-
PDF
- Split View
-
Views
-
Cite
Cite
Linda Houhamdi-Hammou, Yvonne Benito, André Boibieux, Damien Dupont, François Delahaye, Françoise Thivolet-Bejui, Martine Wallon, François Vandenesch, Coralie Bouchiat, Malassezia restricta: An Underdiagnosed Causative Agent of Blood Culture-Negative Infective Endocarditis, Clinical Infectious Diseases, Volume 73, Issue 7, 1 October 2021, Pages 1223–1230, https://doi.org/10.1093/cid/ciab377
Close - Share Icon Share
Abstract
Infective endocarditis (IE) is a severe disease requiring microbial identification to successfully adapt its treatment. Currently, identification of its etiological microorganism remains unresolved in 5.2% of cases. We aimed to improve IE diagnosis using an ultra-sensitive molecular technique on cardiac samples in microbiologically nondocumented (culture and conventional polymerase chain reaction [PCR]) IE (NDIE) cases.
Cardiac samples explanted in a tertiary hospital in Lyon, France, from patients with definite IE over a 5-year period were retrospectively analyzed. NDIE was defined as Duke definite-IE associated with negative explorations including cardiac samples culture, bacterial amplification, and serologies. Ultrasensitive molecular diagnosis was achieved using the Universal Microbe Detection kit (Molzym®). Fungal identification was confirmed using 26S-rDNA and internal transcribed spacer amplifications. Fungal infection was confirmed using Grocott-Gromori staining, auto-immunohistochemistry on cardiac samples, and mannan serologies.
Among 88 included patients, microbial DNA was detected in all 16 NDIE cases. Bacterial taxa typical of IE etiologies were detected in 13/16 cases and Malassezia restricta in the 3 other cases. In these 3 cases, histological examination confirmed the presence of fungi pathognomonic of Malassezia that reacted with patient sera in an auto-immunohistochemistry assay and cross-reacted with Candida albicans in an indirect immunofluorescent assay.
M. restricta appears to be an underestimated causative agent of NDIE. Importantly, serological cross-reaction of M. restricta with C. albicans may lead to its misdiagnosis. This is of major concern since M. restricta is intrinsically resistant to echinocandins; the reference treatment for Candida-fungal IE.
INTRODUCTION
Infective endocarditis (IE) is a poor-outcome infectious disease, fatal when left untreated [1]. It is defined by the infection of a native or prosthetic heart valve, an endocardial surface, or an indwelling cardiac device. IE is usually caused by bacteria and secondarily by fungi, commonly Candida.
Rapid microbial identification is critical as antibiotic and antifungal IE treatments must be adapted to the etiologic agent to ensure successful management [2]. Blood cultures, positive in more than 90% of cases, constitute the cornerstone of IE microbiological diagnosis [3]. When they remain negative, blood culture-negative endocarditis (BCNE) are diagnosed using other microbiological tools. Serological testing is used for the detection of fastidious agents (Coxiella burnetii, Bartonella, Brucella, etc) [2, 4]. Histological examination [5], auto-immunohistochemistry [6], and broad-range polymerase chain reaction (PCR) can be applied on excised valvular tissues and vegetations [7, 8]. Despite the use of these tools, BCNE diagnosis still remains challenging, as highlighted by the 5.2% rate of nondocumented IE (NDIE) cases [1]. To address this issue we used a highly sensitive universal microbe detection (UMD) biomolecular kit (Molzym®, Bremen, Germany) allowing the simultaneous detection of bacteria and fungi [9], which permitted us the diagnosis of 3 cases of Malassezia restricta BCNE from cardiac samples excised in a context of NDIE.
METHODS
Study Design
All cardiac samples (valves and vegetations) excised from patients who had undergone surgery for IE suspicion at the cardiac surgery department of the University Hospital in Lyon, France, between 1 January 2011 and 31 December 2015, were retrospectively reviewed (Figure 1). Patients were considered to have definite NDIE when clinical and echocardiographic findings met the Duke criteria for the diagnosis of definite IE [3], along with histopathologic evidence of inflammation compatible with IE infection on the resected cardiac samples, without microbiological identification irrespective of the technique performed (10 days-incubated blood-cultures, 14 days-incubated cardiac sample cultures, in-house 16S-rDNA PCR performed on cardiac samples, and Coxiella burnetii, Bartonella, and Brucella serologies).
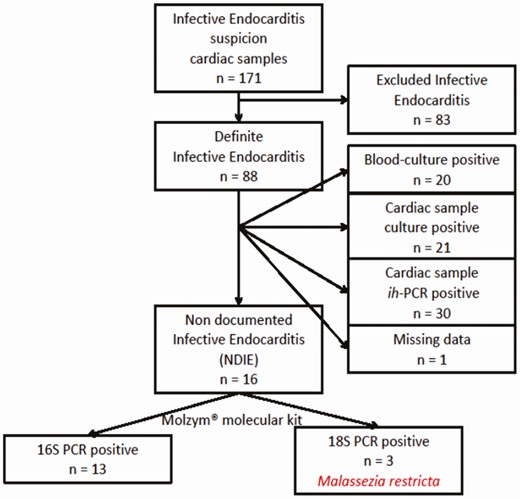
Flow chart for inclusion of cardiac samples explanted in cardiac surgery department of the University Hospital of Lyon, France, between 1 January 2011 and 31 December 2015. Excluded IE cases (n = 83) were negative on culture, histopathological examination and in-house PCR. Abbreviation: ih-PCR, in-house valvular polymerase chain reaction.
Medical history, dates of disease occurrence, clinical symptoms at presentation, echocardiographic signs, antibiotic treatment, and outcome concerning the 3 Malassezia IE patients was retrieved from their medical files. T0 was defined as the day of surgery when the a posteriori Malassezia-positive valve was excised.
Biomolecular Investigations
According to the manufacturer’s instructions, the UMD kit (Molzym®, Bremen, Germany) was used for human DNA removal, microbial DNA extraction, and Gram-positive-16S, Gram-negative-16S, and 18S-rDNA gene-amplification from the excised cardiac samples. Molecular investigations were performed under ultraviolet-decontaminated laminar flow and PCR cabinets with standard precautions to avoid DNA contamination. Amplicons from positive reactions were first purified using MinElute PCR Purification kit (Qiagen®, Courtaboeuf, France) before Sanger sequencing by a DNA sequencing provider (Biofidal®, Vaulx-en-Velin, France).
When polymicrobial infections were detected, Molzym®-PCR products were cloned into Escherichia coli using pGEM T-easy vector (Promega®, Charbonnières-les-Bains, France). Plasmid DNA were extracted, and 8–10 samples were sequenced using the Sanger method and universal T7 and M13rev primers.
Bacterial identification was performed by analyzing the 16S-rDNA sequences using “leBIBIQuick BioInformatic Phylogeny of Prokaryotes” database (https://umr5558-bibiserv.univ-lyon1.fr/lebibi/lebibi.cgi) [10]. Fungal identification was performed by analyzing the 18S-rDNA sequences using 2 public softwares: BLAST (version 2.0; National Center for Biotechnology Information; available at: http://www.ncbi.nlm.gov/BLAST/) [11] and CBS (http://www.cbs.knaw.nl/Collections/BioloMICSSequences.aspx?file=all). Moreover, fungal identification was confirmed by 2 other gene-targeted PCRs: NL1/NL4 primers targeting the D1/D2 domain of the 26S-rDNA [12], and ITS1/ITS4 targeting the full internal transcribed spacer (ITS) regions [13].
In addition to the positive and negative controls included in the UMD kit, 4 IE excluded valves were used as negative controls, and 3 definite IE valves (2 Staphylococcus aureus and 1 Tropheryma whipplei) were used as positive controls.
Histological and Auto-Immunohistochemistry Investigations
Five µm-thick sections were prepared from each formalin-fixed, paraffin-embedded excised Malassezia-positive cardiac samples. In addition to usual hematoxyline-eosin and hematoxyline-phloxine-saffron stains, these sections were used for Grocott-Gomori methenamine silver (GMS) staining and auto-immunohistochemistry [6]. Briefly, each section was incubated with the patient’s own serum, diluted to 1:500 and 1:2000, as primary antibody and goat secondary antibody directed against human immunoglobulin G (IgG) (Fc fragment specific) conjugated to peroxidase (Sigma-Aldrich®, St. Louis, MO, USA) diluted to 1:200. For each tissue section, negative and positive controls were performed using serum from an excluded IE and a Staphylococcus aureus IE, respectively.
Serological Analysis
We assessed the potential cross-reactivity between Candida albicans and M. restricta using the sera sampled from the 3 Malassezia BCNE patients. One sample from patient 1 taken at time of surgery (T0), 2 samples from patient 2 taken at T0 and 2 months before (M-2), and 4 samples from patient 3 taken at M-7, T0, 3 (M + 3) and 4 (M + 4) months after T0 were tested (Table 1).
Characteristics of the 3 Patients Infected With Malassezia restricta Blood Culture-Negative Endocarditis
| Patient no. BCNE T0-Year . | Age (years)/Sex . | Valve . | Valve Type . | Interval Between Valve Implant and Malassezia-positive Valve t0 (months) . | Relevant Clinical Features . | TTE/TOE Vegetation/abscess . | TTE/TOECardiac Dysfunction . | Histopathology . | Grocott-Gomori Staining . | Auto-immunohisto-chemistry . | 16s-rDNA PCR . | T0: 18S-26S-rDNA and ITS PCRs . | Candida albicans Serologies: 1. Mannan antigens 2. Anti-mannan antibodies (IIF-titer*, IEP-arcs**) . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. 2011 | 66/M | Aortic | Mechanical prosthesis | 15 | - Acute cardiac failure - Itching cutaneous lesions | Not applicable | - Severe paravalvular leak - Aortic insufficiency | Acute IE | Yeast-form cells (Figure 2E) | Positive | Negative | Positive: Malassezia restricta | T0 1.Negative 2.Negative (1/80, 2 arcs) | T0: Vancomycin, gentamicin and rifampicin | M + 72: Death of undocumented sepsis |
| 2. 2013 | 72/F | Aortic | Biological prosthesis | 2 | - Acute cardiac failure - Multiple septic emboli - Itching cutaneous lesions - On lipid parenteral nutrition | Peri-valvular and trigon abscess | Massive aortic insufficiency | - Acute IE - Necrosis | - Yeast-form cells - Mycelial pseudohyphae (Figures 3A/3B) | Positive | Negative | Positive: Malassezia restricta | M-2 1.Negative 2.Negative (1/80, 2 arcs) T0 1.Negative 2.Positive (1/160, 2 arcs) | M-2: Daptomycin, gentamicin, and ceftriaxone T0: Daptomycin, imipenem, and amikacin Two weeks later: Daptomyin and caspofungin | M + 1: sepsis and right flank nodule (Figure 3D) with Malassezia-positive PCR M + 6: Death of undocumented iterative sepsis |
| 3. 2015 | 42/F | Mitral | Biological valve | 7 | - Acute cardiac failure - Multiple septic emboli - On lipid parenteral nutrition | Two vegetations | Severe paravalvular leak | Acute IE | - Yeast-form cells | Positive | Negative | Positive: Malassezia restricta | M-7 1.Negative 2.Negative (1/80, 2 arcs) T0 1.Negative 2.Positive (1/160, 2 arcs) M + 3 1.Negative 2.Positive (1/320, 2 arcs) M + 4 1.Negative 2.Negative (1/40, 2 arcs) | M-7: Daptomycin, fosfomycin, and ciprofloxacin T0: Daptomycin, imipenem, and ciprofloxacin M + 3: Amphotrericin B-fluorocytosin, daptomycin, imipenem, and ciprofloxacin M + 4: Fluconazole | M + 3: New vegetation. Introduction of amphotericin B-fluorocytosin. M + 4: Recovery |
| Patient no. BCNE T0-Year . | Age (years)/Sex . | Valve . | Valve Type . | Interval Between Valve Implant and Malassezia-positive Valve t0 (months) . | Relevant Clinical Features . | TTE/TOE Vegetation/abscess . | TTE/TOECardiac Dysfunction . | Histopathology . | Grocott-Gomori Staining . | Auto-immunohisto-chemistry . | 16s-rDNA PCR . | T0: 18S-26S-rDNA and ITS PCRs . | Candida albicans Serologies: 1. Mannan antigens 2. Anti-mannan antibodies (IIF-titer*, IEP-arcs**) . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. 2011 | 66/M | Aortic | Mechanical prosthesis | 15 | - Acute cardiac failure - Itching cutaneous lesions | Not applicable | - Severe paravalvular leak - Aortic insufficiency | Acute IE | Yeast-form cells (Figure 2E) | Positive | Negative | Positive: Malassezia restricta | T0 1.Negative 2.Negative (1/80, 2 arcs) | T0: Vancomycin, gentamicin and rifampicin | M + 72: Death of undocumented sepsis |
| 2. 2013 | 72/F | Aortic | Biological prosthesis | 2 | - Acute cardiac failure - Multiple septic emboli - Itching cutaneous lesions - On lipid parenteral nutrition | Peri-valvular and trigon abscess | Massive aortic insufficiency | - Acute IE - Necrosis | - Yeast-form cells - Mycelial pseudohyphae (Figures 3A/3B) | Positive | Negative | Positive: Malassezia restricta | M-2 1.Negative 2.Negative (1/80, 2 arcs) T0 1.Negative 2.Positive (1/160, 2 arcs) | M-2: Daptomycin, gentamicin, and ceftriaxone T0: Daptomycin, imipenem, and amikacin Two weeks later: Daptomyin and caspofungin | M + 1: sepsis and right flank nodule (Figure 3D) with Malassezia-positive PCR M + 6: Death of undocumented iterative sepsis |
| 3. 2015 | 42/F | Mitral | Biological valve | 7 | - Acute cardiac failure - Multiple septic emboli - On lipid parenteral nutrition | Two vegetations | Severe paravalvular leak | Acute IE | - Yeast-form cells | Positive | Negative | Positive: Malassezia restricta | M-7 1.Negative 2.Negative (1/80, 2 arcs) T0 1.Negative 2.Positive (1/160, 2 arcs) M + 3 1.Negative 2.Positive (1/320, 2 arcs) M + 4 1.Negative 2.Negative (1/40, 2 arcs) | M-7: Daptomycin, fosfomycin, and ciprofloxacin T0: Daptomycin, imipenem, and ciprofloxacin M + 3: Amphotrericin B-fluorocytosin, daptomycin, imipenem, and ciprofloxacin M + 4: Fluconazole | M + 3: New vegetation. Introduction of amphotericin B-fluorocytosin. M + 4: Recovery |
T0: Date of the heart surgery where Malassezia restricta was detected in the cardiac sample. M-X: X months before heart surgery performed on T = T0. M + X: X months following heart surgery performed on T = T0. IIF-titer*: Indirect immunofluorescence-screening assay; positive if titer ≥ 1/160. IEP-arcs**: Immunoelectrophoresis-confirmation assay; positive if ≥ 1 arc.
Abbreviations: BCNE, blood culture-negative endocarditis; ITS, internal transcribed spacer; TOE, transoesophageal echocardiography; TTE, transthoracic echocardiography.
Characteristics of the 3 Patients Infected With Malassezia restricta Blood Culture-Negative Endocarditis
| Patient no. BCNE T0-Year . | Age (years)/Sex . | Valve . | Valve Type . | Interval Between Valve Implant and Malassezia-positive Valve t0 (months) . | Relevant Clinical Features . | TTE/TOE Vegetation/abscess . | TTE/TOECardiac Dysfunction . | Histopathology . | Grocott-Gomori Staining . | Auto-immunohisto-chemistry . | 16s-rDNA PCR . | T0: 18S-26S-rDNA and ITS PCRs . | Candida albicans Serologies: 1. Mannan antigens 2. Anti-mannan antibodies (IIF-titer*, IEP-arcs**) . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. 2011 | 66/M | Aortic | Mechanical prosthesis | 15 | - Acute cardiac failure - Itching cutaneous lesions | Not applicable | - Severe paravalvular leak - Aortic insufficiency | Acute IE | Yeast-form cells (Figure 2E) | Positive | Negative | Positive: Malassezia restricta | T0 1.Negative 2.Negative (1/80, 2 arcs) | T0: Vancomycin, gentamicin and rifampicin | M + 72: Death of undocumented sepsis |
| 2. 2013 | 72/F | Aortic | Biological prosthesis | 2 | - Acute cardiac failure - Multiple septic emboli - Itching cutaneous lesions - On lipid parenteral nutrition | Peri-valvular and trigon abscess | Massive aortic insufficiency | - Acute IE - Necrosis | - Yeast-form cells - Mycelial pseudohyphae (Figures 3A/3B) | Positive | Negative | Positive: Malassezia restricta | M-2 1.Negative 2.Negative (1/80, 2 arcs) T0 1.Negative 2.Positive (1/160, 2 arcs) | M-2: Daptomycin, gentamicin, and ceftriaxone T0: Daptomycin, imipenem, and amikacin Two weeks later: Daptomyin and caspofungin | M + 1: sepsis and right flank nodule (Figure 3D) with Malassezia-positive PCR M + 6: Death of undocumented iterative sepsis |
| 3. 2015 | 42/F | Mitral | Biological valve | 7 | - Acute cardiac failure - Multiple septic emboli - On lipid parenteral nutrition | Two vegetations | Severe paravalvular leak | Acute IE | - Yeast-form cells | Positive | Negative | Positive: Malassezia restricta | M-7 1.Negative 2.Negative (1/80, 2 arcs) T0 1.Negative 2.Positive (1/160, 2 arcs) M + 3 1.Negative 2.Positive (1/320, 2 arcs) M + 4 1.Negative 2.Negative (1/40, 2 arcs) | M-7: Daptomycin, fosfomycin, and ciprofloxacin T0: Daptomycin, imipenem, and ciprofloxacin M + 3: Amphotrericin B-fluorocytosin, daptomycin, imipenem, and ciprofloxacin M + 4: Fluconazole | M + 3: New vegetation. Introduction of amphotericin B-fluorocytosin. M + 4: Recovery |
| Patient no. BCNE T0-Year . | Age (years)/Sex . | Valve . | Valve Type . | Interval Between Valve Implant and Malassezia-positive Valve t0 (months) . | Relevant Clinical Features . | TTE/TOE Vegetation/abscess . | TTE/TOECardiac Dysfunction . | Histopathology . | Grocott-Gomori Staining . | Auto-immunohisto-chemistry . | 16s-rDNA PCR . | T0: 18S-26S-rDNA and ITS PCRs . | Candida albicans Serologies: 1. Mannan antigens 2. Anti-mannan antibodies (IIF-titer*, IEP-arcs**) . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. 2011 | 66/M | Aortic | Mechanical prosthesis | 15 | - Acute cardiac failure - Itching cutaneous lesions | Not applicable | - Severe paravalvular leak - Aortic insufficiency | Acute IE | Yeast-form cells (Figure 2E) | Positive | Negative | Positive: Malassezia restricta | T0 1.Negative 2.Negative (1/80, 2 arcs) | T0: Vancomycin, gentamicin and rifampicin | M + 72: Death of undocumented sepsis |
| 2. 2013 | 72/F | Aortic | Biological prosthesis | 2 | - Acute cardiac failure - Multiple septic emboli - Itching cutaneous lesions - On lipid parenteral nutrition | Peri-valvular and trigon abscess | Massive aortic insufficiency | - Acute IE - Necrosis | - Yeast-form cells - Mycelial pseudohyphae (Figures 3A/3B) | Positive | Negative | Positive: Malassezia restricta | M-2 1.Negative 2.Negative (1/80, 2 arcs) T0 1.Negative 2.Positive (1/160, 2 arcs) | M-2: Daptomycin, gentamicin, and ceftriaxone T0: Daptomycin, imipenem, and amikacin Two weeks later: Daptomyin and caspofungin | M + 1: sepsis and right flank nodule (Figure 3D) with Malassezia-positive PCR M + 6: Death of undocumented iterative sepsis |
| 3. 2015 | 42/F | Mitral | Biological valve | 7 | - Acute cardiac failure - Multiple septic emboli - On lipid parenteral nutrition | Two vegetations | Severe paravalvular leak | Acute IE | - Yeast-form cells | Positive | Negative | Positive: Malassezia restricta | M-7 1.Negative 2.Negative (1/80, 2 arcs) T0 1.Negative 2.Positive (1/160, 2 arcs) M + 3 1.Negative 2.Positive (1/320, 2 arcs) M + 4 1.Negative 2.Negative (1/40, 2 arcs) | M-7: Daptomycin, fosfomycin, and ciprofloxacin T0: Daptomycin, imipenem, and ciprofloxacin M + 3: Amphotrericin B-fluorocytosin, daptomycin, imipenem, and ciprofloxacin M + 4: Fluconazole | M + 3: New vegetation. Introduction of amphotericin B-fluorocytosin. M + 4: Recovery |
T0: Date of the heart surgery where Malassezia restricta was detected in the cardiac sample. M-X: X months before heart surgery performed on T = T0. M + X: X months following heart surgery performed on T = T0. IIF-titer*: Indirect immunofluorescence-screening assay; positive if titer ≥ 1/160. IEP-arcs**: Immunoelectrophoresis-confirmation assay; positive if ≥ 1 arc.
Abbreviations: BCNE, blood culture-negative endocarditis; ITS, internal transcribed spacer; TOE, transoesophageal echocardiography; TTE, transthoracic echocardiography.
C. albicans mannan-antigens were assessed by a quantitative immunoenzymatic assay using SERION ELISA® antigen Candida kit (Institut Virion-Serion®, GmbH, Würzburg, Germany), according to the manufacturer’s instructions. C. albicans anti-mannan antibodies were assessed simultaneously by screening and confirmation assays. Screening assay was performed by indirect immunofluorescence (IIF) using the routine in-house technical protocol demonstrating the binding of the antibodies (serum diluted to 1/20) on a C. albicans-antigens suspension using a fluorescent conjugate (anti-IgG human antiglobulins labeled with fluorescein isothiocyanate). Negative and C. albicans antigen-positive controls were used in both assays. Confirmation assay was performed by immunoelectrophoresis, as described previously by Graber and Williams [14].
Ethics Committee Approval
This project was conducted according to the French law in place at the time of the study and ethical principles outlined in the Declaration of Helsinki. It obtained approval by the institutional ethics committee.
RESULTS
Biomolecular Investigations
Between January 2011 and December 2015, 171 cardiac samples were excised for IE suspicion (Figure 1). Of these, 88 were excised from patients who met the Duke criteria for definite IE [3], and for whom the microbiological etiology was retrieved either by blood (n = 20), cardiac sample (n = 21) cultures, or in-house PCR on cardiac samples (n = 30; missing data, n = 1). The 16 remaining cardiac samples were thus considered NDIE-samples (ie, culture- and in-house 16S-rDNA PCR-negative), which originated from prosthetic mitral (n = 9) or aortic (n = 7) valves. Cardiac samples were explanted from 6 men (37.5%), the mean age of whom was 58 (range: 25–82) years.
All 16 NDIE cardiac samples were positive using the UMD-biomolecular test (Figure 1). Thirteen samples were 16S-PCR positive and 18S/26S/ITS-PCR negative, yielding typical IE causative bacteria such as Streptococcus (n = 9; Table 2). Eight of these 13 samples showed a polymicrobial etiology. Interestingly, the 3 other samples belonging to 3 different patients over 5 years were found to be 16S-PCR negative but 18S-PCR positive with Malassezia restricta identification (accession numbers: MW767157, MW767158, and MW767159), confirmed using ITS- and 26S-PCRs.
Microorganisms Identified in the 16 Cardiac Samples Explanted From Patients With Nondocumented Infective Endocarditis Between 1 January 2011 and 31 December 2015
| Identified Microorganisms . | No. of Positive Samples N = 16 . |
|---|---|
| Streptococci | 3 |
| Streptococcus mitis | 2 |
| Streptococcus cristatus | 1 |
| Enterococcus cecorum | 1 |
| Moraxella spp. | 1 |
| ≥ 2 microorganisms | 8 |
| Streptococcus Oral taxon + S. ralis | 4 |
| Streptococcus sinensis + S. oligofermentans | 1 |
| Enterococcus faecalis + E. cecorum | 1 |
| Abiotrophia defective + Neisseria pharyngia | 1 |
| Streptococcus Oral Taxon + Neisseria subflava/sicca | 1 |
| Malassezia restricta | 3 |
| Identified Microorganisms . | No. of Positive Samples N = 16 . |
|---|---|
| Streptococci | 3 |
| Streptococcus mitis | 2 |
| Streptococcus cristatus | 1 |
| Enterococcus cecorum | 1 |
| Moraxella spp. | 1 |
| ≥ 2 microorganisms | 8 |
| Streptococcus Oral taxon + S. ralis | 4 |
| Streptococcus sinensis + S. oligofermentans | 1 |
| Enterococcus faecalis + E. cecorum | 1 |
| Abiotrophia defective + Neisseria pharyngia | 1 |
| Streptococcus Oral Taxon + Neisseria subflava/sicca | 1 |
| Malassezia restricta | 3 |
Microorganisms Identified in the 16 Cardiac Samples Explanted From Patients With Nondocumented Infective Endocarditis Between 1 January 2011 and 31 December 2015
| Identified Microorganisms . | No. of Positive Samples N = 16 . |
|---|---|
| Streptococci | 3 |
| Streptococcus mitis | 2 |
| Streptococcus cristatus | 1 |
| Enterococcus cecorum | 1 |
| Moraxella spp. | 1 |
| ≥ 2 microorganisms | 8 |
| Streptococcus Oral taxon + S. ralis | 4 |
| Streptococcus sinensis + S. oligofermentans | 1 |
| Enterococcus faecalis + E. cecorum | 1 |
| Abiotrophia defective + Neisseria pharyngia | 1 |
| Streptococcus Oral Taxon + Neisseria subflava/sicca | 1 |
| Malassezia restricta | 3 |
| Identified Microorganisms . | No. of Positive Samples N = 16 . |
|---|---|
| Streptococci | 3 |
| Streptococcus mitis | 2 |
| Streptococcus cristatus | 1 |
| Enterococcus cecorum | 1 |
| Moraxella spp. | 1 |
| ≥ 2 microorganisms | 8 |
| Streptococcus Oral taxon + S. ralis | 4 |
| Streptococcus sinensis + S. oligofermentans | 1 |
| Enterococcus faecalis + E. cecorum | 1 |
| Abiotrophia defective + Neisseria pharyngia | 1 |
| Streptococcus Oral Taxon + Neisseria subflava/sicca | 1 |
| Malassezia restricta | 3 |
Histological and Auto-Immunohistochemistry Investigations
IE was histologically proven for all 16 patients based on the presence of inflammatory infiltrates (neutrophils) and fibrin layers in cardiac samples (Figures 2A–D, F, and G). Histological examination of GMS-stained cardiac sample sections from the 3 M. restricta patients yielded single rounded or oval yeast-form cells 2–3μm in length axis in patients 1 (Figure 2E) and 3 (not shown), and multiple short unbranched mycelial pseudohyphae with some budding yeasts in patient 2 (Figure 3A, B).
![Excised prosthetic aortic valve of patient 1 infected with Malassezia restricta blood culture-negative endocarditis. A, B, Hematoxyline-eosin stain showing superficial vegetations (asterisk) and fibrosis of the connective valve tissue (black arrow) (original magnification, ×200 [A] and ×400 [B]). C, D, Hematoxyline-phloxine-saffron stain showing the vegetation (asterisk) and calcifications (black arrows) on the valve surface (original magnification, ×100 [C], ×200 [D]). E, Grocott-Gomori methenamine silver stain showing single spherical black yeasts in the valvular vegetation, with some budding cells (red arrow) (original magnification, ×400). F, G, Periodic Acid-Schiff stain showing dense fibrocollagenous tissue, sometimes punctuated by macrophagic granulomas and fibronoleucocyte infiltrates (original magnification, ×200 [F] and ×400 [G]). H, Auto-immunohistochemistry using the patient’s own serum diluted to 1:500. Note the brown vegetation (red arrow) (original magnification, ×100).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/73/7/10.1093_cid_ciab377/1/m_ciab377f0002.jpeg?Expires=1750228828&Signature=ZIPFCbZ4AygVjl91hR3IpRWd~AFgIPNZk2tvFgicI2JY8N0L29kzlnIAQZ9X23B1hobp~h2dSxjkjt9X0rC4kwqSWd1zW9XcFDbQotpBtnplrDqZ2sj2xtSHVNrllrPEv-aSwDrPt~7iablNB3-u~SXwpN1LQm0F7bXAYHG1K4DJM9Ry2lTpGZLtjuqzDn5nH5jyDx8O~UewcdSboK41q-W0HFF7ZjiPa1XiX6e1doODdaPLwMuUTj~ZJWKqvsBJ91-Mmxcn9wvyYGyZCY11nV92AgHm58HTaiqMBMdY4K4Yl42BWwv89RYxJVgluK0Js9ldoNz3ThKQPubsFqicxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Excised prosthetic aortic valve of patient 1 infected with Malassezia restricta blood culture-negative endocarditis. A, B, Hematoxyline-eosin stain showing superficial vegetations (asterisk) and fibrosis of the connective valve tissue (black arrow) (original magnification, ×200 [A] and ×400 [B]). C, D, Hematoxyline-phloxine-saffron stain showing the vegetation (asterisk) and calcifications (black arrows) on the valve surface (original magnification, ×100 [C], ×200 [D]). E, Grocott-Gomori methenamine silver stain showing single spherical black yeasts in the valvular vegetation, with some budding cells (red arrow) (original magnification, ×400). F, G, Periodic Acid-Schiff stain showing dense fibrocollagenous tissue, sometimes punctuated by macrophagic granulomas and fibronoleucocyte infiltrates (original magnification, ×200 [F] and ×400 [G]). H, Auto-immunohistochemistry using the patient’s own serum diluted to 1:500. Note the brown vegetation (red arrow) (original magnification, ×100).
![Excised aortic valve (A–C) and right flank nodule (D) of patient 2 infected with Malassezia restricta blood culture negative endocarditis. A, B, Grocott-Gomori methenamine silver stain showing fungal elements stained darkly and organized in short pseudohyphae disclosing their invasive character (red arrows), with some budding yeasts (blue arrowhead). Original magnification, ×200 (A) and ×1000 (B). C, D, Auto-immunohistochemistry using the patient’s own serum diluted to 1:500 in the excised aortic cardiac valve (C. Note the brown vegetations [red arrows]) and the right flank nodule developed 5 months before her death (D) (original magnification, ×25 [C] and ×200 [D]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/73/7/10.1093_cid_ciab377/1/m_ciab377f0003.jpeg?Expires=1750228828&Signature=MvbeouSPWbaCeOPe7rVo0Qp-OXlUQ-hRLF2WL~T08nPwyw1qrSGf2Wr7-iXf158P9jAvOll1EEIaoktVjb5trfeIM6XRyHS9n7oC2MKPF-SHqTRNNGQjHHF-wrIWhYqSH~rnZ0WB6wA5EOmsumD~r9k3FgCheexpLco8G-LH2egV8681GArs-W6n60GoxHCYKxkz5h52IiTS5jLdgB58IDHcHLKW4BR4KJfqWclSMkfSf7xj~qQGNlXn4a~WUJG8e080V-0rLo4ZB0LrEi~GZO4nhL3k8zQPiwuyV2RIXvOW50YkYkFkp6doDYoQByIudoO4PH26OtHFsTQM11okfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Excised aortic valve (A–C) and right flank nodule (D) of patient 2 infected with Malassezia restricta blood culture negative endocarditis. A, B, Grocott-Gomori methenamine silver stain showing fungal elements stained darkly and organized in short pseudohyphae disclosing their invasive character (red arrows), with some budding yeasts (blue arrowhead). Original magnification, ×200 (A) and ×1000 (B). C, D, Auto-immunohistochemistry using the patient’s own serum diluted to 1:500 in the excised aortic cardiac valve (C. Note the brown vegetations [red arrows]) and the right flank nodule developed 5 months before her death (D) (original magnification, ×25 [C] and ×200 [D]).
Auto-immunohistochemistry reactions using the patients’ own sera were positive in their respective cardiac samples (Figures 2H and 3C), whereas sera from negative and positive control patients gave expected results.
Serological Analysis
Serological analyses of the 3 patients did not show any cross-reaction with mannan-antigens (Table 1). However, sera sampled from patients 2 and 3 cross-reacted with C. albicans anti-mannan antibodies in both the screening and confirmation assays.
Anti-mannan antibody serology kinetics was investigated for patients 2 and 3, revealing that both patients were slightly positive at T0 (IIF-screening titer of 1/160), although they were negative 2 and 7 months before, respectively. Interestingly, IIF-screening antibody titers of patient 3 increased 3 months after surgery (1/320) and were negative 4 months after surgery, that is, 1 month after amphotericin B-fluorocytosin therapy initiation.
Malassezia restricta Blood Culture-Negative Endocarditis: Three-Cases Presentation
When reviewing the medical history of the 3 patients, all of them had undergone cardiac valvular prosthesis implantation in the 2–15 months before T0. They were admitted to the hospital for an impaired general condition and moderate-to-severe heart failure with fever episodes (Table 1). Transthoracic echocardiography (TTE) and/or transoesophageal echocardiography (TEE) revealed paravalvular regurgitation due to prosthesis dehiscence in all patients, a trigone abscess (patient 2), and 2 vegetations (patient 3). Whole body computerized tomography scan showed that patients 2 and 3 had images compatible with multiple septic emboli.
Patient 1 (BCNE occurred in 2011), a 66-year-old male, had a new mechanical prosthesis implanted and was treated by vancomycin, gentamicin, and rifampicin at T0. He never received any antifungal therapy due to the lack of microbiological documentation. He died 6 years later of heart failure in a nondocumented febrile context.
Patient 2 (BCNE occurred in 2013), a 72-year-old female, had a first valvular replacement 2 months before T0 in a context of NDIE, which was treated by daptomycin, gentamicin, and ceftriaxone for 4 weeks. At T0, daptomycin, amikacin, and imipenem were introduced, and the patient underwent another biological valvular replacement. Caspofungin was added 2 weeks later when she suffered from an undocumented sepsis and the appearance of a subcutaneous nodule on the right flank (for which 18S-PCR as well as autoimmunochemistry turned out to be positive for M. restricta; Figure 3D). Two months later, TEE found the occurrence of para-prosthetic abscess. The patient died 6 months later in a context of iterative nondocumented sepsis.
Patient 3 (BCNE occurred in 2015), a 42-year-old female, underwent 2 mitral valvular replacements in the 10 months before T0 for iterative NDIE initially treated by amoxicillin and gentamicin and then by daptomycin, ciprofloxacin, and fosfomycin. At T0, daptomycin, ciprofloxacin, and imipenem were initiated concomitantly with the third mitral surgery. Three months later, the presence of a new vegetation on the prosthesis found upon TEE led to the addition of amphotericin B and fluorocytosin. Clearance of the vegetation was seen one month later; the patient was maintained on fluconazole thereafter, according to the available information during investigations.
The 3 patients had either itchy skin lesions or lipid parenteral nutrition weeks to months before the onset of clinical symptoms.
DISCUSSION
Approximately 20% of nondocumented IE (NDIE) cardiac samples were found positive using UMD Molzym® ultrasensitive biomolecular kit, confirming its capacity to overcome diagnostic dead-ends, and 3 of these were positive for M. restricta. A combination of clinical and molecular evidence, along with the results of GMS, autoimmunohistochemistry, and serologies confirmed the diagnosis of M. restricta IE.
A bacterial etiology was established in about 80% of cases, mainly yielding streptococci. This finding is in line with reported etiologies of BCNE that are often caused by empiric antibiotic treatments that impair the growth of streptococci and other beta-lactam highly susceptible microorganisms [2]. Importantly, half of NDIE samples were positive with 2 bacteria, usually including 2 streptococci or oral Neisseria species. Although it is plausible that both these bacteria were the causative agents, histological examinations of the samples concluded to an acute IE in all cases.
Malassezia are lipophilic lipodependent yeasts belonging to the class of Basidiomycetes, which are part of the normal skin microbiota. Among the 14 identified Malassezia species, M. restricta, M. globosa, and M. sympodialis constitute the most often isolated species on healthy human skin [15–18]. Malassezia are best known as fungi of low pathogenicity responsible for superficial cutaneous infections, such as pityriasis versicolor, seborrheic dermatitis, or atopic dermatitis [15]. However, their clinical spectrum has expanded drastically because fatal pulmonary vasculitis reported in a premature infant receiving total parenteral nutrition via an indwelling catheter [19]. Currently, there are at least 50 reported cases of M. furfur and M. pachydermatis (but never M. restricta) in neonates [20–22], infants [21, 23–25] and adults [21, 26–31], responsible for systemic infections such as peritonitis [29], pulmonary vasculitis [19], pneumonia [21, 22], and fungemia with or without IE [24, 25, 30, 31]. Recently, 1 case of adult IE caused by M. furfur has been reported [31], but none were caused by M. restricta.
Here we aimed to ascertain the diagnosis of M. restricta IE using multiple tools. First, fungal species identification was performed using 3 different fungal genes (18S, 26S, and ITS) that are known to be sufficiently variable to allow reliable discrimination between clinically relevant yeast species [12]. Because of their low pathogenicity, Malassezia can easily be considered as a contaminant when they are detected in human clinical samples. In the present study, the use of several controls including positive and negative cardiac samples, in addition to the absence of Malassezia DNA detection in all the other 13 BCNE cardiac samples allowed us to rule out the contamination hypothesis. Second, proven invasive fungal disease requires demonstration in diseased tissue either by histo/cytopathology microscopic analysis or culture [32]. Here GMS and periodic acid Schiff were positive in all 3 samples in the same cardiac sample zone where histological examination revealed neutrophil infiltrates confirming the clinical significance of the findings. Moreover, auto-immunohistochemistry demonstrated that the patients had mounted an immune response to the organisms identified in the tissues. However, routine culture of cardiac samples remained sterile. This could be explained by the fact that Malassezia lack the ability to synthesize medium and long-chain fatty acids and thus require dedicated culture medium containing lipids to grow. Interestingly, all 3 Malassezia-IE patients presented potential portals of entry, that is, the use of SmofKabiven®-lipidic parenteral nutrition (different batches) for weeks to months before diagnosis for 2 patients and presence of skin lesions for the third. This suggests that patients under lipid hyperalimentation regimen and presenting a sepsis should be investigated for lipophylic yeast infections. Although the exact mechanism for Malassezia IE remains unknown, there are many hypotheses. The most likely source of infection is the human skin, from the patient or the healthcare worker, which contaminates the patient indwelling catheters [24, 33]. Another hypothesized infection source includes a prior contamination of the parenteral nutrition [24, 33]. In this series, nosocomial transmission from healthcare workers’ skin seems to be unlikely as the 3 IE cases occurred at different times (an interval of 2 years) with different cardiac surgeons, and no other case has been detected to date in our institution.
All 3 patients with M. restricta IE presented 1 (patients 1 and 2) or 2 (patient 3) episode(s) of IE prior to T0. Unfortunately, no prior cardiac sample was available to test whether M. restricta may have been detected in previous episodes. Indeed, detection of another pathogen in theses samples would indicate that M. restricta could act as an opportunist, whereas its prior and/or subsequent detection would have proven its ability to persist, recur, and/or evade clearance.
The present findings raise the issue of the diagnosis of such Malassezia IE. Symptoms appear to be nonspecific, with fever and cardiac failure as the only manifestations for all 3 patients reported here. Moreover, because Malassezia does not grow on routine culture media unless supplemented with fatty acids, the Malassezia species needs to be specifically searched for [34]. Novel metagenomic next-generation sequencing-based cell-free DNA plasma assays may also be a promising approach [35, 36]. In addition, serum samples from 2 patients cross-reacted with C. albicans at T0 and even 3 months later for 1 of them, although they were negative before T0. This cross-reactivity has already been reported between Candida albicans and M. furfur [37] due to the similarity of mannan-antigens among yeasts. Unfortunately, there was no serum leftovers in the present study to test 1,3-β-D-glucan cross-reaction between these fungal species. Because fungal serology constitutes a precious diagnostic tool when valve tissues are unavailable, this sero-cross-reactivity can dramatically impact IE management and specifically the use of echinocandins; international guidelines recommend high-dose echinocandins to treat Candida IE [38–40], although Malassezia is intrinsically resistant to echinocandins. Here 2 of the 3 patients had received antifungals. Although 1 died from recurrent nondocumented sepsis under caspofungin, the other recovered with clinical and TEE improvement on amphotericin B-fluorocytosine. Interestingly, the serum of the latter was negative 1 month following the beginning of the antifungal bitherapy.
In conclusion, to the best of our knowledge, this study reports for the first time the commensal skin-yeast M. restricta as an IE causative etiology. Diagnosis of Malassezia IE is not easy, and its prevalence could be underestimated due to serological cross-reaction with C. albicans. Although rare, Malassezia etiology should be better recognized and actively searched for, particularly in case of BCNE. Therefore, GMS staining and fungal PCR should be systematically performed on cardiac samples when usual IE etiologies are not retrieved, as recommended [2]. This might represent a key point for the successful management of fungal IE that requires the use of appropriate antifungal therapy adapted to the causative agent.
Notes
Financial support. The research described in this manuscript was not supported by a specific grant.
Conflicts of interest. F. V. reports grants from Pathoquest and bioMérieux, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data availaibility statement. 18S sequences have been deposited on ENA with the following accession numbers MW767157 for patient 1, MW767158 for patient 2, and MW767159 for patient 3.
References
Author notes
Equal senior authorship.




