-
PDF
- Split View
-
Views
-
Cite
Cite
Arthur W Baker, Jason E Stout, Deverick J Anderson, Daniel J Sexton, Becky Smith, Rebekah W Moehring, Kirk Huslage, Christopher J Hostler, Sarah S Lewis, Tap Water Avoidance Decreases Rates of Hospital-onset Pulmonary Nontuberculous Mycobacteria, Clinical Infectious Diseases, Volume 73, Issue 3, 1 August 2021, Pages 524–527, https://doi.org/10.1093/cid/ciaa1237
Close - Share Icon Share
Abstract
We analyzed the impact of a hospital tap water avoidance protocol on respiratory isolation of nontuberculous mycobacteria (NTM). After protocol implementation, hospital-onset episodes of respiratory NTM isolation on high-risk units decreased from 41.0 to 9.9 episodes per 10 000 patient-days (incidence rate ratio, 0.24; 95% confidence interval, .17–.34; P < .0001).
(See the Editorial Commentary by Arduino on pages 528–30.)
Epidemiologists have traditionally considered acquisition of nontuberculous mycobacteria (NTM) to occur in the community [1]. However, reports of healthcare facility-acquired NTM and associated outbreaks have become increasingly common [2, 3]. NTM acquisition at healthcare facilities typically occurs after vulnerable patients have contact with water colonized with NTM [4]. Prevention of hospital-acquired NTM infections is critical because patients with these infections commonly fail to achieve cure and experience substantial morbidity and mortality [5].
Investigators at our hospital mitigated a large outbreak of Mycobacterium abscessus complex (MABC) that occurred from 2013 to 2015 [6]. This outbreak was linked to a colonized water supply at a new hospital addition where cultures of biofilms from hospital water outlets grew MABC. Molecular fingerprinting, including pulsed-field gel electrophoresis, confirmed that environmental isolates from the new hospital addition were clonally related to clinical MABC isolates. The first phase of the outbreak was characterized by hospital-onset pulmonary colonization or infection with MABC, which occurred in over 50 patients, including more than 30 lung transplant recipients. In response to the outbreak, we implemented a tap water avoidance protocol designed to decrease the risk of pulmonary acquisition of MABC. This protocol provided sterile water for consumption and patient-care activities requiring water to all patients receiving care on 3 intensive care units (ICUs) and 1 intermediate unit at the new hospital addition. Ice was also avoided in these units.
Rates of hospital-onset pulmonary MABC demonstrably declined following institution of the preceding tap water avoidance protocol [6]. We hypothesized that avoidance of tap water would likewise be associated with decreased respiratory isolation of other NTM species. We performed this follow-up study to analyze rates of hospital-onset episodes of pulmonary NTM in ICU and intermediate unit patients during the MABC outbreak and in the post-outbreak time period, following the introduction of sterile water use. We also evaluated if NTM species commonly obtained from patient specimens were also isolated from hospital water outlets.
METHODS
Duke University Hospital (DUH) is a 957-bed tertiary care hospital in central North Carolina. In late July 2013, a new hospital addition opened, which included 160 ICU and intermediate beds and provided early postoperative care for all lung transplant recipients. DUH utilizes the municipal water supply.
The tap water avoidance protocol was implemented in late May 2014. Strict unit-wide tap water avoidance occurred for all patients in 3 ICUs and 1 intermediate unit where new lung transplant recipients received care following their “stepdown” from the cardiothoracic surgery ICU. On these 4 units, sterile water that was commercially produced for irrigation replaced tap water for routine activities such as oral care, rinsing of suction catheters, and enteral tube irrigation. Patients were restricted from showering, and bathing was performed with waterless bath products or sterile water. Ice use was also avoided on these units and was not provided for consumption or patient care activities, such as speech therapy assessments.
Beginning in April 2015, 11 months after implementation of the tap water avoidance protocol, we made other changes to the hospital’s water management plan to improve water flow rates, temperature gradients, and disinfectant levels, as previously described [6]. Throughout the study, hospitalized patients were not placed on contact or other specialized isolation precautions due to NTM colonization or infection.
We retrospectively identified all patients who had positive cultures for NTM obtained at our hospital from the beginning of the MABC outbreak in August 2013 through December 2015. The pre-intervention outbreak period was defined as August 2013 through May 2014; the tap water avoidance intervention period was defined as June 2014 through December 2015. For both time periods, an episode of respiratory NTM isolation was defined as a positive culture from a respiratory specimen obtained from a patient actively receiving care on any of the 4 units using unit-wide tap water avoidance protocols.
The date of culture collection was considered to be the date of NTM isolation. We excluded cultures obtained prior to day 3 of hospitalization and also excluded NTM isolates from patients who had prior growth of the same NTM species in respiratory cultures previously obtained from January 2012 onward at our 3-hospital health system.
We performed mycobacterial cultures of biofilms obtained from water outlets located on the 4 intervention units at periodic times during the outbreak and intervention time periods from April 2014 through May 2015. Water outlets sampled included faucets, shower heads, ice machines, and a water basin.
Incidence rate ratios (IRRs) were estimated assuming that episodes of respiratory NTM isolation followed the Poisson distribution. Calculations were performed in SAS software, version 9.4 (SAS Institute, Cary, North Carolina, USA). Standard mycobacterial culture methods were used for clinical and environmental specimens, as previously described [6]. The Duke University institutional review board approved this investigation and research.
RESULTS
Over the 29-month study period, 105 patients experienced 137 unique episodes of hospital-onset respiratory NTM isolation that occurred over 70 168 total patient-days on the 4 intervention units (Figure 1). The incidence rate of NTM isolation decreased from 41.0 episodes per 10 000 patient-days during the 10-month outbreak period to 9.9 patients per 10 000 patient-days during the 19-month intervention period (IRR, 0.24; 95% confidence interval [CI], .17–.34; P < .0001).
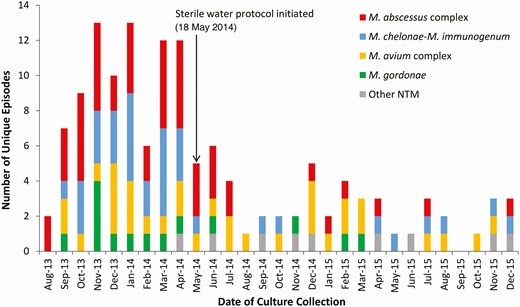
Epidemic curve of 137 unique episodes of nontuberculous mycobacteria isolated from the respiratory tract of 105 patients hospitalized on 4 intensive care or intermediate units. The 4 study units opened for patient care in late July 2013. The outbreak period occurred from August 2013 through May 2014, and the tap water avoidance intervention period occurred from June 2014 through December 2015. Other NTM include M. mucogenicum (n=4), M. fortuitum (n=3), M. arupense (n=1), and M. terrae (n=1). Abbreviations: M. abscessus, Mycobacterium abscessus; M. arupense, Mycobacterium arupense; M. avium, Mycobacterium avium; M. chelonae, Mycobacterium chelonae; M. fortuitum, Mycobacterium fortunitum; M. gordonae, Mycobacterium gordonae; M. immunogenum, Mycobacterium immunogenum; M. mucogenicum, Mycobacterium mucogenicum; Mycobacterium abscessus; M. terrae, Mycobacterium terrae; NTM, nontuberculous mycobacteria.
Among lung transplant recipients, respiratory NTM isolation occurred in 56 patients, who accounted for 78 (57%) of 137 total episodes of NTM isolation. The incidence rate of NTM isolation for lung transplant recipients decreased from 28.6 episodes per 10 000 patient-days during the outbreak period to 3.3 episodes per 10 000 patient-days during the intervention period (IRR, 0.12; 95% CI, .07–.20; P < .0001). Volume of lung transplant surgeries performed remained stable during the outbreak (118 transplants per 12 months) and intervention (109 transplants per 12 months) time periods.
Of the 137 total episodes of NTM isolation, 4 NTM species were responsible for 128 (93%) episodes. Incidence rates for respiratory isolation of each of these NTM markedly decreased immediately after initiation of the tap water avoidance protocol (Figure 1): Rates of hospital-onset episodes of respiratory NTM isolation per 10 000 patient-days decreased from 16.6 to 2.3 episodes for MABC (IRR, 0.14; P < .0001); from 12.0 to 1.7 for Mycobacterium chelonae-Mycobacterium immunogenum (IRR, 0.14; P < .0001); from 7.4 to 3.5 for Mycobacterium avium complex (MAC) (IRR, 0.48; P = .03); and from 4.6 to 0.8 for Mycobacterium gordonae (IRR, 0.18; P = .004).
Mycobacterial biofilm cultures were positive for at least 1 NTM species in 25 of 33 (76%) total water outlets sampled throughout the 4 study units. Environmental NTM species isolated included MABC (n = 11, 33%), M. chelonae-M. immunogenum (n = 11, 33%), and M. gordonae (n = 11, 33%). The prevalence of positive biofilm cultures for NTM was not significantly different for cultures performed during the outbreak period (10/12 [83%]) versus the intervention period (15/21 [71%]) (prevalence ratio, 0.86; P = .41), and the same NTM species were isolated during both time periods.
Discussion
Implementation of a tap water avoidance protocol promptly mitigated a clonal outbreak of pulmonary MABC linked to a colonized water system at a new hospital addition. In addition, use of sterile water for ICU and intermediate unit patient care and consumption also substantially decreased hospital-onset respiratory isolation of other NTM species, including M. chelonae-M. immunogenum, MAC, and M. gordonae. In fact, after implementation of the protocol, overall pulmonary NTM acquisition on the 4 study units decreased by 76%.
Three primary factors strongly indicate that tap water avoidance led directly to decreased respiratory acquisition of waterborne NTM among hospitalized patients on high risk units. First, environmental sampling of biofilms taken from study unit water outlets confirmed persistent water system colonization with the primary clone of MABC responsible for the outbreak and other NTM species commonly acquired by patients during the study time period. Second, other changes to the hospital’s water management plan did not occur until months after the substantial decrease in respiratory isolation of NTM occurred. Third, although potential for indirect person-to-person transmission of MABC exists [7–9], our study showed an immediate and sustained decrease in respiratory NTM acquisition from multiple species, including the outbreak strain of MABC, following the single initial intervention of hospital tap water avoidance. Thus, these data complement evidence from other studies that exposure to water aerosols from plumbing systems represents a key risk factor in pulmonary NTM infections [10, 11]. Furthermore, results from this study uniquely demonstrate that tap water avoidance can be effective in reducing that exposure and risk of subsequent disease.
Hospitalized patients exposed to oral or enteral tap water colonized with NTM are at risk for pulmonary NTM colonization, presumably due to micro-aspiration [12, 13]. Pulmonary NTM colonization with pathogenic NTM can progress to invasive pulmonary infection or even disseminated infection, especially in lung transplant recipients and other immunosuppressed patients [14]. However, prevention of hospital acquisition of pulmonary NTM also has important implications for patients who do not progress from pulmonary NTM colonization to invasive infection. For example, because differentiating colonization from invasive pulmonary NTM infection is challenging, particularly in hospitalized patients, patients with pulmonary colonization alone often receive combination antibiotic therapy associated with numerous adverse events [15]. Furthermore, although NTM species such as M. gordonae rarely cause disease, laboratory identification of specific NTM species can require several weeks of incubation following initial growth in culture. While awaiting species identification, clinicians at times prescribe complicated empiric antibiotic regimens but subsequently learn that antibiotic therapy for the identified NTM species was unnecessary.
This study raised several important questions that require further study. First, we need to better understand which hospitals, units, and patients would benefit most from tap water avoidance. Patients at high risk for pulmonary NTM infection, such as lung transplant recipients, may additionally benefit from community tap water avoidance [6]. Also, use of 0.2-μm water filters or certain water system decontamination procedures [16, 17] might prevent respiratory isolation of NTM as successfully as use of a sterile water protocol, which is resource-intensive and expensive. Finally, more data are needed to determine which hospital water systems should be screened for NTM and how best to perform clinical surveillance for healthcare facility-acquired NTM.
This study had 2 primary limitations. First, we were unable to conclusively differentiate NTM airway colonization from invasive pulmonary infection in the affected patient population. We presumed that decreasing incidence of hospital-acquired respiratory NTM isolation would reduce risk of symptomatic infection, adverse events from antibiotic therapy, and other morbidities associated with NTM infection but did not assess these outcomes. Second, some episodes defined as hospital-onset respiratory NTM isolation could have instead represented delayed diagnoses of community-acquired NTM. Inclusion of community-acquired NTM episodes would have led to underestimation of the effect of hospital tap water avoidance.
In conclusion, we demonstrated a substantial decrease in respiratory NTM isolation among ICU and intermediate unit patients after introduction of a protocol for tap water avoidance. Other hospitals with endemic NTM, new water systems, or patients at high risk for pulmonary NTM infection, such as lung transplant recipients, should consider implementation of similar protocols.
Notes
Presented in part: An abstract containing preliminary data was presented at IDWeek, San Francisco, California; 5 October 2018, abstract 927.
Financial support. A. W. B. was supported in part by the Transplant Infectious Disease Interdisciplinary Research Training Grant of the National Institutes of Health [grant number 5T32AI100851-02]. A. W. B. also reports Prevention Epicenters Program grants from Centers for Disease Control and Prevention (CDC) and grants from Agency for Healthcare Research and Quality (AHRQ), outside the submitted work. D. A. reports grants from CDC, Agency for Healthcare Research and Quality (AHRQ), and Antibacterial Resistance Leadership Group (ARLG); and royalties from UpToDate Online, outside the submitted work.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.




