| Female | 304 (41.6) | 259 (57.3) | 45 (16.2) | 0.41 (.35–.47) | |
| Other | 5 (0.7) | 3 (0.7) | 2 (0.7) | −0.0006 (−.02 to .01) | |
| Race/ethnicity | |||||
| Black, non-Hispanic | 85 (11.6) | 44 (9.7) | 41 (14.7) | −0.05 (−.103 to −.002) | <.001 |
| White, non-Hispanic | 321 (44.0) | 177 (39.2) | 144 (51.8) | 0.13 (−.20 to −.05) | |
| Asian, non-Hispanic | 17 (2.3) | 12 (2.7) | 5 (1.8) | 0.01 (−.02 to .03) | |
| Hispanic | 257 (35.2) | 199 (44.0) | 58 (20.9) | 0.23 (.16–.30) | |
| Native American/Alaska Native, non-Hispanic | 14 (1.9) | 4 (0.9) | 10 (3.6) | −0.03 (−.06 to −.01) | |
| Other, non-Hispanic | 33 (4.5) | 15 (3.3) | 18 (6.5) | -0.03 (−.07 to −.0005) | |
| New or worsening symptoms reported at testinga | |||||
| Any symptom | 269 (36.8) | 216 (47.8) | 53 (19.1) | 0.29 (.22–.35) | <.001 |
| Cough, shortness of breath or difficulty breathing, anosmia, or ageusia | 168 (23.0) | 139 (30.8) | 29 (10.4) | 0.20 (.15–.26) | <.001 |
| ≥2 Symptoms, including fever (measured or subjective) or chills, rigors, myalgia, headache, sore throat, nausea or vomiting, diarrhea, fatigue, and congestion or nasal discharge | 187 (25.6) | 172 (38.1) | 15 (5.4) | 0.33 (.27–.38) | <.001 |
| Flulike symptoms (fever plus either cough or sore throat) | 66 (9.0) | 62 (13.7) | 4 (1.4) | 0.12 (.09–.16) | <.001 |
| Fever (measured or subjective) or chills | 90 (12.3) | 84 (18.6) | 6 (2.2) | 0.16 (.12–.21) | <.001 |
| Cough | 79 (10.8) | 61 (13.5) | 18 (6.5) | 0.07 (.03–.11) | .004 |
| Shortness of breath or difficulty breathing | 86 (11.8) | 69 (15.3) | 17 (6.1) | 0.09 (.05–.13) | <.001 |
| Fatigue | 87 (11.9) | 76 (16.8) | 11 (4.0) | 0.13 (.09–.17) | <.001 |
| Myalgia | 108 (14.8) | 97 (21.5) | 11 (4.0) | 0.18 (.13–.22) | <.001 |
| Headache | 66 (9.0) | 54 (11.9) | 12 (4.3) | 0.08 (.04–.11) | .001 |
| Anosmia or ageusia | 76 (10.4) | 73 (16.2) | 3 (1.1) | 0.15 (.12–.19) | <.001 |
| Sore throat | 116 (15.9) | 109 (24.1) | 7 (2.5) | 0.22 (.39–.49) | <.001 |
| Congestion or nasal discharge | 66 (9.0) | 42 (9.3) | 24 (8.6) | 0.01 (−.04 to .05) | .87 |
| Nausea or vomiting | 30 (4.1) | 21 (4.6) | 9 (3.2) | 0.01 (−.02 to .04) | .46 |
| Diarrhea | 165 (22.6) | 156 (34.5) | 9 (3.2) | 0.31 (.26–.36) | <.001 |
| Close contact with known COVID-19 case within prior 2 wk | 159 (21.8) | 149 (33.0) | 10 (3.6) | 0.29 (.24–.34) | <.001 |
| Preferred specimen type | |||||
| Self-collected ANS | 272 (37.3) | 174 (38.5) | 98 (35.3) | 0.03 (−.04 to .10) | .67 |
| Self-collected SS | 335 (45.9) | 205 (45.4) | 130 (46.8) | −0.01 (−.09 to .06) | |
| Healthcare provider–collected NPS | 98 (13.4) | 64 (14.2) | 34 (12.2) | 0.02 (−.03 to .07) | |
| Willingness to be tested again by specimen type, if it were the only type being collected | |||||
| Self-collected ANS | 704 (96.4) | 445 (98.5) | 259 (93.2) | 0.05 (.02–.09) | <.001 |
| Self-collected SS | 707 (96.8) | 444 (98.2) | 263 (94.6) | 0.04 (.01–.07) | .07 |
| Healthcare provider–collected NPS | 641 (87.8) | 403 (89.2) | 238 (85.6) | 0.04 (−.01 to .09) | .44 |
| Positive for SARS-COV-2 by ≥1 specimen typeb | 84 (11.5) | 82 (18.1) | 2 (0.7) | 0.17 (.14–.21) | <.001 |
Abbreviations: ANS, anterior nasal specimen; CI, confidence interval; COVID-19, coronavirus disease 2019; NPS, nasopharyngeal specimen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SS, saliva specimen.
aThe difference between proportions of participants enrolled at homeless service sites and participants enrolled at community sites, within each category.
bSymptoms defined as new or worsening fever (subjective or objective) or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new anosmia or ageusia, sore throat, congestion or nasal discharge, nausea or vomiting, and diarrhea.
cIncluding participants who had a missing, invalid, or inconclusive test result for any specimen type (ANS, SS, or NPS).
Self-Reported Demographic, Clinical, and Epidemiologic Characteristics, Specimen Preference, and Overall Severe Acute Respiratory Syndrome Coronavirus 2 Positivity With Real-Time Reverse-Transcription Polymerase Chain Reaction by Testing Site
| Participant Characteristic . | Participants by Testing Site, No. (%) . | . | . | ||
|---|---|---|---|---|---|
| . | All Sites (N = 730) . | Community Sites (n = 452) . | Homeless Service Sites (n = 278 ) . | Difference of Proportionsa (95% CI) . | P Value . |
| Age group, y | |||||
| 18–40 | 330 (45.2) | 245 (54.2) | 85 (30.6) | 0.24 (.16–.31) | <.001 |
| 41–65 | 334 (45.8) | 167 (36.9) | 167 (60.1) | −0.23 (−.30 to −.16) | |
| >65 | 66 (9.0) | 40 (8.8) | 26 (9.4) | −0.01 (−.05 to .04) | |
| Sex | |||||
| Male | 420 (57.5) | 190 (42.0) | 230 (82.7) | −0.41 (−.47 to −.34) | <.001 |
| Female | 304 (41.6) | 259 (57.3) | 45 (16.2) | 0.41 (.35–.47) | |
| Other | 5 (0.7) | 3 (0.7) | 2 (0.7) | −0.0006 (−.02 to .01) | |
| Race/ethnicity | |||||
| Black, non-Hispanic | 85 (11.6) | 44 (9.7) | 41 (14.7) | −0.05 (−.103 to −.002) | <.001 |
| White, non-Hispanic | 321 (44.0) | 177 (39.2) | 144 (51.8) | 0.13 (−.20 to −.05) | |
| Asian, non-Hispanic | 17 (2.3) | 12 (2.7) | 5 (1.8) | 0.01 (−.02 to .03) | |
| Hispanic | 257 (35.2) | 199 (44.0) | 58 (20.9) | 0.23 (.16–.30) | |
| Native American/Alaska Native, non-Hispanic | 14 (1.9) | 4 (0.9) | 10 (3.6) | −0.03 (−.06 to −.01) | |
| Other, non-Hispanic | 33 (4.5) | 15 (3.3) | 18 (6.5) | -0.03 (−.07 to −.0005) | |
| New or worsening symptoms reported at testinga | |||||
| Any symptom | 269 (36.8) | 216 (47.8) | 53 (19.1) | 0.29 (.22–.35) | <.001 |
| Cough, shortness of breath or difficulty breathing, anosmia, or ageusia | 168 (23.0) | 139 (30.8) | 29 (10.4) | 0.20 (.15–.26) | <.001 |
| ≥2 Symptoms, including fever (measured or subjective) or chills, rigors, myalgia, headache, sore throat, nausea or vomiting, diarrhea, fatigue, and congestion or nasal discharge | 187 (25.6) | 172 (38.1) | 15 (5.4) | 0.33 (.27–.38) | <.001 |
| Flulike symptoms (fever plus either cough or sore throat) | 66 (9.0) | 62 (13.7) | 4 (1.4) | 0.12 (.09–.16) | <.001 |
| Fever (measured or subjective) or chills | 90 (12.3) | 84 (18.6) | 6 (2.2) | 0.16 (.12–.21) | <.001 |
| Cough | 79 (10.8) | 61 (13.5) | 18 (6.5) | 0.07 (.03–.11) | .004 |
| Shortness of breath or difficulty breathing | 86 (11.8) | 69 (15.3) | 17 (6.1) | 0.09 (.05–.13) | <.001 |
| Fatigue | 87 (11.9) | 76 (16.8) | 11 (4.0) | 0.13 (.09–.17) | <.001 |
| Myalgia | 108 (14.8) | 97 (21.5) | 11 (4.0) | 0.18 (.13–.22) | <.001 |
| Headache | 66 (9.0) | 54 (11.9) | 12 (4.3) | 0.08 (.04–.11) | .001 |
| Anosmia or ageusia | 76 (10.4) | 73 (16.2) | 3 (1.1) | 0.15 (.12–.19) | <.001 |
| Sore throat | 116 (15.9) | 109 (24.1) | 7 (2.5) | 0.22 (.39–.49) | <.001 |
| Congestion or nasal discharge | 66 (9.0) | 42 (9.3) | 24 (8.6) | 0.01 (−.04 to .05) | .87 |
| Nausea or vomiting | 30 (4.1) | 21 (4.6) | 9 (3.2) | 0.01 (−.02 to .04) | .46 |
| Diarrhea | 165 (22.6) | 156 (34.5) | 9 (3.2) | 0.31 (.26–.36) | <.001 |
| Close contact with known COVID-19 case within prior 2 wk | 159 (21.8) | 149 (33.0) | 10 (3.6) | 0.29 (.24–.34) | <.001 |
| Preferred specimen type | |||||
| Self-collected ANS | 272 (37.3) | 174 (38.5) | 98 (35.3) | 0.03 (−.04 to .10) | .67 |
| Self-collected SS | 335 (45.9) | 205 (45.4) | 130 (46.8) | −0.01 (−.09 to .06) | |
| Healthcare provider–collected NPS | 98 (13.4) | 64 (14.2) | 34 (12.2) | 0.02 (−.03 to .07) | |
| Willingness to be tested again by specimen type, if it were the only type being collected | |||||
| Self-collected ANS | 704 (96.4) | 445 (98.5) | 259 (93.2) | 0.05 (.02–.09) | <.001 |
| Self-collected SS | 707 (96.8) | 444 (98.2) | 263 (94.6) | 0.04 (.01–.07) | .07 |
| Healthcare provider–collected NPS | 641 (87.8) | 403 (89.2) | 238 (85.6) | 0.04 (−.01 to .09) | .44 |
| Positive for SARS-COV-2 by ≥1 specimen typeb | 84 (11.5) | 82 (18.1) | 2 (0.7) | 0.17 (.14–.21) | <.001 |
| Participant Characteristic . | Participants by Testing Site, No. (%) . | . | . | ||
|---|---|---|---|---|---|
| . | All Sites (N = 730) . | Community Sites (n = 452) . | Homeless Service Sites (n = 278 ) . | Difference of Proportionsa (95% CI) . | P Value . |
| Age group, y | |||||
| 18–40 | 330 (45.2) | 245 (54.2) | 85 (30.6) | 0.24 (.16–.31) | <.001 |
| 41–65 | 334 (45.8) | 167 (36.9) | 167 (60.1) | −0.23 (−.30 to −.16) | |
| >65 | 66 (9.0) | 40 (8.8) | 26 (9.4) | −0.01 (−.05 to .04) | |
| Sex | |||||
| Male | 420 (57.5) | 190 (42.0) | 230 (82.7) | −0.41 (−.47 to −.34) | <.001 |
| Female | 304 (41.6) | 259 (57.3) | 45 (16.2) | 0.41 (.35–.47) | |
| Other | 5 (0.7) | 3 (0.7) | 2 (0.7) | −0.0006 (−.02 to .01) | |
| Race/ethnicity | |||||
| Black, non-Hispanic | 85 (11.6) | 44 (9.7) | 41 (14.7) | −0.05 (−.103 to −.002) | <.001 |
| White, non-Hispanic | 321 (44.0) | 177 (39.2) | 144 (51.8) | 0.13 (−.20 to −.05) | |
| Asian, non-Hispanic | 17 (2.3) | 12 (2.7) | 5 (1.8) | 0.01 (−.02 to .03) | |
| Hispanic | 257 (35.2) | 199 (44.0) | 58 (20.9) | 0.23 (.16–.30) | |
| Native American/Alaska Native, non-Hispanic | 14 (1.9) | 4 (0.9) | 10 (3.6) | −0.03 (−.06 to −.01) | |
| Other, non-Hispanic | 33 (4.5) | 15 (3.3) | 18 (6.5) | -0.03 (−.07 to −.0005) | |
| New or worsening symptoms reported at testinga | |||||
| Any symptom | 269 (36.8) | 216 (47.8) | 53 (19.1) | 0.29 (.22–.35) | <.001 |
| Cough, shortness of breath or difficulty breathing, anosmia, or ageusia | 168 (23.0) | 139 (30.8) | 29 (10.4) | 0.20 (.15–.26) | <.001 |
| ≥2 Symptoms, including fever (measured or subjective) or chills, rigors, myalgia, headache, sore throat, nausea or vomiting, diarrhea, fatigue, and congestion or nasal discharge | 187 (25.6) | 172 (38.1) | 15 (5.4) | 0.33 (.27–.38) | <.001 |
| Flulike symptoms (fever plus either cough or sore throat) | 66 (9.0) | 62 (13.7) | 4 (1.4) | 0.12 (.09–.16) | <.001 |
| Fever (measured or subjective) or chills | 90 (12.3) | 84 (18.6) | 6 (2.2) | 0.16 (.12–.21) | <.001 |
| Cough | 79 (10.8) | 61 (13.5) | 18 (6.5) | 0.07 (.03–.11) | .004 |
| Shortness of breath or difficulty breathing | 86 (11.8) | 69 (15.3) | 17 (6.1) | 0.09 (.05–.13) | <.001 |
| Fatigue | 87 (11.9) | 76 (16.8) | 11 (4.0) | 0.13 (.09–.17) | <.001 |
| Myalgia | 108 (14.8) | 97 (21.5) | 11 (4.0) | 0.18 (.13–.22) | <.001 |
| Headache | 66 (9.0) | 54 (11.9) | 12 (4.3) | 0.08 (.04–.11) | .001 |
| Anosmia or ageusia | 76 (10.4) | 73 (16.2) | 3 (1.1) | 0.15 (.12–.19) | <.001 |
| Sore throat | 116 (15.9) | 109 (24.1) | 7 (2.5) | 0.22 (.39–.49) | <.001 |
| Congestion or nasal discharge | 66 (9.0) | 42 (9.3) | 24 (8.6) | 0.01 (−.04 to .05) | .87 |
| Nausea or vomiting | 30 (4.1) | 21 (4.6) | 9 (3.2) | 0.01 (−.02 to .04) | .46 |
| Diarrhea | 165 (22.6) | 156 (34.5) | 9 (3.2) | 0.31 (.26–.36) | <.001 |
| Close contact with known COVID-19 case within prior 2 wk | 159 (21.8) | 149 (33.0) | 10 (3.6) | 0.29 (.24–.34) | <.001 |
| Preferred specimen type | |||||
| Self-collected ANS | 272 (37.3) | 174 (38.5) | 98 (35.3) | 0.03 (−.04 to .10) | .67 |
| Self-collected SS | 335 (45.9) | 205 (45.4) | 130 (46.8) | −0.01 (−.09 to .06) | |
| Healthcare provider–collected NPS | 98 (13.4) | 64 (14.2) | 34 (12.2) | 0.02 (−.03 to .07) | |
| Willingness to be tested again by specimen type, if it were the only type being collected | |||||
| Self-collected ANS | 704 (96.4) | 445 (98.5) | 259 (93.2) | 0.05 (.02–.09) | <.001 |
| Self-collected SS | 707 (96.8) | 444 (98.2) | 263 (94.6) | 0.04 (.01–.07) | .07 |
| Healthcare provider–collected NPS | 641 (87.8) | 403 (89.2) | 238 (85.6) | 0.04 (−.01 to .09) | .44 |
| Positive for SARS-COV-2 by ≥1 specimen typeb | 84 (11.5) | 82 (18.1) | 2 (0.7) | 0.17 (.14–.21) | <.001 |
Abbreviations: ANS, anterior nasal specimen; CI, confidence interval; COVID-19, coronavirus disease 2019; NPS, nasopharyngeal specimen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SS, saliva specimen.
aThe difference between proportions of participants enrolled at homeless service sites and participants enrolled at community sites, within each category.
bSymptoms defined as new or worsening fever (subjective or objective) or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new anosmia or ageusia, sore throat, congestion or nasal discharge, nausea or vomiting, and diarrhea.
cIncluding participants who had a missing, invalid, or inconclusive test result for any specimen type (ANS, SS, or NPS).
Specimen Type Preference and Willingness to Be Tested Again by Specimen Type
SS were the most preferred specimen type overall (45.9%), followed by ANS (37.3%), and NPS (13.4%). Specimen type preference did not differ by testing site type. The majority of participants from both sites indicated they would be willing to be tested again with ANS (98.5% at community sites and 93.2% at homeless shelters), SS (98.2% at community sites and 94.6% at homeless shelters), and NPS (89.2% at community sites and 85.6% at homeless shelters) if each was the only specimen type being collected for testing.
Testing Performance of ANS and SS With rRT-PCR
Conclusive (positive or negative) results were available for 544 SS, 609 ANS, and 665 NPS; 467 participants (64.0%) had conclusive results for all 3 specimen types (Figure 1). Compared with NPS, the overall sensitivity for rRT-PCR detection of SARS-CoV-2 was 85.2% (95% CI, 73.4%–92.3%) for SS and 80.0% (68.7%–87.9%) for ANS (Table 2). Sensitivity was higher among participants reporting ≥1 COVID-19 symptom at the time of specimen collection (93.6% [95% CI, 82.8%–97.8%] for SS; 86.8% [75.1%–93.5%] for ANS) and also when results from SS and ANS were combined (87.5% [77.2%–93.5%]). Among asymptomatic participants, sensitivity was low (28.5% for SS and 50.0% for ANS). Compared with NPS, the specificity and negative predictive value were ≥96% for both SS and ANS, including among asymptomatic participants. Analysis of test performance by testing location type was limited by low numbers of positive test results among participants at homeless shelters during the study period (Supplementary Table). Using the Cohen kappa coefficient, agreement was 0.85 (95% CI, .77–.93) between SS and NPS and 0.83 (.76–.91) between ANS and NPS.
Performance of Self-Collected Anterior Nasal and Saliva Specimens for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 With Real-Time Reverse-Transcription Polymerase Chain Reaction, Using Healthcare Provider–Collected Nasopharyngeal Specimens as Reference
| Test Characteristic . | Proportion, No./Total (% [95% CI]) . | |||||
|---|---|---|---|---|---|---|
| . | ANSa (n = 563) . | SSb (n = 497) . | Asymptomatic at Testing . | Any Symptomsc at Testing . | ||
| . | . | . | ANSa (n = 332) . | SSb (n = 299) . | ANSa (n = 230) . | SSb (n = 197) . |
| Sensitivity | 52/65 (80.0 [68.7–87.9]) | 46/54 (85.2 [73.4–92.3]) | 6/12 (50 [25.3–74.6]) | 2/7 (28.5 [8.2–64.1]) | 46/53 (86.8 [75.1–93.5]) | 44/47 (93.6 [82.8–97.8]) |
| Specificity | 492/497 (99.0 [97.6–99.6]) | 436/442 (98.6 [97.1–99.4]) | 317/320 (99.1 [97.2–99.7]) | 289/292 (99.0 [97.0–99.7]) | 175/177 (98.9 [96.0–99.7]) | 147/150 (98.0 [94.3–99.3]) |
| PLR | 79.7 [34.1–187.6] | 62.9 [28.9–137.7] | 53.3 [15.7–173.0] | 27.8 [5.8–114.4] | 76.8 [21.5–280.5] | 46.8 [16.3–137.5] |
| NLR | 0.20 [.12–.31] | 0.15 [.08–.27] | 0.50 [.26–.75] | 0.72 [.36–.93] | 0.13 [.07–.25] | 0.065 [.02–.18] |
| PPV | 52/57 (91.2 [81.0–96.2]) | 46/52 (88.5 [77.0–94.6]) | 6/9 (66.7 [35.4–87.9]) | 2/5 (40 [11.8–76.9]) | 46/48 (95.8 [86.0–98.8]) | 44/47 (93.6 [82.8–97.8]) |
| NPV | 492/505 (97.4 [95.6–98.5]) | 436/444 (98.2 [96.5–99.1]) | 317/323 (98.1 [96.0–99.2]) | 289/294 (98.3 [96.1–99.3]) | 175/182 (96.2 [92.2–98.1]) | 147/150 (98.0 [94.3–99.3]) |
| Agreementd | 0.83 [.76–.91] | 0.85 [.77–.93] | 0.56 [.27–.84] | 0.32 [.00–.79] | 0.89 [.81–.96] | 0.92 [.85–.98] |
| Test Characteristic . | Proportion, No./Total (% [95% CI]) . | |||||
|---|---|---|---|---|---|---|
| . | ANSa (n = 563) . | SSb (n = 497) . | Asymptomatic at Testing . | Any Symptomsc at Testing . | ||
| . | . | . | ANSa (n = 332) . | SSb (n = 299) . | ANSa (n = 230) . | SSb (n = 197) . |
| Sensitivity | 52/65 (80.0 [68.7–87.9]) | 46/54 (85.2 [73.4–92.3]) | 6/12 (50 [25.3–74.6]) | 2/7 (28.5 [8.2–64.1]) | 46/53 (86.8 [75.1–93.5]) | 44/47 (93.6 [82.8–97.8]) |
| Specificity | 492/497 (99.0 [97.6–99.6]) | 436/442 (98.6 [97.1–99.4]) | 317/320 (99.1 [97.2–99.7]) | 289/292 (99.0 [97.0–99.7]) | 175/177 (98.9 [96.0–99.7]) | 147/150 (98.0 [94.3–99.3]) |
| PLR | 79.7 [34.1–187.6] | 62.9 [28.9–137.7] | 53.3 [15.7–173.0] | 27.8 [5.8–114.4] | 76.8 [21.5–280.5] | 46.8 [16.3–137.5] |
| NLR | 0.20 [.12–.31] | 0.15 [.08–.27] | 0.50 [.26–.75] | 0.72 [.36–.93] | 0.13 [.07–.25] | 0.065 [.02–.18] |
| PPV | 52/57 (91.2 [81.0–96.2]) | 46/52 (88.5 [77.0–94.6]) | 6/9 (66.7 [35.4–87.9]) | 2/5 (40 [11.8–76.9]) | 46/48 (95.8 [86.0–98.8]) | 44/47 (93.6 [82.8–97.8]) |
| NPV | 492/505 (97.4 [95.6–98.5]) | 436/444 (98.2 [96.5–99.1]) | 317/323 (98.1 [96.0–99.2]) | 289/294 (98.3 [96.1–99.3]) | 175/182 (96.2 [92.2–98.1]) | 147/150 (98.0 [94.3–99.3]) |
| Agreementd | 0.83 [.76–.91] | 0.85 [.77–.93] | 0.56 [.27–.84] | 0.32 [.00–.79] | 0.89 [.81–.96] | 0.92 [.85–.98] |
Abbreviations: ANS, anterior nasal specimens; CI, confidence interval; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; SS, saliva specimens.
aAnalysis restricted to participants with conclusive positive or negative results with both ANS and nasopharyngeal specimens (NPS).
bAnalysis restricted to participants with conclusive positive or negative results with both SS and NPS.
cSymptoms defined as new or worsening fever (subjective or objective) or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new anosmia or ageusia, sore throat, congestion or nasal discharge, nausea or vomiting, and diarrhea.
dAgreement calculated using Cohen kappa correlation coefficient (CI computed by means of bootstrapping).
Performance of Self-Collected Anterior Nasal and Saliva Specimens for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 With Real-Time Reverse-Transcription Polymerase Chain Reaction, Using Healthcare Provider–Collected Nasopharyngeal Specimens as Reference
| Test Characteristic . | Proportion, No./Total (% [95% CI]) . | |||||
|---|---|---|---|---|---|---|
| . | ANSa (n = 563) . | SSb (n = 497) . | Asymptomatic at Testing . | Any Symptomsc at Testing . | ||
| . | . | . | ANSa (n = 332) . | SSb (n = 299) . | ANSa (n = 230) . | SSb (n = 197) . |
| Sensitivity | 52/65 (80.0 [68.7–87.9]) | 46/54 (85.2 [73.4–92.3]) | 6/12 (50 [25.3–74.6]) | 2/7 (28.5 [8.2–64.1]) | 46/53 (86.8 [75.1–93.5]) | 44/47 (93.6 [82.8–97.8]) |
| Specificity | 492/497 (99.0 [97.6–99.6]) | 436/442 (98.6 [97.1–99.4]) | 317/320 (99.1 [97.2–99.7]) | 289/292 (99.0 [97.0–99.7]) | 175/177 (98.9 [96.0–99.7]) | 147/150 (98.0 [94.3–99.3]) |
| PLR | 79.7 [34.1–187.6] | 62.9 [28.9–137.7] | 53.3 [15.7–173.0] | 27.8 [5.8–114.4] | 76.8 [21.5–280.5] | 46.8 [16.3–137.5] |
| NLR | 0.20 [.12–.31] | 0.15 [.08–.27] | 0.50 [.26–.75] | 0.72 [.36–.93] | 0.13 [.07–.25] | 0.065 [.02–.18] |
| PPV | 52/57 (91.2 [81.0–96.2]) | 46/52 (88.5 [77.0–94.6]) | 6/9 (66.7 [35.4–87.9]) | 2/5 (40 [11.8–76.9]) | 46/48 (95.8 [86.0–98.8]) | 44/47 (93.6 [82.8–97.8]) |
| NPV | 492/505 (97.4 [95.6–98.5]) | 436/444 (98.2 [96.5–99.1]) | 317/323 (98.1 [96.0–99.2]) | 289/294 (98.3 [96.1–99.3]) | 175/182 (96.2 [92.2–98.1]) | 147/150 (98.0 [94.3–99.3]) |
| Agreementd | 0.83 [.76–.91] | 0.85 [.77–.93] | 0.56 [.27–.84] | 0.32 [.00–.79] | 0.89 [.81–.96] | 0.92 [.85–.98] |
| Test Characteristic . | Proportion, No./Total (% [95% CI]) . | |||||
|---|---|---|---|---|---|---|
| . | ANSa (n = 563) . | SSb (n = 497) . | Asymptomatic at Testing . | Any Symptomsc at Testing . | ||
| . | . | . | ANSa (n = 332) . | SSb (n = 299) . | ANSa (n = 230) . | SSb (n = 197) . |
| Sensitivity | 52/65 (80.0 [68.7–87.9]) | 46/54 (85.2 [73.4–92.3]) | 6/12 (50 [25.3–74.6]) | 2/7 (28.5 [8.2–64.1]) | 46/53 (86.8 [75.1–93.5]) | 44/47 (93.6 [82.8–97.8]) |
| Specificity | 492/497 (99.0 [97.6–99.6]) | 436/442 (98.6 [97.1–99.4]) | 317/320 (99.1 [97.2–99.7]) | 289/292 (99.0 [97.0–99.7]) | 175/177 (98.9 [96.0–99.7]) | 147/150 (98.0 [94.3–99.3]) |
| PLR | 79.7 [34.1–187.6] | 62.9 [28.9–137.7] | 53.3 [15.7–173.0] | 27.8 [5.8–114.4] | 76.8 [21.5–280.5] | 46.8 [16.3–137.5] |
| NLR | 0.20 [.12–.31] | 0.15 [.08–.27] | 0.50 [.26–.75] | 0.72 [.36–.93] | 0.13 [.07–.25] | 0.065 [.02–.18] |
| PPV | 52/57 (91.2 [81.0–96.2]) | 46/52 (88.5 [77.0–94.6]) | 6/9 (66.7 [35.4–87.9]) | 2/5 (40 [11.8–76.9]) | 46/48 (95.8 [86.0–98.8]) | 44/47 (93.6 [82.8–97.8]) |
| NPV | 492/505 (97.4 [95.6–98.5]) | 436/444 (98.2 [96.5–99.1]) | 317/323 (98.1 [96.0–99.2]) | 289/294 (98.3 [96.1–99.3]) | 175/182 (96.2 [92.2–98.1]) | 147/150 (98.0 [94.3–99.3]) |
| Agreementd | 0.83 [.76–.91] | 0.85 [.77–.93] | 0.56 [.27–.84] | 0.32 [.00–.79] | 0.89 [.81–.96] | 0.92 [.85–.98] |
Abbreviations: ANS, anterior nasal specimens; CI, confidence interval; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; SS, saliva specimens.
aAnalysis restricted to participants with conclusive positive or negative results with both ANS and nasopharyngeal specimens (NPS).
bAnalysis restricted to participants with conclusive positive or negative results with both SS and NPS.
cSymptoms defined as new or worsening fever (subjective or objective) or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new anosmia or ageusia, sore throat, congestion or nasal discharge, nausea or vomiting, and diarrhea.
dAgreement calculated using Cohen kappa correlation coefficient (CI computed by means of bootstrapping).
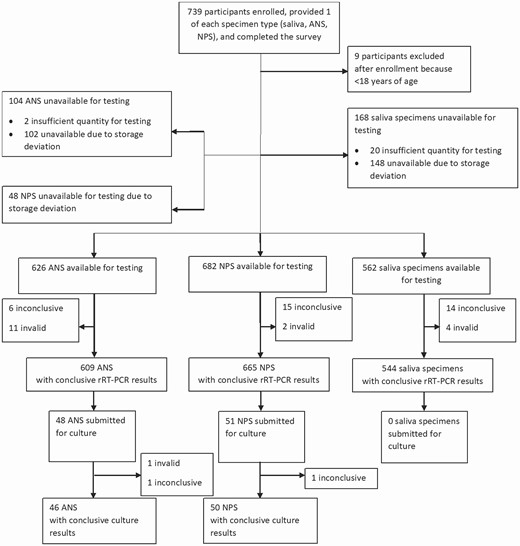
Flowchart of the participants enrolled and the specimens submitted for severe acute respiratory syndrome coronavirus 2 real-time reverse-transcription polymerase chain reaction (rRT-PCR) and viral culture. Abbreviations: ANS, anterior nasal specimens; NPS, nasopharyngeal specimens; SS, saliva specimens.
Comparison of Mean Ct Values by Specimen Type
For all participants, Ct values for the N1 and N2 rRT-PCR targets were strongly correlated (r = 0.994 [95% CI, .990–.996]) (Supplementary Figure 2); thus, only N1 Ct values are reported. Among participants with a valid result for all 3 specimen types and with SARS-CoV-2 detected in the NPS, mean Ct values of NPS were lower (indicating a higher concentration of target nucleic acid in the specimen) among participants positive with all 3 specimen types (18.1 [95% CI, 16.9–19.3]) than among those positive only with the NPS (32.4 [25.4–39.4]). The difference between mean Ct values in participants positive only by NPS and those in participants positive by all 3 specimen types was 14.3 (95% CI, 5.8–22.8).
SARS-CoV-2 Detection by Viral Culture
A total of 48 ANS and 51 NPS from 51 participants were submitted for viral culture (Figure 1); of these, conclusive (positive or negative) culture results were available for 50 NPS and 46 ANS from 50 participants (Table 3). Culturable virus was detected in specimens from 19 participants (38%), 6 in both NPS and ANS, 12 in NPS only, and 1 in ANS only. Using culturable virus in any specimen type as the reference, sensitivity for SARS-CoV-2 detection by rRT-PCR was 100% (95% CI, 83.2%–100%) for both NPS and ANS and 93.8% (71.7%–99.7%) for SS. In addition, among the 19 participants with culturable virus, 1 rRT-PCR result for SS was inconclusive and 1 was invalid. Ct values for the N1 target were associated with overall culture result. Receiver operating characteristic analysis showed that a Ct value of 18.7 was the optimal cutoff for detection of culturable SARS-CoV-2 (Supplementary Figure 3); among specimens positive for SARS-CoV-2 by rRT-PCR, those with lower Ct values (<19) were more often positive by culture, while specimens with higher Ct values (≥19) were more often negative by culture (Figure 2).
Severe Acute Respiratory Syndrome Coronavirus 2 Detection by Real-Time Reverse-Transcription Polymerase Chain Reaction for Nasopharyngeal Specimens (NPS), Anterior Nasal Specimens (ANS), and Saliva Specimens and by Culture for NPS and ANS in Participants With ≥1 Valid Culture Result for Either NPS or ANS
| Participant . | rRT-PCR Resultsa . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | NPS . | ANS . | SS . | Culture Results . | |||||||
| . | Result . | Ct (N1) . | Ct (N2) . | Result . | Ct (N1) . | Ct (N2) . | Result . | Ct (N1) . | Ct (N2) . | NPS . | ANS . |
| 1 | Pos | 16.4 | 16.0 | Pos | 14.6 | 14.2 | Pos | 27.5 | 27.7 | Neg | Pos |
| 2 | Pos | 28.1 | 28.0 | Pos | 31.5 | 31.8 | Inc | 35.1 | >40.0 | Neg | Neg |
| 3 | Neg | >40.0 | >40.0 | Pos | 31.6 | 36.2 | Neg | >40.0 | >40.0 | Neg | Neg |
| 4 | Pos | 27.8 | 28.1 | Inc | 38.9 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 5 | Pos | 18.8 | 17.8 | Pos | 28.7 | 28.7 | Pos | 31.6 | 30.7 | Pos | Neg |
| 6 | Pos | 25.6 | 25.6 | Pos | 28.1 | 28.7 | Pos | 37.4 | 35.4 | Neg | Neg |
| 7 | Pos | 17.1 | 16.1 | Pos | 16.8 | 15.6 | Neg | >40.0 | >40.0 | Pos | Pos |
| 8 | Pos | 32.3 | 31.6 | Neg | >40.0 | >40.0 | Pos | 30.2 | 28.6 | Neg | Neg |
| 9 | Pos | 25.2 | 24.9 | Neg | >40.0 | >40.0 | Pos | 30.7 | 31.7 | Neg | Neg |
| 10 | Pos | 18.8 | 18.0 | Pos | 13.6 | 12.2 | Pos | 12.4 | 11.0 | Pos | Neg |
| 11 | Pos | 17.5 | 16.4 | Pos | 18.7 | 17.8 | Invalid | >40.0 | >40.0 | Pos | Pos |
| 12 | Pos | 19.9 | 18.8 | Pos | 24.0 | 22.9 | Pos | 28.1 | 27.0 | Neg | Neg |
| 13 | Pos | 27.7 | 28.0 | Pos | 32.9 | 33.0 | Pos | 24.4 | 23.9 | Neg | Neg |
| 14 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 30.2 | 29.2 | Neg | Neg |
| 15 | Pos | 36.3 | 37.4 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 16 | Pos | 15.4 | 14.7 | Pos | 18.4 | 18.2 | Pos | 19.0 | 19.4 | Pos | Neg |
| 17 | Pos | 33.6 | 33.9 | Pos | 27.7 | 27.7 | Inc | >40.0 | 36.6 | Neg | Neg |
| 18 | Pos | 19.7 | 18.4 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 19 | Pos | 32.7 | 33.8 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 20 | Pos | 21.7 | 22.9 | Pos | 28.7 | 28.6 | Pos | 24.2 | 24.2 | Pos | Neg |
| 21 | Pos | 34.6 | 35.1 | Neg | >40.0 | >40.0 | ...b | … | … | Neg | Neg |
| 22 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 19.7 | 22.4 | Neg | … |
| 23 | Pos | 13.4 | 12.6 | Pos | 16.7 | 14.9 | … | … | … | Pos | Neg |
| 24 | Pos | 16.8 | 15.8 | Pos | 19.2 | 17.9 | Pos | 25.8 | 27.0 | Pos | Neg |
| 25 | Neg | >40.0 | >40.0 | Pos | 19.1 | 19.3 | Inc | >40.0 | 39.2 | Neg | Neg |
| 26 | Neg | >40.0 | >40.0 | Pos | 18.8 | 19.5 | … | … | … | Neg | Neg |
| 27 | Pos | 14.5 | 13.6 | Pos | 17.6 | 16.4 | Pos | 26.7 | 25.8 | Neg | Neg |
| 28 | Pos | 16.6 | 15.6 | Pos | 16.8 | 15.8 | Pos | 23.7 | 23.0 | Pos | Pos |
| 29 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 33.3 | 32.7 | Neg | Neg |
| 30 | Pos | 29.8 | 30.7 | Neg | >40.0 | >40.0 | Pos | 31.9 | 32.7 | Neg | Neg |
| 31 | Pos | 16.6 | 14.5 | Pos | 15.3 | 13.3 | Pos | 33.2 | 39.2 | Pos | Neg |
| 32 | Pos | 13.8 | 12.6 | Pos | 16.9 | 15.0 | Pos | 18.0 | 17.1 | Pos | Neg |
| 33 | Pos | 26.1 | 26.4 | Inc | 35.6 | >40.0 | Inc | >40.0 | 34.7 | Neg | Neg |
| 34 | Pos | 19.4 | 19.0 | Pos | 26.1 | 25.2 | Pos | 26.8 | 26.0 | Neg | Neg |
| 35 | Neg | >40.0 | >40.0 | Pos | 32.2 | 30.9 | Pos | 25.3 | 23.7 | Neg | Neg |
| 36 | Neg | >40.0 | >40.0 | Inc | >40.0 | 36.0 | Pos | 31.2 | 30.5 | Neg | Neg |
| 37 | Pos | 23.7 | 22.9 | Pos | 30.5 | 29.6 | Pos | 33.2 | 34.5 | Neg | Neg |
| 38 | Pos | 25.1 | 25.4 | Neg | >40.0 | >40.0 | Pos | 36.5 | 36.4 | Neg | Neg |
| 39 | Pos | 20.6 | 19.9 | Pos | 19.3 | 18.5 | Pos | 22.5 | 21.7 | Neg | Neg |
| 40 | Pos | 31.8 | 33.6 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 41 | Pos | 18.3 | 16.7 | Pos | 20.7 | 18.7 | Inc | 25.9 | >40.0 | Pos | Neg |
| 42 | Pos | 13.4 | 11.9 | Pos | 13.4 | 11.9 | Pos | 10.3 | 9.2 | Pos | Pos |
| 43 | Pos | 16.6 | 15.9 | Pos | 20.4 | 19.7 | Pos | 29.5 | 28.8 | Pos | Neg |
| 44 | Pos | 15.9 | 15.1 | Pos | 21.7 | 20.7 | Pos | 26.9 | 26.5 | Neg | Neg |
| 45 | Pos | 18.6 | 17.7 | Pos | 24.7 | 24.0 | Pos | 21.0 | 20.3 | Neg | Neg |
| 46 | Pos | 15.1 | 14.1 | Pos | 14.6 | 13.0 | Pos | 27.8 | 27.8 | Pos | Pos |
| 47 | Pos | 12.9 | 12.2 | Pos | 15.9 | 15.2 | Pos | 22.3 | 22.2 | Pos | Pos |
| 48 | Pos | 16.9 | 16.1 | Pos | 17.5 | 16.8 | Pos | 13.5 | 12.4 | Pos | … |
| 49 | Pos | 35.9 | 36.2 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | … |
| 50 | Pos | 19.1 | 18.2 | Pos | 17.4 | 16.5 | Pos | 28.2 | 27.7 | Pos | … |
| Participant . | rRT-PCR Resultsa . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | NPS . | ANS . | SS . | Culture Results . | |||||||
| . | Result . | Ct (N1) . | Ct (N2) . | Result . | Ct (N1) . | Ct (N2) . | Result . | Ct (N1) . | Ct (N2) . | NPS . | ANS . |
| 1 | Pos | 16.4 | 16.0 | Pos | 14.6 | 14.2 | Pos | 27.5 | 27.7 | Neg | Pos |
| 2 | Pos | 28.1 | 28.0 | Pos | 31.5 | 31.8 | Inc | 35.1 | >40.0 | Neg | Neg |
| 3 | Neg | >40.0 | >40.0 | Pos | 31.6 | 36.2 | Neg | >40.0 | >40.0 | Neg | Neg |
| 4 | Pos | 27.8 | 28.1 | Inc | 38.9 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 5 | Pos | 18.8 | 17.8 | Pos | 28.7 | 28.7 | Pos | 31.6 | 30.7 | Pos | Neg |
| 6 | Pos | 25.6 | 25.6 | Pos | 28.1 | 28.7 | Pos | 37.4 | 35.4 | Neg | Neg |
| 7 | Pos | 17.1 | 16.1 | Pos | 16.8 | 15.6 | Neg | >40.0 | >40.0 | Pos | Pos |
| 8 | Pos | 32.3 | 31.6 | Neg | >40.0 | >40.0 | Pos | 30.2 | 28.6 | Neg | Neg |
| 9 | Pos | 25.2 | 24.9 | Neg | >40.0 | >40.0 | Pos | 30.7 | 31.7 | Neg | Neg |
| 10 | Pos | 18.8 | 18.0 | Pos | 13.6 | 12.2 | Pos | 12.4 | 11.0 | Pos | Neg |
| 11 | Pos | 17.5 | 16.4 | Pos | 18.7 | 17.8 | Invalid | >40.0 | >40.0 | Pos | Pos |
| 12 | Pos | 19.9 | 18.8 | Pos | 24.0 | 22.9 | Pos | 28.1 | 27.0 | Neg | Neg |
| 13 | Pos | 27.7 | 28.0 | Pos | 32.9 | 33.0 | Pos | 24.4 | 23.9 | Neg | Neg |
| 14 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 30.2 | 29.2 | Neg | Neg |
| 15 | Pos | 36.3 | 37.4 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 16 | Pos | 15.4 | 14.7 | Pos | 18.4 | 18.2 | Pos | 19.0 | 19.4 | Pos | Neg |
| 17 | Pos | 33.6 | 33.9 | Pos | 27.7 | 27.7 | Inc | >40.0 | 36.6 | Neg | Neg |
| 18 | Pos | 19.7 | 18.4 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 19 | Pos | 32.7 | 33.8 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 20 | Pos | 21.7 | 22.9 | Pos | 28.7 | 28.6 | Pos | 24.2 | 24.2 | Pos | Neg |
| 21 | Pos | 34.6 | 35.1 | Neg | >40.0 | >40.0 | ...b | … | … | Neg | Neg |
| 22 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 19.7 | 22.4 | Neg | … |
| 23 | Pos | 13.4 | 12.6 | Pos | 16.7 | 14.9 | … | … | … | Pos | Neg |
| 24 | Pos | 16.8 | 15.8 | Pos | 19.2 | 17.9 | Pos | 25.8 | 27.0 | Pos | Neg |
| 25 | Neg | >40.0 | >40.0 | Pos | 19.1 | 19.3 | Inc | >40.0 | 39.2 | Neg | Neg |
| 26 | Neg | >40.0 | >40.0 | Pos | 18.8 | 19.5 | … | … | … | Neg | Neg |
| 27 | Pos | 14.5 | 13.6 | Pos | 17.6 | 16.4 | Pos | 26.7 | 25.8 | Neg | Neg |
| 28 | Pos | 16.6 | 15.6 | Pos | 16.8 | 15.8 | Pos | 23.7 | 23.0 | Pos | Pos |
| 29 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 33.3 | 32.7 | Neg | Neg |
| 30 | Pos | 29.8 | 30.7 | Neg | >40.0 | >40.0 | Pos | 31.9 | 32.7 | Neg | Neg |
| 31 | Pos | 16.6 | 14.5 | Pos | 15.3 | 13.3 | Pos | 33.2 | 39.2 | Pos | Neg |
| 32 | Pos | 13.8 | 12.6 | Pos | 16.9 | 15.0 | Pos | 18.0 | 17.1 | Pos | Neg |
| 33 | Pos | 26.1 | 26.4 | Inc | 35.6 | >40.0 | Inc | >40.0 | 34.7 | Neg | Neg |
| 34 | Pos | 19.4 | 19.0 | Pos | 26.1 | 25.2 | Pos | 26.8 | 26.0 | Neg | Neg |
| 35 | Neg | >40.0 | >40.0 | Pos | 32.2 | 30.9 | Pos | 25.3 | 23.7 | Neg | Neg |
| 36 | Neg | >40.0 | >40.0 | Inc | >40.0 | 36.0 | Pos | 31.2 | 30.5 | Neg | Neg |
| 37 | Pos | 23.7 | 22.9 | Pos | 30.5 | 29.6 | Pos | 33.2 | 34.5 | Neg | Neg |
| 38 | Pos | 25.1 | 25.4 | Neg | >40.0 | >40.0 | Pos | 36.5 | 36.4 | Neg | Neg |
| 39 | Pos | 20.6 | 19.9 | Pos | 19.3 | 18.5 | Pos | 22.5 | 21.7 | Neg | Neg |
| 40 | Pos | 31.8 | 33.6 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 41 | Pos | 18.3 | 16.7 | Pos | 20.7 | 18.7 | Inc | 25.9 | >40.0 | Pos | Neg |
| 42 | Pos | 13.4 | 11.9 | Pos | 13.4 | 11.9 | Pos | 10.3 | 9.2 | Pos | Pos |
| 43 | Pos | 16.6 | 15.9 | Pos | 20.4 | 19.7 | Pos | 29.5 | 28.8 | Pos | Neg |
| 44 | Pos | 15.9 | 15.1 | Pos | 21.7 | 20.7 | Pos | 26.9 | 26.5 | Neg | Neg |
| 45 | Pos | 18.6 | 17.7 | Pos | 24.7 | 24.0 | Pos | 21.0 | 20.3 | Neg | Neg |
| 46 | Pos | 15.1 | 14.1 | Pos | 14.6 | 13.0 | Pos | 27.8 | 27.8 | Pos | Pos |
| 47 | Pos | 12.9 | 12.2 | Pos | 15.9 | 15.2 | Pos | 22.3 | 22.2 | Pos | Pos |
| 48 | Pos | 16.9 | 16.1 | Pos | 17.5 | 16.8 | Pos | 13.5 | 12.4 | Pos | … |
| 49 | Pos | 35.9 | 36.2 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | … |
| 50 | Pos | 19.1 | 18.2 | Pos | 17.4 | 16.5 | Pos | 28.2 | 27.7 | Pos | … |
Abbreviations: ANS, anterior nasal specimens; Ct, cycle threshold; Inc, inconclusive; N1, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid genetic target 1; N2, SARS-CoV-2 nucleocapsid genetic target 2; Neg, negative; NPS, nasopharyngeal specimens; Pos, positive; rRT-PCR, real-time reverse-transcription polymerase chain reaction; SS, saliva specimens.
aOutcomes defined according to the emergency use authorization for the Centers for Disease Control and Prevention 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel [1], as follows: positive, Ct values for N1 and N2 <40.00; negative, Ct values for N1 and N2 ≥40.00 and positive control RNase P <40.00; invalid, Ct values for N1, N2, and positive control RNase P ≥40.00; and inconclusive, Ct values for either N1 or N2 (but not both) <40.00.
bEllipses (…) indicate not tested because of unavailable specimen.
Severe Acute Respiratory Syndrome Coronavirus 2 Detection by Real-Time Reverse-Transcription Polymerase Chain Reaction for Nasopharyngeal Specimens (NPS), Anterior Nasal Specimens (ANS), and Saliva Specimens and by Culture for NPS and ANS in Participants With ≥1 Valid Culture Result for Either NPS or ANS
| Participant . | rRT-PCR Resultsa . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | NPS . | ANS . | SS . | Culture Results . | |||||||
| . | Result . | Ct (N1) . | Ct (N2) . | Result . | Ct (N1) . | Ct (N2) . | Result . | Ct (N1) . | Ct (N2) . | NPS . | ANS . |
| 1 | Pos | 16.4 | 16.0 | Pos | 14.6 | 14.2 | Pos | 27.5 | 27.7 | Neg | Pos |
| 2 | Pos | 28.1 | 28.0 | Pos | 31.5 | 31.8 | Inc | 35.1 | >40.0 | Neg | Neg |
| 3 | Neg | >40.0 | >40.0 | Pos | 31.6 | 36.2 | Neg | >40.0 | >40.0 | Neg | Neg |
| 4 | Pos | 27.8 | 28.1 | Inc | 38.9 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 5 | Pos | 18.8 | 17.8 | Pos | 28.7 | 28.7 | Pos | 31.6 | 30.7 | Pos | Neg |
| 6 | Pos | 25.6 | 25.6 | Pos | 28.1 | 28.7 | Pos | 37.4 | 35.4 | Neg | Neg |
| 7 | Pos | 17.1 | 16.1 | Pos | 16.8 | 15.6 | Neg | >40.0 | >40.0 | Pos | Pos |
| 8 | Pos | 32.3 | 31.6 | Neg | >40.0 | >40.0 | Pos | 30.2 | 28.6 | Neg | Neg |
| 9 | Pos | 25.2 | 24.9 | Neg | >40.0 | >40.0 | Pos | 30.7 | 31.7 | Neg | Neg |
| 10 | Pos | 18.8 | 18.0 | Pos | 13.6 | 12.2 | Pos | 12.4 | 11.0 | Pos | Neg |
| 11 | Pos | 17.5 | 16.4 | Pos | 18.7 | 17.8 | Invalid | >40.0 | >40.0 | Pos | Pos |
| 12 | Pos | 19.9 | 18.8 | Pos | 24.0 | 22.9 | Pos | 28.1 | 27.0 | Neg | Neg |
| 13 | Pos | 27.7 | 28.0 | Pos | 32.9 | 33.0 | Pos | 24.4 | 23.9 | Neg | Neg |
| 14 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 30.2 | 29.2 | Neg | Neg |
| 15 | Pos | 36.3 | 37.4 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 16 | Pos | 15.4 | 14.7 | Pos | 18.4 | 18.2 | Pos | 19.0 | 19.4 | Pos | Neg |
| 17 | Pos | 33.6 | 33.9 | Pos | 27.7 | 27.7 | Inc | >40.0 | 36.6 | Neg | Neg |
| 18 | Pos | 19.7 | 18.4 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 19 | Pos | 32.7 | 33.8 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 20 | Pos | 21.7 | 22.9 | Pos | 28.7 | 28.6 | Pos | 24.2 | 24.2 | Pos | Neg |
| 21 | Pos | 34.6 | 35.1 | Neg | >40.0 | >40.0 | ...b | … | … | Neg | Neg |
| 22 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 19.7 | 22.4 | Neg | … |
| 23 | Pos | 13.4 | 12.6 | Pos | 16.7 | 14.9 | … | … | … | Pos | Neg |
| 24 | Pos | 16.8 | 15.8 | Pos | 19.2 | 17.9 | Pos | 25.8 | 27.0 | Pos | Neg |
| 25 | Neg | >40.0 | >40.0 | Pos | 19.1 | 19.3 | Inc | >40.0 | 39.2 | Neg | Neg |
| 26 | Neg | >40.0 | >40.0 | Pos | 18.8 | 19.5 | … | … | … | Neg | Neg |
| 27 | Pos | 14.5 | 13.6 | Pos | 17.6 | 16.4 | Pos | 26.7 | 25.8 | Neg | Neg |
| 28 | Pos | 16.6 | 15.6 | Pos | 16.8 | 15.8 | Pos | 23.7 | 23.0 | Pos | Pos |
| 29 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 33.3 | 32.7 | Neg | Neg |
| 30 | Pos | 29.8 | 30.7 | Neg | >40.0 | >40.0 | Pos | 31.9 | 32.7 | Neg | Neg |
| 31 | Pos | 16.6 | 14.5 | Pos | 15.3 | 13.3 | Pos | 33.2 | 39.2 | Pos | Neg |
| 32 | Pos | 13.8 | 12.6 | Pos | 16.9 | 15.0 | Pos | 18.0 | 17.1 | Pos | Neg |
| 33 | Pos | 26.1 | 26.4 | Inc | 35.6 | >40.0 | Inc | >40.0 | 34.7 | Neg | Neg |
| 34 | Pos | 19.4 | 19.0 | Pos | 26.1 | 25.2 | Pos | 26.8 | 26.0 | Neg | Neg |
| 35 | Neg | >40.0 | >40.0 | Pos | 32.2 | 30.9 | Pos | 25.3 | 23.7 | Neg | Neg |
| 36 | Neg | >40.0 | >40.0 | Inc | >40.0 | 36.0 | Pos | 31.2 | 30.5 | Neg | Neg |
| 37 | Pos | 23.7 | 22.9 | Pos | 30.5 | 29.6 | Pos | 33.2 | 34.5 | Neg | Neg |
| 38 | Pos | 25.1 | 25.4 | Neg | >40.0 | >40.0 | Pos | 36.5 | 36.4 | Neg | Neg |
| 39 | Pos | 20.6 | 19.9 | Pos | 19.3 | 18.5 | Pos | 22.5 | 21.7 | Neg | Neg |
| 40 | Pos | 31.8 | 33.6 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 41 | Pos | 18.3 | 16.7 | Pos | 20.7 | 18.7 | Inc | 25.9 | >40.0 | Pos | Neg |
| 42 | Pos | 13.4 | 11.9 | Pos | 13.4 | 11.9 | Pos | 10.3 | 9.2 | Pos | Pos |
| 43 | Pos | 16.6 | 15.9 | Pos | 20.4 | 19.7 | Pos | 29.5 | 28.8 | Pos | Neg |
| 44 | Pos | 15.9 | 15.1 | Pos | 21.7 | 20.7 | Pos | 26.9 | 26.5 | Neg | Neg |
| 45 | Pos | 18.6 | 17.7 | Pos | 24.7 | 24.0 | Pos | 21.0 | 20.3 | Neg | Neg |
| 46 | Pos | 15.1 | 14.1 | Pos | 14.6 | 13.0 | Pos | 27.8 | 27.8 | Pos | Pos |
| 47 | Pos | 12.9 | 12.2 | Pos | 15.9 | 15.2 | Pos | 22.3 | 22.2 | Pos | Pos |
| 48 | Pos | 16.9 | 16.1 | Pos | 17.5 | 16.8 | Pos | 13.5 | 12.4 | Pos | … |
| 49 | Pos | 35.9 | 36.2 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | … |
| 50 | Pos | 19.1 | 18.2 | Pos | 17.4 | 16.5 | Pos | 28.2 | 27.7 | Pos | … |
| Participant . | rRT-PCR Resultsa . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | NPS . | ANS . | SS . | Culture Results . | |||||||
| . | Result . | Ct (N1) . | Ct (N2) . | Result . | Ct (N1) . | Ct (N2) . | Result . | Ct (N1) . | Ct (N2) . | NPS . | ANS . |
| 1 | Pos | 16.4 | 16.0 | Pos | 14.6 | 14.2 | Pos | 27.5 | 27.7 | Neg | Pos |
| 2 | Pos | 28.1 | 28.0 | Pos | 31.5 | 31.8 | Inc | 35.1 | >40.0 | Neg | Neg |
| 3 | Neg | >40.0 | >40.0 | Pos | 31.6 | 36.2 | Neg | >40.0 | >40.0 | Neg | Neg |
| 4 | Pos | 27.8 | 28.1 | Inc | 38.9 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 5 | Pos | 18.8 | 17.8 | Pos | 28.7 | 28.7 | Pos | 31.6 | 30.7 | Pos | Neg |
| 6 | Pos | 25.6 | 25.6 | Pos | 28.1 | 28.7 | Pos | 37.4 | 35.4 | Neg | Neg |
| 7 | Pos | 17.1 | 16.1 | Pos | 16.8 | 15.6 | Neg | >40.0 | >40.0 | Pos | Pos |
| 8 | Pos | 32.3 | 31.6 | Neg | >40.0 | >40.0 | Pos | 30.2 | 28.6 | Neg | Neg |
| 9 | Pos | 25.2 | 24.9 | Neg | >40.0 | >40.0 | Pos | 30.7 | 31.7 | Neg | Neg |
| 10 | Pos | 18.8 | 18.0 | Pos | 13.6 | 12.2 | Pos | 12.4 | 11.0 | Pos | Neg |
| 11 | Pos | 17.5 | 16.4 | Pos | 18.7 | 17.8 | Invalid | >40.0 | >40.0 | Pos | Pos |
| 12 | Pos | 19.9 | 18.8 | Pos | 24.0 | 22.9 | Pos | 28.1 | 27.0 | Neg | Neg |
| 13 | Pos | 27.7 | 28.0 | Pos | 32.9 | 33.0 | Pos | 24.4 | 23.9 | Neg | Neg |
| 14 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 30.2 | 29.2 | Neg | Neg |
| 15 | Pos | 36.3 | 37.4 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 16 | Pos | 15.4 | 14.7 | Pos | 18.4 | 18.2 | Pos | 19.0 | 19.4 | Pos | Neg |
| 17 | Pos | 33.6 | 33.9 | Pos | 27.7 | 27.7 | Inc | >40.0 | 36.6 | Neg | Neg |
| 18 | Pos | 19.7 | 18.4 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 19 | Pos | 32.7 | 33.8 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 20 | Pos | 21.7 | 22.9 | Pos | 28.7 | 28.6 | Pos | 24.2 | 24.2 | Pos | Neg |
| 21 | Pos | 34.6 | 35.1 | Neg | >40.0 | >40.0 | ...b | … | … | Neg | Neg |
| 22 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 19.7 | 22.4 | Neg | … |
| 23 | Pos | 13.4 | 12.6 | Pos | 16.7 | 14.9 | … | … | … | Pos | Neg |
| 24 | Pos | 16.8 | 15.8 | Pos | 19.2 | 17.9 | Pos | 25.8 | 27.0 | Pos | Neg |
| 25 | Neg | >40.0 | >40.0 | Pos | 19.1 | 19.3 | Inc | >40.0 | 39.2 | Neg | Neg |
| 26 | Neg | >40.0 | >40.0 | Pos | 18.8 | 19.5 | … | … | … | Neg | Neg |
| 27 | Pos | 14.5 | 13.6 | Pos | 17.6 | 16.4 | Pos | 26.7 | 25.8 | Neg | Neg |
| 28 | Pos | 16.6 | 15.6 | Pos | 16.8 | 15.8 | Pos | 23.7 | 23.0 | Pos | Pos |
| 29 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Pos | 33.3 | 32.7 | Neg | Neg |
| 30 | Pos | 29.8 | 30.7 | Neg | >40.0 | >40.0 | Pos | 31.9 | 32.7 | Neg | Neg |
| 31 | Pos | 16.6 | 14.5 | Pos | 15.3 | 13.3 | Pos | 33.2 | 39.2 | Pos | Neg |
| 32 | Pos | 13.8 | 12.6 | Pos | 16.9 | 15.0 | Pos | 18.0 | 17.1 | Pos | Neg |
| 33 | Pos | 26.1 | 26.4 | Inc | 35.6 | >40.0 | Inc | >40.0 | 34.7 | Neg | Neg |
| 34 | Pos | 19.4 | 19.0 | Pos | 26.1 | 25.2 | Pos | 26.8 | 26.0 | Neg | Neg |
| 35 | Neg | >40.0 | >40.0 | Pos | 32.2 | 30.9 | Pos | 25.3 | 23.7 | Neg | Neg |
| 36 | Neg | >40.0 | >40.0 | Inc | >40.0 | 36.0 | Pos | 31.2 | 30.5 | Neg | Neg |
| 37 | Pos | 23.7 | 22.9 | Pos | 30.5 | 29.6 | Pos | 33.2 | 34.5 | Neg | Neg |
| 38 | Pos | 25.1 | 25.4 | Neg | >40.0 | >40.0 | Pos | 36.5 | 36.4 | Neg | Neg |
| 39 | Pos | 20.6 | 19.9 | Pos | 19.3 | 18.5 | Pos | 22.5 | 21.7 | Neg | Neg |
| 40 | Pos | 31.8 | 33.6 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | Neg |
| 41 | Pos | 18.3 | 16.7 | Pos | 20.7 | 18.7 | Inc | 25.9 | >40.0 | Pos | Neg |
| 42 | Pos | 13.4 | 11.9 | Pos | 13.4 | 11.9 | Pos | 10.3 | 9.2 | Pos | Pos |
| 43 | Pos | 16.6 | 15.9 | Pos | 20.4 | 19.7 | Pos | 29.5 | 28.8 | Pos | Neg |
| 44 | Pos | 15.9 | 15.1 | Pos | 21.7 | 20.7 | Pos | 26.9 | 26.5 | Neg | Neg |
| 45 | Pos | 18.6 | 17.7 | Pos | 24.7 | 24.0 | Pos | 21.0 | 20.3 | Neg | Neg |
| 46 | Pos | 15.1 | 14.1 | Pos | 14.6 | 13.0 | Pos | 27.8 | 27.8 | Pos | Pos |
| 47 | Pos | 12.9 | 12.2 | Pos | 15.9 | 15.2 | Pos | 22.3 | 22.2 | Pos | Pos |
| 48 | Pos | 16.9 | 16.1 | Pos | 17.5 | 16.8 | Pos | 13.5 | 12.4 | Pos | … |
| 49 | Pos | 35.9 | 36.2 | Neg | >40.0 | >40.0 | Neg | >40.0 | >40.0 | Neg | … |
| 50 | Pos | 19.1 | 18.2 | Pos | 17.4 | 16.5 | Pos | 28.2 | 27.7 | Pos | … |
Abbreviations: ANS, anterior nasal specimens; Ct, cycle threshold; Inc, inconclusive; N1, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid genetic target 1; N2, SARS-CoV-2 nucleocapsid genetic target 2; Neg, negative; NPS, nasopharyngeal specimens; Pos, positive; rRT-PCR, real-time reverse-transcription polymerase chain reaction; SS, saliva specimens.
aOutcomes defined according to the emergency use authorization for the Centers for Disease Control and Prevention 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel [1], as follows: positive, Ct values for N1 and N2 <40.00; negative, Ct values for N1 and N2 ≥40.00 and positive control RNase P <40.00; invalid, Ct values for N1, N2, and positive control RNase P ≥40.00; and inconclusive, Ct values for either N1 or N2 (but not both) <40.00.
bEllipses (…) indicate not tested because of unavailable specimen.
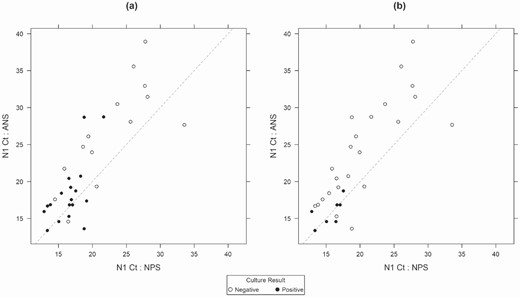
Correlation of cycle threshold (Ct) values for the severe acute respiratory syndrome coronavirus 2 genetic target N1 with real-time reverse-transcription polymerase chain reaction for nasopharyngeal specimens (NPS) and anterior nasal specimens (ANS), by culture result. A, Viral culture results from NPS. B, Viral culture results from ANS.
DISCUSSION
In the current investigation, most participants positive for SARS-CoV-2 by rRT-PCR with NPS also tested positive by self-collected SS and ANS, especially participants reporting current symptoms. Agreement of results between specimen types (SS and NPS; ANS and NPS) was high for participants reporting current symptoms. Adopting less invasive specimen collection could improve testing uptake, but the benefits of SS and ANS for testing should be weighed carefully against the loss of sensitivity in asymptomatic people.
These findings suggest that several strategies might be applied to optimize SARS-CoV-2 detection in self-collected SS or ANS. When patients are unable or unwilling to undergo NPS collection, or when PPE or trained healthcare personnel are limited, self-collected SS or ANS could be offered, acknowledging that some infections might be missed that would have been detected by NPS. Notably, most participants with discordant results between the 3 specimens had NPS Ct values >30, consistent with decreased genetic material in the sample and potentially nonviable virus, which is also consistent with findings of prior studies [16–18]. In addition, when evaluating the limited number of specimens that were tested for SARS-CoV-2 by viral culture and found to have culturable virus present, rRT-PCR sensitivities were high for both SS and ANS (94% and 100%, respectively), suggesting that the lower sensitivity of SS and ANS may not be clinically relevant.
Given the low sensitivity among asymptomatic individuals, self-collected SS or ANS specimens are likely to be most useful to test people reporting current COVID-19 symptoms. Testing both SS and ANS in parallel for individual participants appeared to increase sensitivity, but this strategy may be impractical given the increased burden on laboratory resources. Pooled SS or ANS could be a subject of future research.
While SS had a modestly higher overall sensitivity than ANS for SARS-CoV-2 detection in this investigation, their sensitivity among asymptomatic participants was notably lower than among symptomatic participants and lower than has been previously reported [19]. Some participants were unable to produce saliva, while others were able to produce saliva but in volumes insufficient for testing (Figure 1). Laboratory personnel reported that SS are difficult to process and often required additional processing to yield valid results, consistent with prior reports [13, 20]. The usefulness of SS might be limited if participants are unable to produce adequate specimens or if the laboratory has limited resources to resolve saliva processing issues. One approach that has shown promise to standardize sampling of the oral cavity for SARS-CoV-2 testing is the saline mouth rinse/gargle method [21], which might overcome challenges when saliva production is limited. Because our study objective was to evaluate test performance during real-life testing events, we did not exclude participants who ate, drank, brushed their teeth, or smoked immediately before sampling, which may have affected test performance of SS [22]. Future studies that optimize sampling of the oral cavity are particularly relevant in light of recent data indicating that the oral cavity might have a direct role in SARS-CoV-2 transmission [23].
The current evaluation had several limitations. First, participants were enrolled in a single large urban area and might not be representative of other communities. Second, the changing dynamics of SARS-CoV-2 test-seeking behavior and SARS-CoV-2 incidence in Denver during the enrollment period [24, 25] limited ability to compare test performance by testing location type. Overall positivity rates in Denver during the period of study enrollment ranged from 2% in early September 2020 to 12% in November 2020. More specifically, at Denver Public Health testing events during this period, the positivity rates ranged from 2% to 33% at community sites and from 0% to 8% in homeless shelters. In addition, survey responses were self-reported; therefore, responses might be subject to social desirability or recall biases. Finally, because only a nonrandom subset of samples was evaluated by viral culture, inferential statements based on the findings reported here should be evaluated in a separate study to ensure generalizability.
Strengths of this analysis include its design using specimens collected from actual community events and inclusion of a diverse group of participants from events that frequently had relatively high positivity rates. The addition of symptom status, Ct values, and viral culture data to the qualitative rRT-PCR results supports a more complete understanding of the usefulness of ANS and SS to detect clinically meaningful SARS-CoV-2 infection.
While use of self-collected SS and ANS offers practical advantages, challenges in collecting and processing SS might be a limitation [13]. Understanding the benefits and limitations of less invasive specimen collection procedures for SARS-CoV-2 testing should inform public health efforts to design testing programs most appropriate to the local context and population and could possibly improve test uptake. Development of SARS-CoV-2 testing programs should consider differences in test sensitivity by specimen type, logistical and practical factors of offering testing in different settings, and specimen preferences by those seeking testing. For high-volume testing events, self-collected ANS are a preferred alternative to more invasive NPS by patients, are easier to collect and process than SS, and reliably detect SARS-CoV-2 among people who are symptomatic.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Participants in Denver, Colorado; Sarah Stella, MD (Denver Health and Hospital Authority); and the Colorado Coalition for the Homeless.
Group authorships. The Colorado Department of Public Health and Environment COVID-19 Laboratory Response Team includes Shannon R. Matzinger, Meghan Hudziec, Molly C. Hetherington-Rauth, Nicholas J. Pysnack, Christopher Delmonico, Kimberly Huynh-Templeman, Tara M. Stitzlein, and Alexandria E. B. Rossheim. The Centers for Disease Control and Prevention COVID-19 Laboratory Response Team includes Jennifer Folster, Magdalena Medrzycki, Phili Wong, Shilpi Jain, and Natalie Thornburg.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Supplement sponsorship. This supplement is supported by the Infectious Diseases Society of America through Cooperative Agreement NU50CK000574 with the U.S. Centers for Disease Control and Prevention.
Financial support. This work was supported by the City and County of Denver and by the Colorado Department of Public Health and Environment (testing kits and laboratory support).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Laboratory response team members are listed in the Acknowledgements.




