-
PDF
- Split View
-
Views
-
Cite
Cite
Vinh Vu Hai, Yusuke Shimakawa, Jin Kim, Hai Do Ngoc, Quang Le Minh, Didier Laureillard, Maud Lemoine, Assessment and Simplification of Treatment Eligibility Among Patients With Chronic Hepatitis B Infection in Vietnam, Clinical Infectious Diseases, Volume 73, Issue 5, 1 September 2021, Pages e1072–e1077, https://doi.org/10.1093/cid/ciaa1814
Close - Share Icon Share
Abstract
Treatment eligibility and the accuracy of its simplified criteria have been poorly documented in patients with chronic hepatitis B virus (HBV) infection worldwide, especially in low- and middle-income countries.
From a cohort of HBV-infected patients in Vietnam, we assessed the proportion of patients eligible for treatment using the national guidelines based on reference tests (HBV DNA quantification and FibroScan); and the accuracy of simplified treatment criteria free from HBV DNA and FibroScan (Treatment Eligibility in Africa for the Hepatitis B Virus [TREAT-B] score and simplified World Health Organization [WHO] criteria) to select patients for antiviral therapy using the national guidelines as a reference.
We analyzed 400 consecutive treatment-naïve HBV-monoinfected patients: 49% males, median age 38 years (range, 18–86), 32% hepatitis B e antigen-positive, median HBV DNA 4.8 log10 IU/mL (undetectable −8.4), median FibroScan 5.3 kPa (3.0–67.8), and 25% having significant liver fibrosis including 12% with cirrhosis. Of these, 167 (42%) fulfilled treatment criteria according to national guidelines. Using the national criteria as a reference, the performance of TREAT-B to select patients for treatment was high (area under the receiver operating characteristic [AUROC], 0.89 [95% confidence interval 0.87-0.92]) with a sensitivity of 74.3% and a specificity of 88.4%. In a subset of patients with 2 alanine aminotransferase measurements over a 6-month period (n = 89), the AUROC of TREAT-B was significantly higher than that of the simplified WHO criteria (P < .001).
Our study suggests that a large proportion of patients with chronic HBV infection require antiviral therapy in Vietnam. Compared with the simplified WHO criteria free from HBV DNA quantification, TREAT-B is a better alternative to easily indicate treatment eligibility and might help scale up treatment intervention in Vietnam.
(See the Editorial Commentary by Vinikoor on pages e1078–9.)
Chronic infection with hepatitis B virus (HBV) affects 292 million people worldwide and is responsible for 887 000 deaths annually, mainly from cirrhosis and hepatocellular carcinoma (HCC) [1].
In 2016, the World Health Organization (WHO) set up an ambitious strategy to eliminate viral hepatitis as a public threat by 2030 [2, 3]. In patients with chronic HBV infection, antiviral treatment initiation is generally recommended if moderate or severe liver inflammation and/or fibrosis coexist with an ongoing viral replication [4–6]. However, the recommended tools to assess these conditions (ie, nucleic acid test to measure HBV DNA levels and liver biopsy or liver elastography [FibroScan]) to evaluate fibrosis stage are expensive and hardly accessible in most low- and middle-income countries (LMICs) [7, 8].
To address this issue and improve treatment coverage in LMICs, in its 2015 guidelines, the WHO added a conditional recommendation to consider treatment based on persistently abnormal alanine transaminase (ALT) levels alone, if HBV DNA testing is not available [6]. Without undertaking expensive tests (HBV DNA quantification and FibroScan), antiviral treatment can be initiated in (1) case of cirrhosis defined clinically or biologically using aspartate aminotransferase-to-platelet ratio index > 2.0 or (2) case of persistently elevated ALT on 3 ALT measurements over a period of 6 to 12 months [6].
Recently, we developed a simple score named Treatment Eligibility in Africa for the Hepatitis B Virus (TREAT-B), free from HBV DNA quantification and liver fibrosis measurements, based on only serum hepatitis B e antigen (HBeAg) serostatus and ALT levels [9], and found higher accuracy of TREAT-B compared with the simplified WHO treatment criteria to identify HBV-infected African patients who require antiviral therapy [9]. The performance of TREAT-B score for the identification of patients with chronic HBV infection in need of antiviral therapy has been also assessed in subsequent African, European, and Australian studies [10–15]; however, this has never been assessed in an Asian country.
The HBV burden is of particular importance in Asia, where >75% of people chronically infected with HBV worldwide reside and from where >70% of new HCC cases diagnosed worldwide arise [16]. The current HBV treatment coverage is below 5% in most Asian LMICs [1, 17], making unrealistic the increase of treatment coverage to 80% by 2030 as recommended by WHO [1, 2].
Vietnam is highly endemic for HBV infection with an estimated prevalence >8% in the general adult population [18] and up to 15% in subgroups such as people who inject drugs or female sex workers [17]. In 2017, Vietnam accounted 15 868 deaths attributable to HBV; between 2007 and 2017, the number of deaths from cirrhosis increased by 26.5%, with HBV being the main cause.
In 2015, the Vietnamese Ministry of Health developed a national strategic plan for viral hepatitis and released guidelines for the diagnosis and treatment of chronic hepatitis B. These guidelines based on complex and expensive tests are difficult to implement in remote and rural areas. Moreover, the proportion of HBV-infected subjects in need of antiviral therapy according to these national guidelines or international criteria has never been documented in Vietnam.
We aimed to fill this gap by assessing, in a cohort of treatment-naïve HBV monoinfected patients in Hai Phong, Northern Vietnam: (1) the proportion of patients with significant liver disease in need of antiviral therapy and (2) the accuracy of simplified criteria free from HBV DNA quantification and FibroScan (TREAT-B score and the simplified WHO treatment criteria) to select patients for antiviral therapy using the national treatment criteria as a reference.
METHODS
Study Population
We conducted a retrospective analysis using an electronic record system of all adult patients who attended the outpatient liver clinic at Viet Tiep hospital, Northern Vietnam, from the 1 January 2017 to 31 December 2018. This hospital is the referral hospital for HBV infection in the region. We excluded from the analysis patients with prior or current HBV antiviral treatment, co-infection with hepatitis C virus (HCV) or human immunodeficiency virus, pregnancy, HCC, and intrahepatic mass, or missing clinical or virological data. Coinfection with hepatitis D virus (HDV) was however not excluded because HDV serology is not done routinely in Vietnam. We also excluded patients whose liver stiffness measurement (LSM) using FibroScan (FibroScan 402, Echosens, France) was unreliable, defined as a ratio of interquartile range divided by the median LSM exceeding 0.3, when the LSM was ≥7.1 kPa. FibroScan was performed in a fasting state in all patients. Liver transaminases (ALT, aspartate transaminase) levels were measured locally at the biochemistry laboratory, HBeAg was detected using a rapid point-of-care test (SD Bioline, South Korea), and HBV DNA was measured using an in-house reverse transcriptase-polymerase chain reaction (Viet-A Corp., Vietnam) approved by the Vietnamese Ministry of Health, with a lower limit of detection of 50 IU/mL. The study was approved by the local ethics committee (ref 03/QĐ-BVVT-HĐKH).
National Hepatitis B Treatment Guidelines
The treatment criteria recommended by the Vietnamese national guidelines largely depend on HBV viral load measurement, ALT level, and fibrosis staging using liver histopathology or LSM. These criteria are summarized in Supplementary Table 1. In case of compensated/decompensated cirrhosis, antivirals are indicated if HBV DNA is detectable, regardless of ALT levels and HBeAg status. In case of noncirrhosis, antivirals are indicated if patients fulfilling both of these criteria: (1) liver disease, defined as ALT >2× upper limit of normal (ULN) or liver fibrosis ≥F2; and (2) high viral replication, defined as HBV DNA ≥20 000 IU/mL for HBeAg-positive patients and HBV DNA ≥2000 IU/mL for HBeAg-negative patients. For those not meeting either of these 2 criteria, antivirals are indicated for the following conditions: (1) >30 years of age, with persistently abnormal ALT >ULN (at least 3 times over a period of 24–48 weeks) and HBV >20 000 IU/mL, regardless HBeAg status; (2) family history of HCC or cirrhosis; (3) presenting extrahepatic manifestations such as glomerulonephritis, polyarthritis, cryoglobulinemia, polyarteritis nodosa, etc.; and (4) relapse after stopping HBV antivirals. In this analysis, we defined significant fibrosis and cirrhosis for the national treatment eligibility criteria as Metavir ≥F2 and F4 using LSM cut-offs of >7.0 and >11.0 kPa, respectively [19]. In the national guidelines, the ULN for ALT were defined as 25 IU/L for women and 35 IU/L for men; these are similar thresholds as the American Association for the Study of Liver Diseases [20].
Simplified Criteria
In the WHO guidelines, there are 2 types of treatment criteria: those with or without HBV DNA quantification [6]. Because our aim was to evaluate treatment eligibility criteria free from HBV DNA measurement, we only considered the latter for this analysis (Supplementary Table 1). In these criteria without HBV DNA, treatment is indicated for: (1) decompensated cirrhosis diagnosed by physical examination or cirrhosis using aminotransferase-to-platelet ratio index >2.0 or (2) persistently elevated ALT alone. The ULN for ALT recommended by the WHO were applied for these criteria: 19 IU/L for women and 30 IU/L for men [6].
TREAT-B score is obtained by adding the HBeAg score (negative [0 point] or positive [1 point]), and ALT score (<20 IU/L [0 point], 20–39 [1 point], 40–79 [2 points], or ≥80 [3 points]). TREAT-B ranged from 0 (HBeAg-negative and ALT <20 IU/L) to 4 (HBeAg-positive and ALT ≥80 IU/L) (Supplementary Table 2) [9].
Statistical Analysis
Descriptive statistics were used to summarize baseline characteristics of study participants. The performance of TREAT-B to identify patients in need of antiviral therapy by the national guidelines was assessed: (1) using a full dataset based on a cross-sectional study and (2) by limiting a subgroup of patients who had a second ALT measurement over a 6-month period. For the first analysis, we considered the eligibility based on parameters assessed at a single time point. For the second analysis, the national guidelines indicated to treat those having “persistently abnormal ALT” if they are aged >30 years with a viral load >200 000 IU/mL, and the simplified WHO criteria indicated to treat those having “persistently abnormal ALT” irrespective of age or HBV DNA levels. Because the WHO treatment criteria require longitudinal data, we restricted the analysis of the performance of the simplified WHO treatment criteria in the subgroup of patients with repeated ALT measurement.
The performances of the simplified criteria (TREAT-B and WHO) were assessed using the national guidelines as the “gold standard,” and the area under the receiver operating characteristic (AUROC), sensitivity, and specificity were calculated.
Statistical analysis was performed using STATA, version 14.2 (StataCorp, College Station, TX). Results were reported in accordance with the Standards for Reporting of Diagnostic Accuracy.
RESULTS
Patient Characteristics
Between 1 January 2017 and 31 December 2018, a total of 2767 patients were seen at the outpatient liver clinic of Viet Tiep Hospital, of whom 1846 (67%) were positive for HBsAg but 1446 were excluded mainly because they were on HBV antiviral therapy. We included 400 consecutive patients who were treatment-naïve and HBV monoinfected with available clinical and laboratory data in the analysis (Figure 1).
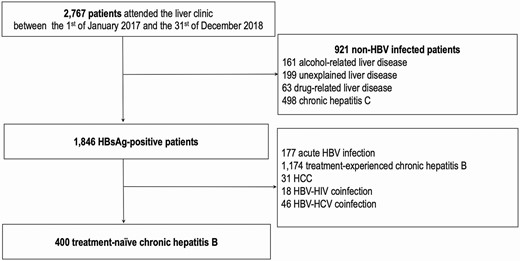
Study flow chart. Abbreviations: HBV, hepatitis B virus; HCC, hepatocellular cancer; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HBsAg, hepatitis B s antigen.
Of these 400 patients, 195 (48.8%) were men, the median age was 38 years (range, 18–86), 128 (32.0%) were positive for HBeAg, and the median viral load was 4.8 log10 IU/mL (range, undetectable −8.4). Among the entire study population, 100 (25.0%) patients had clinically significant fibrosis including 49 (12.3%) with suspected cirrhosis (Table 1).
Characteristics of the Study Population (n = 400) by the Number of ALT Measurements
| Variables . | . | Entire Cohort (N = 400) . | With 2 ALT Measurements (n = 89) . | With 1 ALT Measurement (n = 311) . | P Value . |
|---|---|---|---|---|---|
| Median age (y) | … | 38 (18–86) | 40 (18–79) | 37 (18–86) | .25 |
| Male, n (%) | … | 195 (48.8) | 39 (43.8) | 156 (50.2) | .29 |
| Median HBV viral load (log10 IU/mL) | … | 4.8 (undetectable −8.4) | 5.4 (undetectable −7.9) | 4.4 (undetectable −8.4) | .04 |
| Positive HBeAg, n (%) | … | 128 (32.0) | 29 (32.6) | 99 (31.8) | .89 |
| Median first ALT (IU/L) | … | 44 (10–456) | 69 (10–456) | 40 (12–356) | <.001 |
| Median second ALT (IU/L) | … | N/A | 69 (14–427) | N/A | N/A |
| Median AST (IU/L) | … | 34 (14–486) | 46 (14–486) | 32 (14–435) | <.001 |
| Median platelet count (109/L) | … | 215 (43–572) | 202 (60–422) | 216 (43–572) | .03 |
| Medial LSM (kPa) | … | 5.3 (3.0–67.8) | 6.3 (3.2–67.8) | 5.0 (3.0–35.3) | <.001 |
| Liver fibrosis, n (%) | No or mild (≤7.0 kPa) | 300 (75.0) | 52 (58.4) | 248 (79.7) | <.001 |
| Significant (7.1–11.0 kPa) | 51 (12.7) | 14 (15.8) | 37 (11.9) | ||
| Cirrhosis (≥11.1 kPa) | 49 (12.3) | 23 (25.8) | 26 (8.4) | ||
| Median APRI | … | 0.44 (0.12–9.37) | 0.61 (0.15–9.37) | 0.40 (0.12–8.74) | .40 |
| Eligible for treatment by the Vietnamese National Guidelines, n (%) | … | 167 (41.8) | 52 (58.4) | 115 (37.0) | <.001 |
| Eligible for simplified WHO treatment criteria without HBV DNA measurement,a n (%) | … | N/A | 81 (91.0) | N/A | N/A |
| TREAT-B score, n (%) | 0 | 22 (5.5) | 3 (3.4) | 19 (6.1) | .001 |
| 1 | 114 (28.5) | 14 (15.7) | 100 (32.2) | ||
| 2 | 113 (28.2) | 23 (25.8) | 90 (28.9) | ||
| 3 | 108 (27.0) | 32 (36.0) | 76 (24.4) | ||
| 4 | 43 (10.7) | 17 (19.1) | 26 (8.4) |
| Variables . | . | Entire Cohort (N = 400) . | With 2 ALT Measurements (n = 89) . | With 1 ALT Measurement (n = 311) . | P Value . |
|---|---|---|---|---|---|
| Median age (y) | … | 38 (18–86) | 40 (18–79) | 37 (18–86) | .25 |
| Male, n (%) | … | 195 (48.8) | 39 (43.8) | 156 (50.2) | .29 |
| Median HBV viral load (log10 IU/mL) | … | 4.8 (undetectable −8.4) | 5.4 (undetectable −7.9) | 4.4 (undetectable −8.4) | .04 |
| Positive HBeAg, n (%) | … | 128 (32.0) | 29 (32.6) | 99 (31.8) | .89 |
| Median first ALT (IU/L) | … | 44 (10–456) | 69 (10–456) | 40 (12–356) | <.001 |
| Median second ALT (IU/L) | … | N/A | 69 (14–427) | N/A | N/A |
| Median AST (IU/L) | … | 34 (14–486) | 46 (14–486) | 32 (14–435) | <.001 |
| Median platelet count (109/L) | … | 215 (43–572) | 202 (60–422) | 216 (43–572) | .03 |
| Medial LSM (kPa) | … | 5.3 (3.0–67.8) | 6.3 (3.2–67.8) | 5.0 (3.0–35.3) | <.001 |
| Liver fibrosis, n (%) | No or mild (≤7.0 kPa) | 300 (75.0) | 52 (58.4) | 248 (79.7) | <.001 |
| Significant (7.1–11.0 kPa) | 51 (12.7) | 14 (15.8) | 37 (11.9) | ||
| Cirrhosis (≥11.1 kPa) | 49 (12.3) | 23 (25.8) | 26 (8.4) | ||
| Median APRI | … | 0.44 (0.12–9.37) | 0.61 (0.15–9.37) | 0.40 (0.12–8.74) | .40 |
| Eligible for treatment by the Vietnamese National Guidelines, n (%) | … | 167 (41.8) | 52 (58.4) | 115 (37.0) | <.001 |
| Eligible for simplified WHO treatment criteria without HBV DNA measurement,a n (%) | … | N/A | 81 (91.0) | N/A | N/A |
| TREAT-B score, n (%) | 0 | 22 (5.5) | 3 (3.4) | 19 (6.1) | .001 |
| 1 | 114 (28.5) | 14 (15.7) | 100 (32.2) | ||
| 2 | 113 (28.2) | 23 (25.8) | 90 (28.9) | ||
| 3 | 108 (27.0) | 32 (36.0) | 76 (24.4) | ||
| 4 | 43 (10.7) | 17 (19.1) | 26 (8.4) |
All treatment criteria are summarized in Supplementary Table 1. Continuous variables are presented as median (range). Categorical variables are presented as number (percentage).
Abbreviations: ALT, alanine aminotransferase; APRI, aminotransferase-to-platelet ratio index; AST, aspartate transaminase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; LSM, least square mean; N/A, not available; TREAT-B, Treatment Eligibility in Africa for the Hepatitis B Virus.
aWe considered the eligibility based on a single ALT measurement.
Characteristics of the Study Population (n = 400) by the Number of ALT Measurements
| Variables . | . | Entire Cohort (N = 400) . | With 2 ALT Measurements (n = 89) . | With 1 ALT Measurement (n = 311) . | P Value . |
|---|---|---|---|---|---|
| Median age (y) | … | 38 (18–86) | 40 (18–79) | 37 (18–86) | .25 |
| Male, n (%) | … | 195 (48.8) | 39 (43.8) | 156 (50.2) | .29 |
| Median HBV viral load (log10 IU/mL) | … | 4.8 (undetectable −8.4) | 5.4 (undetectable −7.9) | 4.4 (undetectable −8.4) | .04 |
| Positive HBeAg, n (%) | … | 128 (32.0) | 29 (32.6) | 99 (31.8) | .89 |
| Median first ALT (IU/L) | … | 44 (10–456) | 69 (10–456) | 40 (12–356) | <.001 |
| Median second ALT (IU/L) | … | N/A | 69 (14–427) | N/A | N/A |
| Median AST (IU/L) | … | 34 (14–486) | 46 (14–486) | 32 (14–435) | <.001 |
| Median platelet count (109/L) | … | 215 (43–572) | 202 (60–422) | 216 (43–572) | .03 |
| Medial LSM (kPa) | … | 5.3 (3.0–67.8) | 6.3 (3.2–67.8) | 5.0 (3.0–35.3) | <.001 |
| Liver fibrosis, n (%) | No or mild (≤7.0 kPa) | 300 (75.0) | 52 (58.4) | 248 (79.7) | <.001 |
| Significant (7.1–11.0 kPa) | 51 (12.7) | 14 (15.8) | 37 (11.9) | ||
| Cirrhosis (≥11.1 kPa) | 49 (12.3) | 23 (25.8) | 26 (8.4) | ||
| Median APRI | … | 0.44 (0.12–9.37) | 0.61 (0.15–9.37) | 0.40 (0.12–8.74) | .40 |
| Eligible for treatment by the Vietnamese National Guidelines, n (%) | … | 167 (41.8) | 52 (58.4) | 115 (37.0) | <.001 |
| Eligible for simplified WHO treatment criteria without HBV DNA measurement,a n (%) | … | N/A | 81 (91.0) | N/A | N/A |
| TREAT-B score, n (%) | 0 | 22 (5.5) | 3 (3.4) | 19 (6.1) | .001 |
| 1 | 114 (28.5) | 14 (15.7) | 100 (32.2) | ||
| 2 | 113 (28.2) | 23 (25.8) | 90 (28.9) | ||
| 3 | 108 (27.0) | 32 (36.0) | 76 (24.4) | ||
| 4 | 43 (10.7) | 17 (19.1) | 26 (8.4) |
| Variables . | . | Entire Cohort (N = 400) . | With 2 ALT Measurements (n = 89) . | With 1 ALT Measurement (n = 311) . | P Value . |
|---|---|---|---|---|---|
| Median age (y) | … | 38 (18–86) | 40 (18–79) | 37 (18–86) | .25 |
| Male, n (%) | … | 195 (48.8) | 39 (43.8) | 156 (50.2) | .29 |
| Median HBV viral load (log10 IU/mL) | … | 4.8 (undetectable −8.4) | 5.4 (undetectable −7.9) | 4.4 (undetectable −8.4) | .04 |
| Positive HBeAg, n (%) | … | 128 (32.0) | 29 (32.6) | 99 (31.8) | .89 |
| Median first ALT (IU/L) | … | 44 (10–456) | 69 (10–456) | 40 (12–356) | <.001 |
| Median second ALT (IU/L) | … | N/A | 69 (14–427) | N/A | N/A |
| Median AST (IU/L) | … | 34 (14–486) | 46 (14–486) | 32 (14–435) | <.001 |
| Median platelet count (109/L) | … | 215 (43–572) | 202 (60–422) | 216 (43–572) | .03 |
| Medial LSM (kPa) | … | 5.3 (3.0–67.8) | 6.3 (3.2–67.8) | 5.0 (3.0–35.3) | <.001 |
| Liver fibrosis, n (%) | No or mild (≤7.0 kPa) | 300 (75.0) | 52 (58.4) | 248 (79.7) | <.001 |
| Significant (7.1–11.0 kPa) | 51 (12.7) | 14 (15.8) | 37 (11.9) | ||
| Cirrhosis (≥11.1 kPa) | 49 (12.3) | 23 (25.8) | 26 (8.4) | ||
| Median APRI | … | 0.44 (0.12–9.37) | 0.61 (0.15–9.37) | 0.40 (0.12–8.74) | .40 |
| Eligible for treatment by the Vietnamese National Guidelines, n (%) | … | 167 (41.8) | 52 (58.4) | 115 (37.0) | <.001 |
| Eligible for simplified WHO treatment criteria without HBV DNA measurement,a n (%) | … | N/A | 81 (91.0) | N/A | N/A |
| TREAT-B score, n (%) | 0 | 22 (5.5) | 3 (3.4) | 19 (6.1) | .001 |
| 1 | 114 (28.5) | 14 (15.7) | 100 (32.2) | ||
| 2 | 113 (28.2) | 23 (25.8) | 90 (28.9) | ||
| 3 | 108 (27.0) | 32 (36.0) | 76 (24.4) | ||
| 4 | 43 (10.7) | 17 (19.1) | 26 (8.4) |
All treatment criteria are summarized in Supplementary Table 1. Continuous variables are presented as median (range). Categorical variables are presented as number (percentage).
Abbreviations: ALT, alanine aminotransferase; APRI, aminotransferase-to-platelet ratio index; AST, aspartate transaminase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; LSM, least square mean; N/A, not available; TREAT-B, Treatment Eligibility in Africa for the Hepatitis B Virus.
aWe considered the eligibility based on a single ALT measurement.
Of the 400 participants, 89 had at least 2 measurements of ALT level more than 6 months apart. Within this subgroup, 39 were men (39.8%), the median age was 40 years (range, 18–79), 29 (32.6%) were HBeAg positive, with a median viral load of 5.4 log10 IU/mL (range, undetectable −7.9), and 37 (41.5%) had clinically significant liver fibrosis including 23 (25.8%) with cirrhosis (Table 1).
HBV Treatment Eligibility and Performance of TREAT-B Score and the WHO Simplified Criteria
In the entire study population, the number of patients eligible for treatment was 167 (41.8%) according to the Vietnamese guidelines. Using the TREAT-B score (cutoff ≥ 2), a larger proportion (264 [65.9%]) of patients had treatment indication (Table 1).
Using the Vietnamese treatment criteria as a reference, the performance of TREAT-B (cutoff ≥ 2) to select patients for treatment was very good with an AUROC at 0.89 (95% confidence interval, 0.87-0.92). A cutoff at 2 had an excellent sensitivity at 98.8% but a poor specificity (57.5%). A cutoff of 3 had a better specificity at 88.4% but lower sensitivity of 74.3% (Table 2).
Performance of the Simplified Criteria to Select Patients Eligible for Antiviral Therapy in Reference to the Vietnamese National Guidelines at a Single Time Point (n = 400)
| . | TREAT-B . | |||
|---|---|---|---|---|
| AUROC . | 0.89 (0.87–0.92) . | |||
| Cutoff | ≥1 | ≥2 | ≥3 | 4 |
| True positive | 167 | 165 | 124 | 43 |
| False negative | 0 | 2 | 43 | 124 |
| True negative | 22 | 134 | 206 | 233 |
| False positive | 211 | 99 | 27 | 0 |
| Sensitivity (%) | 100 (97.8–100) | 98.8 (95.7– 99.9) | 74.3 (66.9–80.7) | 25.7 (19.3–33.1) |
| Specificity (%) | 9.4 (6.0–13.9) | 57.5 (50.9– 63.9) | 88.4 (83.6–92.2) | 100 (98.4–100) |
| . | TREAT-B . | |||
|---|---|---|---|---|
| AUROC . | 0.89 (0.87–0.92) . | |||
| Cutoff | ≥1 | ≥2 | ≥3 | 4 |
| True positive | 167 | 165 | 124 | 43 |
| False negative | 0 | 2 | 43 | 124 |
| True negative | 22 | 134 | 206 | 233 |
| False positive | 211 | 99 | 27 | 0 |
| Sensitivity (%) | 100 (97.8–100) | 98.8 (95.7– 99.9) | 74.3 (66.9–80.7) | 25.7 (19.3–33.1) |
| Specificity (%) | 9.4 (6.0–13.9) | 57.5 (50.9– 63.9) | 88.4 (83.6–92.2) | 100 (98.4–100) |
The values in parentheses are the 95% confidence intervals.
Abbreviations: AUROC, area under the receiver operating characteristic; TREAT-B, Treatment Eligibility in Africa for the Hepatitis B Virus.
Performance of the Simplified Criteria to Select Patients Eligible for Antiviral Therapy in Reference to the Vietnamese National Guidelines at a Single Time Point (n = 400)
| . | TREAT-B . | |||
|---|---|---|---|---|
| AUROC . | 0.89 (0.87–0.92) . | |||
| Cutoff | ≥1 | ≥2 | ≥3 | 4 |
| True positive | 167 | 165 | 124 | 43 |
| False negative | 0 | 2 | 43 | 124 |
| True negative | 22 | 134 | 206 | 233 |
| False positive | 211 | 99 | 27 | 0 |
| Sensitivity (%) | 100 (97.8–100) | 98.8 (95.7– 99.9) | 74.3 (66.9–80.7) | 25.7 (19.3–33.1) |
| Specificity (%) | 9.4 (6.0–13.9) | 57.5 (50.9– 63.9) | 88.4 (83.6–92.2) | 100 (98.4–100) |
| . | TREAT-B . | |||
|---|---|---|---|---|
| AUROC . | 0.89 (0.87–0.92) . | |||
| Cutoff | ≥1 | ≥2 | ≥3 | 4 |
| True positive | 167 | 165 | 124 | 43 |
| False negative | 0 | 2 | 43 | 124 |
| True negative | 22 | 134 | 206 | 233 |
| False positive | 211 | 99 | 27 | 0 |
| Sensitivity (%) | 100 (97.8–100) | 98.8 (95.7– 99.9) | 74.3 (66.9–80.7) | 25.7 (19.3–33.1) |
| Specificity (%) | 9.4 (6.0–13.9) | 57.5 (50.9– 63.9) | 88.4 (83.6–92.2) | 100 (98.4–100) |
The values in parentheses are the 95% confidence intervals.
Abbreviations: AUROC, area under the receiver operating characteristic; TREAT-B, Treatment Eligibility in Africa for the Hepatitis B Virus.
Within the subgroup of 89 participants with 2 ALT level measurements, the number of patients eligible for treatment was 52 (58.4%) based on the Vietnamese criteria, 81 (90%) according to the simplified WHO criteria, and 72 (80.9%) according to TREAT-B (cutoff ≥ 2) (Table 1). In this subgroup, using the Vietnamese treatment criteria as a reference, the AUROC of TREAT-B (0.87 [95% confidence interval, 0.80–0.94]) was significantly higher than that of the simplified WHO criteria (0.63 [0.55-0.71], P < .001) (Table 3). When using a cutoff of 3, the performance of the TREAT-B had higher specificity (86.7%) but a lower sensitivity compared with a cutoff of 2 (Table 3).
Performance of the Simplified Criteria to Select Patients Eligible for Antiviral Therapy in Reference to the Vietnamese National Guidelines in a Subgroup of Patients With 2 ALT Measurements Over 6 Months (n = 89)
| . | TREAT-B . | Simplified WHO Criteria Without HBV DNA . | |||
|---|---|---|---|---|---|
| AUROC . | 0.87 (0.80–0.94) . | 0.63 (0.55–0.71) . | |||
| P value (compared with TREAT-B) | N/A | <.001 | |||
| Cutoff | ≥1 | ≥2 | ≥3 | 4 | N/A |
| True positive | 59 | 56 | 45 | 17 | 59 |
| False negative | 0 | 3 | 14 | 42 | 0 |
| True negative | 3 | 14 | 26 | 30 | 8 |
| False positive | 27 | 16 | 4 | 0 | 22 |
| Sensitivity (%) | 100 (93.9–100) | 94.9 (85.9–98.9) | 76.3 (63.4–86.4) | 28.8 (17.8–42.1) | 100 (93.9–100) |
| Specificity (%) | 10.0 (2.1–26.5) | 46.7 (28.3–65.7) | 86.7 (69.3–96.2) | 100 (88.4–100) | 26.7 (12.3–45.9) |
| . | TREAT-B . | Simplified WHO Criteria Without HBV DNA . | |||
|---|---|---|---|---|---|
| AUROC . | 0.87 (0.80–0.94) . | 0.63 (0.55–0.71) . | |||
| P value (compared with TREAT-B) | N/A | <.001 | |||
| Cutoff | ≥1 | ≥2 | ≥3 | 4 | N/A |
| True positive | 59 | 56 | 45 | 17 | 59 |
| False negative | 0 | 3 | 14 | 42 | 0 |
| True negative | 3 | 14 | 26 | 30 | 8 |
| False positive | 27 | 16 | 4 | 0 | 22 |
| Sensitivity (%) | 100 (93.9–100) | 94.9 (85.9–98.9) | 76.3 (63.4–86.4) | 28.8 (17.8–42.1) | 100 (93.9–100) |
| Specificity (%) | 10.0 (2.1–26.5) | 46.7 (28.3–65.7) | 86.7 (69.3–96.2) | 100 (88.4–100) | 26.7 (12.3–45.9) |
The values in parentheses are the 95% confidence intervals.
Abbreviations: AUROC, area under the receiver operating characteristic; HBV, hepatitis B virus; N/A, not available; TREAT-B, Treatment Eligibility in Africa for the Hepatitis B Virus.
Performance of the Simplified Criteria to Select Patients Eligible for Antiviral Therapy in Reference to the Vietnamese National Guidelines in a Subgroup of Patients With 2 ALT Measurements Over 6 Months (n = 89)
| . | TREAT-B . | Simplified WHO Criteria Without HBV DNA . | |||
|---|---|---|---|---|---|
| AUROC . | 0.87 (0.80–0.94) . | 0.63 (0.55–0.71) . | |||
| P value (compared with TREAT-B) | N/A | <.001 | |||
| Cutoff | ≥1 | ≥2 | ≥3 | 4 | N/A |
| True positive | 59 | 56 | 45 | 17 | 59 |
| False negative | 0 | 3 | 14 | 42 | 0 |
| True negative | 3 | 14 | 26 | 30 | 8 |
| False positive | 27 | 16 | 4 | 0 | 22 |
| Sensitivity (%) | 100 (93.9–100) | 94.9 (85.9–98.9) | 76.3 (63.4–86.4) | 28.8 (17.8–42.1) | 100 (93.9–100) |
| Specificity (%) | 10.0 (2.1–26.5) | 46.7 (28.3–65.7) | 86.7 (69.3–96.2) | 100 (88.4–100) | 26.7 (12.3–45.9) |
| . | TREAT-B . | Simplified WHO Criteria Without HBV DNA . | |||
|---|---|---|---|---|---|
| AUROC . | 0.87 (0.80–0.94) . | 0.63 (0.55–0.71) . | |||
| P value (compared with TREAT-B) | N/A | <.001 | |||
| Cutoff | ≥1 | ≥2 | ≥3 | 4 | N/A |
| True positive | 59 | 56 | 45 | 17 | 59 |
| False negative | 0 | 3 | 14 | 42 | 0 |
| True negative | 3 | 14 | 26 | 30 | 8 |
| False positive | 27 | 16 | 4 | 0 | 22 |
| Sensitivity (%) | 100 (93.9–100) | 94.9 (85.9–98.9) | 76.3 (63.4–86.4) | 28.8 (17.8–42.1) | 100 (93.9–100) |
| Specificity (%) | 10.0 (2.1–26.5) | 46.7 (28.3–65.7) | 86.7 (69.3–96.2) | 100 (88.4–100) | 26.7 (12.3–45.9) |
The values in parentheses are the 95% confidence intervals.
Abbreviations: AUROC, area under the receiver operating characteristic; HBV, hepatitis B virus; N/A, not available; TREAT-B, Treatment Eligibility in Africa for the Hepatitis B Virus.
DISCUSSION
In a large cohort of Vietnamese treatment-naïve patients with chronic HBV infection, we found (1) a substantial proportion of patients in need of antiviral therapy (around 40%), (2) a significant proportion (12%) of patients with cirrhosis, and (3) a good performance of TREAT-B, higher than the simplified WHO treatment criteria free of HBV DNA, for the identification of HBV-infected patients eligible for antiviral therapy.
To the best of our knowledge, this is the first study analyzing the liver disease severity and the proportion of patients requiring antiviral therapy in HBV-monoinfected patients referred to one of the largest outpatient liver clinics in Vietnam. This is also the first study reporting the performance of simplified criteria for the identification of HBV-infected patients in need of antiviral therapy in a LMIC in Asia. Studies on HBV infection conducted in Vietnam are mainly sero-surveys assessing the prevalence of HBsAg in the general population or in selected groups (eg, injecting drug users, blood donors), or virological studies analyzing the characteristics of HBV in infected individuals in Vietnam [17, 21–25]. None of these studies described the severity of liver disease in people with chronic HBV infection. As a result, the Vietnamese national hepatitis program can only develop a national plan on the basis of estimates generated by modeling studies, in which parameters were supplied from other countries [1].
Our finding of about 40% of HBV-infected patients eligible for treatment is slightly higher than the estimate in the South East Asian Region made by the Polaris group (32%) but similar to estimates made by the same group in Vietnam (41.6%) [1]. The proportion of patients with chronic HBV infection who meet treatment eligibility using international guidelines has been poorly documented in Asia. For example, in Hong Kong, Fung et al reported that 64% of 1400 patients with chronic HBV infection seen in a hepatitis unit in a central hospital were eligible for treatment [26]. Care must be taken to interpret these hospital-based estimates, which may overestimate such a proportion. Population-based data from Asia are critically missing.
With an estimated 7 764 000, people chronically infected with HBV in Vietnam, it is currently considered—based on the Polaris data—that 3 232 000 (ie, 41.6%) people require antiviral therapy; however, only 45 000 people (1%) are currently receiving antiviral therapy [1]. Achieving the 2030 WHO targets indicates that 2 585 600 HBV-infected individuals (80% treatment coverage among those who are eligible for treatment) should be on treatment by 2030 in Vietnam; in other words, over the next decade, an additional 2 540 600 people will have to initiate antiviral therapy. This will not be feasible without simplified treatment algorithms and decentralization of screen-and-treat interventions. HBV treatment initiation in Vietnam is mainly provided by hospitals or urban centers. By contrast to human immunodeficiency virus-infected patients, HBV monoinfected patients are hardly managed in primary health care centers in Vietnam. This is partly because of the complexity of the current diagnostic and treatment algorithms, which are based on highly accurate but expensive tests (ie, polymerase chain reaction and FibroScan), which are not accessible in rural areas.
Simplified cascade of care for viral hepatitis has been poorly developed and evaluated in Asia. In Vietnam, only a few simplified diagnostic tools have been validated for HCV infection (eg, dry blood spots, HCV core antigen) but none for HBV infection, especially alternatives to the standard HBV DNA measurement [27, 28].
Our study suggests that the use of TREAT-B is an accurate alternative to easily select patients for antiviral therapy using only 2 simple tests (ALT ad HBeAg) without required liver imaging, which is an enormous advantage in remote areas. Importantly, our study shows for the first time that TREAT-B is accurate when using a rapid diagnostic HBeAg point-of-care test. In Vietnam, ALT measurement is widely accessible and can be performed in rural areas; therefore, TREAT-B only based on rapid HBeAg point-of-care and ALT, could be easily integrated at a decentralized hepatitis care in rural areas.
Our findings also suggest that a large proportion of HBV monoinfected patients might be unnecessarily treated with antivirals when using simplified treatment criteria. Considering that in Vietnam 3 232 000 HBsAg-positive subjects are eligible for treatment [1], our results mean that using the simplified WHO treatment guidelines, 1 178 950 (36.5%) or 7 999 929 (24.75%) using TREAT-B score will receive unnecessary antiviral therapy. Obviously, this poses medical, logistical, and financial burdens because the treatment is currently life-long and requires regular monitoring with potential toxicity. However, expanding treatment coverage might also reduce infectivity rate and improve quality of life. Cost-effectiveness analysis of treatment interventions using simplified clinical staging based on TREAT-B in resource-limited countries are required.
Our study has some limitations. First, it is not representative of the Vietnamese general population because we restricted our analysis to 1 outpatient clinic in Northern Vietnam. Second, most patients had a single liver assessment; however, we were able to confirm our findings in a subgroup of patients with 2 ALT measurements. Third, despite a high prevalence of HDV infection in Vietnam [29], we were unable to exclude HDV-coinfected patients because HDV serology is not done routinely in Vietnam. However, we believe that this study provides important information about HBV infection in Vietnam and should be used to inform the development of further simplified diagnostic and treatment algorithms in Vietnam. Finally, we were unable to provide the proportion of patients with excessive alcohol intake or metabolic disorders that may overestimate the degree of liver inflammation and fibrosis.
Currently, the control of the HBV epidemic in Vietnam mainly relies on universal infant hepatitis B vaccination program introduced in 2002. However, modeling studies have shown that HBV vaccination alone will not be enough to achieve HBV elimination over the next decade and it is crucial to significantly scale up screen-and-treat interventions [30]. Simplification of HBV cascade of care is therefore urgently needed. We believe that TREAT-B is an accurate alternative to the current complex treatment criteria and performs better than the simplified WHO criteria free from HBV DNA measurement.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to Echosens, Paris, France, for the donation of the Fibroscan machine and the technical support. They thank the staff of Viet Tiep hospital who contribute daily to the care of the patients. The authors are also grateful to the French Agency for Research on AIDS and Viral Hepatitis (Inserm-ANRS) site in Vietnam.
Potential conflicts of interest. M. L. and Y. S. received consultant fees from Gilead US company. Gilead US company provides financial and drug support to the PROLIFICA program (www.prolifica.africa) led by M. L. and Y. S. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
V. V. H. and Y. S. contributed equally to this work.




