-
PDF
- Split View
-
Views
-
Cite
Cite
Xavier Duval, Vincent Le Moing, Sarah Tubiana, Marina Esposito-Farèse, Emila Ilic-Habensus, Florence Leclercq, Aurélie Bourdon, François Goehringer, Christine Selton-Suty, Elodie Chevalier, David Boutoille, Nicolas Piriou, Thierry Le Tourneau, Catherine Chirouze, Marie-France Seronde, Olivier Morel, Lionel Piroth, Jean-Christophe Eicher, Olivier Humbert, Matthieu Revest, Elise Thébault, Anne Devillers, François Delahaye, André Boibieux, Bastien Grégoire, Bruno Hoen, Cédric Laouenan, Bernard Iung, François Rouzet, AEPEI-TEPvENDO study group , Impact of Systematic Whole-body 18F-Fluorodeoxyglucose PET/CT on the Management of Patients Suspected of Infective Endocarditis: The Prospective Multicenter TEPvENDO Study, Clinical Infectious Diseases, Volume 73, Issue 3, 1 August 2021, Pages 393–403, https://doi.org/10.1093/cid/ciaa666
Close - Share Icon Share
Abstract
Diagnostic and patients’ management modifications induced by whole-body 18F-FDG-PET/CT had not been evaluated so far in prosthetic valve (PV) or native valve (NV) infective endocarditis (IE)-suspected patients.
In sum, 140 consecutive patients in 8 tertiary care hospitals underwent 18F-FDG-PET/CT. ESC-2015-modified Duke criteria and patients’ management plan were established jointly by 2 experts before 18F-FDG-PET/CT. The same experts reestablished Duke classification and patients’ management plan immediately after qualitative interpretation of 18F-FDG-PET/CT. A 6-month final Duke classification was established.
Among the 70 PV and 70 NV patients, 34 and 46 were classified as definite IE before 18F-FDG-PET/CT. Abnormal perivalvular 18F-FDG uptake was recorded in 67.2% PV and 24.3% NV patients respectively (P < .001) and extracardiac uptake in 44.3% PV and 51.4% NV patients. IE classification was modified in 24.3% and 5.7% patients (P = .005) (net reclassification index 20% and 4.3%). Patients’ managements were modified in 21.4% PV and 31.4% NV patients (P = .25). It was mainly due to perivalvular uptake in PV patients and to extra-cardiac uptake in NV patients and consisted in surgery plan modifications in 7 patients, antibiotic plan modifications in 22 patients and both in 5 patients. Altogether, 18F-FDG-PET/CT modified classification and/or care in 40% of the patients (95% confidence interval: 32–48), which was most likely to occur in those with a noncontributing echocardiography (P < .001) or IE classified as possible at baseline (P = .04), while there was no difference between NV and PV.
Systematic 18F-FDG-PET/CT did significantly and appropriately impact diagnostic classification and/or IE management in PV and NV-IE suspected patients.
NCT02287792.
(See the Editorial Commentary by Rojas-Moreno on pages 404–5.)
Infective endocarditis (IE) diagnosis is often challenging, particularly when the causative microorganism is difficult to identify and/or when echocardiography is noncontributing [1, 2]. In such situations, guidelines recommend resorting to other imaging techniques to confirm or exclude valve involvement and/or search for clinically silent IE extracardiac manifestations [3, 4]. These investigations may help practitioners establish or rule out the IE diagnosis and adapt also patients’ management, especially regarding antibiotic choice and indication for and timing of valve surgery.
Several observational series have reported the diagnostic value of 18-fluorine-fluorodeoxyglucose positron emission tomography coupled with computed tomography (18F-FDG-PET/CT) in prosthetic valve (PV) infection [5–10]. This led the European Society of Cardiology to include in the ESC-2015 modified-Duke classification 18F-FDG perivalvular uptake as a major criterion of prosthetic valve infective endocarditis (PVIE) after 3 months of valve implantation, and extracardiac uptake as a minor criterion for both PV and native valve (NV) patients [4]. The American Heart Association guidelines of the same year, however, argued that “more study was needed to define the utility of 18F-FDG-PET/CT in the diagnosis and management of IE” [3]. Furthermore, the diagnostic value of FDG PET/CT in NV endocarditis has been much less investigated so far [11, 12]. In addition, beyond the diagnostic reclassification associated with 18F-FDG-PET/CT, what seems most important is its impact on patient management, which has not been evaluated to date.
Since the above-mentioned guidelines publications, the specificity of 18F-FDG-PET/CT cardiac uptake has been challenged by the evidence that perivalvular 18F-FDG uptake was frequently present in patients with PV and with no infection, regardless of the time span (<3 or >3 months) since valve implantation, due to inflammation surrounding a foreign body or use of surgical adhesives [13]. This may lead to false positives in PVIE patients when the perivalvular uptake is considered as a major Duke criterion without taking into account the uptake pattern and the use of surgical adhesive during cardiac surgery [14].
The aim of the TEPvENDO multicenter prospective study was to assess both diagnostic and patients’ management modifications induced by 18F-FDG-PET/CT using a qualitative reading of perivalvular uptake in patients suspected of NV IE or PV IE, using systematic whole body 18F-FDG-PET/CT including brain and lower limbs.
METHODS
Patients
From April 2015 to March 2016, all adult patients with high clinical suspicion of IE, hospitalized in 8 French tertiary care hospitals having a local IE team (which involved at least a cardiologist, an infectious diseases specialist, a cardiac surgeon, and a microbiologist) were included. The inclusion criteria are detailed in Supplementary Data. Written informed consent was obtained from all participants. The protocol was approved by the French CPP 1 Ethics committee (IRB no. 2014-sept-13685). 18F-FDG-PET/CT was to be performed within 7 days of inclusion. Transthoracic and/or transoesophageal echocardiography (TTE/TOE) were performed as clinically indicated [15].
IE Classifications
IE classification was established 3 times during the course of IE by 2 IE experts:
- 1.
Before 18F-FDG-PET/CT scan using Duke classification modified by Li, hereafter referred to as “Duke-Li classification at inclusion” [16];
- 2.
After 18F-FDG-PET/CT completion using a modified-ESC-2015 IE classification, hereafter referred to as “m-ESC2015 18F-FDG-PET/CT classification.” In the ESC-2015 classification, any valvular uptake is considered as a major Duke criterion only in PV patients, and emboli or aneurysms detected by 18F-FDG-PET/CT as a minor Duke criterion in PV and NV patients [3, 4]. In the present m-ESC-2015 18F-FDG-PET/CT classification, a positive valvular uptake was also considered as a major Duke criterion in NV patients.
- 3.
The final IE classification was established at month 6, hereafter referred to as “final classification.”
18F-Fluorodeoxyglucose PET/CT
Acquisition Procedure
All patients underwent a high-fat low-carbohydrate diet followed by >12 hours fasting in order to suppress physiological myocardial 18F-FDG uptake [17]. Sixty minutes after 18F-FDG injection (3.5 to 4 MBq/kg) without heparin, a low-dose computed tomography (CT) was acquired followed by whole-body PET (vertex to toes). An additional cerebral step (8-minute single bed position) was acquired 3 hours after 18F-FDG injection. Transverse PET slices were reconstructed into a 256 × 256 matrix with (AC) and without (NAC) attenuation correction.
Qualitative Analysis
Any detectable FDG uptake was considered abnormal on NV. On PV, the periprosthetic FDG uptake was qualitatively assessed on images corrected (AC) or not (NAC) for attenuation, on oblique views reoriented so that the plane of the slice coincides with the plane of the PV, and considered normal when absent or homogeneous (ie, diffuse FDG signal around the PV ring without focal enhancement) regardless of uptake intensity [14] and abnormal when heterogeneous and/or extending beyond the peri-annular area. To standardize image interpretation across participating centers, a 1-day training session was held before initiation of the study with all nuclear medicine physicians involved in the study.
Quantitative Analysis
In case of positive 18F-FDG-PET/CT, maximal standardized uptake value (SUVmax) was measured. Mean standardized uptake value (SUV) of blood-pool was calculated as the average of mean SUVs in three adjacent axial slices within the right atrium in areas devoid of significant spillover activity from surrounding tissues. Valve-to-background ratio was calculated by dividing the SUVmax of the valve area by the mean SUV of blood-pool.
18F-Fluorodeoxyglucose PET/CT Diagnostic and Management Modifications
Within 24 hours before 18F-FDG PET/CT, a cardiologist specialist and an infectious diseases specialist, both experts in IE, visited the patients and filled in a standardized questionnaire. They jointly established Duke-Li classification at inclusion according to the Duke-Li criteria, outlined an antimicrobial therapy plan and, if necessary, a surgery plan (timeline, type of surgery, indications) [16]. Within 24 hours following 18F-FDG-PET/CT completion, the 2 experts jointly reassessed IE classification (assessing the modifications related to FDG PET/CT results) and proposed diagnostic and/or therapeutic modifications when appropriate (Figure 1).
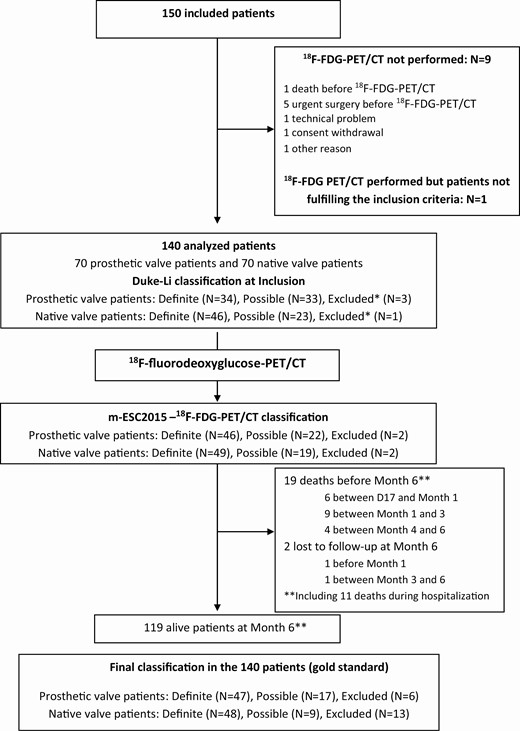
Study Flow chart. *Not fulfilling the definite and possible IE Duke-Li definition at inclusion. Abbreviations: FDG-PET-CT, fluorodeoxyglucose-positron emission tomography-computed tomography; IE, infective endocarditis.
IE Classification Gold Standard
The gold standard was the 6-month final classification that took into account all available data except the results of the FDG PET/CT if they were not confirmed by another additional exploration, to avoid tautology.
Statistical Analysis
Quantitative variables were presented as median and interquartile range (IQR). Baseline and follow-up characteristics were described by standard methods. Comparisons were performed by χ 2 or Student t test as required, or their nonparametric versions, Fisher exact or Wilcoxon/Mann-Whitney tests as appropriate.
We considered that any modification in the Duke-Li classification due to 18F-FDG PET/CT was a change in diagnosis, whatever the direction of the change (upgrade or downgrade diagnosis). To estimate whether 18F-FDG-PET/CT helped physicians to properly reclassify patients according to the gold standard, we calculated the net reclassification index (NRI) [18] (definite IE vs others). Several diagnostic performances were calculated and expressed with their 95% confidence interval (CI).
Patients’ characteristics were compared according to whether they would benefit from 18F-FDG-PET/CT. Patients benefiting from 18F-FDG-PET/CT were defined as those whose Duke-Li classification was correctly reclassified after 18F-FDG-PET/CT according to the gold standard, and/or for whom 18F-FDG-PET/CT revealed a previously unknown IE portal of entry, and/or led to a management modification.
The significant statistical level was 2-sided 5%. All statistical analyses were performed using R software v 3.4 (R Foundation for Statistical Computing Platform). The analyses were performed according to the Standards for Reporting of Diagnostic Accuracy guidelines (STARD initiative) [19].
Sample Size
Based on the literature, we assumed that 18F-FDG-PET/CT would detect otherwise undiagnosed complications in 20% of patients. We anticipated that findings in 75% of these patients would lead to a change in their therapeutic management, which corresponded to 15% of all the patients. The enrollment of 150 patients would allow 5.7% (95% CI: 9.3–20.7) accuracy of the estimated rate of change in patients’ management.
RESULTS
Baseline Characteristics
One hundred and forty patients were analyzed (70 PV patients and 70 NV patients) (Figure 1, Table 1). At inclusion, according to the Duke-Li classification, IE was classified as definite in 80 patients (34 PVIE and 46 native valve infective endocarditis [NVIE]) (P = .095), possible in 56 patients (33 PVIE and 23 NVIE) and excluded in 4 patients (3 PV and 1 NV) who did not fulfill any of these 2 categories but considered as probable IE by attending physicians (Figure 1, Table S1). In the 70 PV patients, the median time span after the last valve implantation was 5.8 years (IQR: 2.9–9.3); 62 out of the 70 cardiac surgery reports were collected, and 6 (9.7%) indicated the use of surgical adhesive. Median C-reactive protein (CRP) was 78 (IQR: 29–146) mg/L.
Baseline Characteristics of the 140 Patients According to the Nature of the Cardiac Valve and to the Final Duke-Li IE Classification (Gold Standard) at 6 Months
| . | All Patients N=140 . | PV Patients N=70 . | . | . | . | NV Patients N=70 . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | . | DefiniteN=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | P-valuea . |
| Age (years) | 67 | 65.3 (15.7) | 69.2 (14.1) | 60.7 (24.5) | 65.83 (16.12) | 62.1 (13.8) | 67.56 (12.01) | 71.31 (13.8) | 64.5 (13.9) | .295 |
| Male | 140 (74.3) | 37 (78.7) | 11 (64.71) | 2 (33.3) | 50 (71.4) | 40 (83.3) | 8 (88.9) | 6 (46.2) | 54 (77.1) | .562 |
| Diabetes | 29 (20.7) | 9 (19.1) | 2 (11.8) | 1 (16.8) | 12 (17.1) | 6 (12.5) | 4 (44.4) | 7 (53.9) | 17 (24.3) | .404 |
| History of cancer | 23 (16.4) | 6 (12.8) | 4 (23.5) | 1 (16.8) | 11 (15.7) | 6 (12.5) | 2 (22.2) | 4 (30.8) | 12 (17.1) | >.999 |
| Severe comorbidityb | 31 (22.1) | 10 (21.3) | 5 (29.4) | 2 (33.3) | 17 (24.3) | 7 (14.6) | 2 (22.2) | 5 (38.5) | 14 (20.0) | .684 |
| Bioprosthetic valve | 39 (27.9) | 25 (53.2) | 9 (52.9) | 5 (83.3) | 39 (55.7) | NA | NA | NA | NA | - |
| Mechanical valve | 31 (22.1) | 22 (46.8) | 7 (41.2) | 2 (33.3) | 31 (44.3) | NA | NA | NA | NA | - |
| CRP >= 40 mg/L | 93 (67.9) | 31 (67.4) | 9 (52.9) | 2 (33.3) | 42 (60.9) | 36 (78.3) | 6 (66.7) | 9 (69.2) | 51 (75) | .100 |
| Causative microorganisms | ||||||||||
| Staphylococcus aureus | 26 (18.6) | 7 (14.9) | 1 (5.9) | 0 (0) | 8 (11.4) | 9 (18.8) | 2 (22.2) | 7 (53.9) | 18 (25.7) | .248 |
| Coagulase-negative staphylococci | 17 (12.1) | 5 (10.6) | 1 (5.9) | 2 (33.3) | 8 (11.4) | 5 (10.4) | 1 (11.1) | 3 (23.1) | 9 (12.9) | |
| Oral streptococci | 25 (17.9) | 11 (23.4) | 2 (11.8) | 0 (0) | 13 (18.6) | 11 (22.9) | 1 (11.1) | 0 (0) | 12 (17.1) | |
| Streptococcus bovis | 11 (7.9) | 6 (12.8) | 0 (0) | 0 (0) | 6 (8.6) | 4 (8.3) | 0 (0) | 1 (7.7) | 5 (7.1) | |
| Enterococcus | 12 (8.6) | 4 (8.5) | 2 (11.8) | 0 (0) | 6 (8.6) | 5 (10.4) | 1 (11.1) | 0 (0) | 6 (8.6) | |
| HACEK | 5 (3.6) | 4 (8.5) | 0 (0) | 0 (0) | 4 (5.7) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1.4) | |
| Other | 23 (16.4) | 8 (17.1) | 2 (11.8) | 1 (16.7) | 11 (15.7) | 10 (20.8) | 2 (22.2) | 0 (0) | 12 (17.1) | |
| Negative blood cultures | 21 (15.0) | 2 (4.7) | 9 (52.9) | 3 (33.3) | 14 (20.0) | 3 (6.3) | 2 (22.2) | 2 (15.4) | 7 (10.0) | |
| Echocardiography | ||||||||||
| Transthoracic alone c | 36 (25.7) | 10 (21.2) | 2 (11.8) | 2 (33.3) | 14 (20.0) | 14 (29.2) | 3 (33.3) | 5 (38.5) | 22 (31.4) | .175 |
| At least transoesophageal | 104 (74.3) | 37 (78.7) | 15 (88.2) | 4 (66.7) | 56 (80.0) | 34 (70.8) | 6 (66.7) | 8 (61.5) | 48 (68.6) | .175 |
| Vegetation | 71 (50.7) | 18 (38.3) | 6 (35.3) | 4 (66.7) | 28 (40.0) | 35 (72.9) | 3 (33.3) | 5 (38.5) | 43 (61.4) | .018 |
| Peri annular complication | 16 (11.4) | 8 (17.0) | 1 (5.9) | 0 (0) | 9 (12.9) | 7 (14.6) | 0 (0) | 0 (0) | 7 (10.0) | .791 |
| New partial dehiscence | 13 (9.3) | 9 (19.2) | 2 (11.8) | 2 (33.3) | 13 (18.6) | NA | NA | NA | NA | |
| New valvular regurgitation | 34 (24.3) | 7 (14.9) | 2 (11.8) | 2 (33.3) | 11 (15.7) | 20 (41.7) | 3 (33.3) | 0 (0) | 23 (32.9) | .029 |
| Noncontributive echocardiography | 53 (37.9) | 19 (40.4) | 9 (52.9) | 1 (16.7) | 29 (41.4) | 11 (22.9) | 5 (55.6) | 8 (61.5) | 24 (34.3) | .486 |
| . | All Patients N=140 . | PV Patients N=70 . | . | . | . | NV Patients N=70 . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | . | DefiniteN=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | P-valuea . |
| Age (years) | 67 | 65.3 (15.7) | 69.2 (14.1) | 60.7 (24.5) | 65.83 (16.12) | 62.1 (13.8) | 67.56 (12.01) | 71.31 (13.8) | 64.5 (13.9) | .295 |
| Male | 140 (74.3) | 37 (78.7) | 11 (64.71) | 2 (33.3) | 50 (71.4) | 40 (83.3) | 8 (88.9) | 6 (46.2) | 54 (77.1) | .562 |
| Diabetes | 29 (20.7) | 9 (19.1) | 2 (11.8) | 1 (16.8) | 12 (17.1) | 6 (12.5) | 4 (44.4) | 7 (53.9) | 17 (24.3) | .404 |
| History of cancer | 23 (16.4) | 6 (12.8) | 4 (23.5) | 1 (16.8) | 11 (15.7) | 6 (12.5) | 2 (22.2) | 4 (30.8) | 12 (17.1) | >.999 |
| Severe comorbidityb | 31 (22.1) | 10 (21.3) | 5 (29.4) | 2 (33.3) | 17 (24.3) | 7 (14.6) | 2 (22.2) | 5 (38.5) | 14 (20.0) | .684 |
| Bioprosthetic valve | 39 (27.9) | 25 (53.2) | 9 (52.9) | 5 (83.3) | 39 (55.7) | NA | NA | NA | NA | - |
| Mechanical valve | 31 (22.1) | 22 (46.8) | 7 (41.2) | 2 (33.3) | 31 (44.3) | NA | NA | NA | NA | - |
| CRP >= 40 mg/L | 93 (67.9) | 31 (67.4) | 9 (52.9) | 2 (33.3) | 42 (60.9) | 36 (78.3) | 6 (66.7) | 9 (69.2) | 51 (75) | .100 |
| Causative microorganisms | ||||||||||
| Staphylococcus aureus | 26 (18.6) | 7 (14.9) | 1 (5.9) | 0 (0) | 8 (11.4) | 9 (18.8) | 2 (22.2) | 7 (53.9) | 18 (25.7) | .248 |
| Coagulase-negative staphylococci | 17 (12.1) | 5 (10.6) | 1 (5.9) | 2 (33.3) | 8 (11.4) | 5 (10.4) | 1 (11.1) | 3 (23.1) | 9 (12.9) | |
| Oral streptococci | 25 (17.9) | 11 (23.4) | 2 (11.8) | 0 (0) | 13 (18.6) | 11 (22.9) | 1 (11.1) | 0 (0) | 12 (17.1) | |
| Streptococcus bovis | 11 (7.9) | 6 (12.8) | 0 (0) | 0 (0) | 6 (8.6) | 4 (8.3) | 0 (0) | 1 (7.7) | 5 (7.1) | |
| Enterococcus | 12 (8.6) | 4 (8.5) | 2 (11.8) | 0 (0) | 6 (8.6) | 5 (10.4) | 1 (11.1) | 0 (0) | 6 (8.6) | |
| HACEK | 5 (3.6) | 4 (8.5) | 0 (0) | 0 (0) | 4 (5.7) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1.4) | |
| Other | 23 (16.4) | 8 (17.1) | 2 (11.8) | 1 (16.7) | 11 (15.7) | 10 (20.8) | 2 (22.2) | 0 (0) | 12 (17.1) | |
| Negative blood cultures | 21 (15.0) | 2 (4.7) | 9 (52.9) | 3 (33.3) | 14 (20.0) | 3 (6.3) | 2 (22.2) | 2 (15.4) | 7 (10.0) | |
| Echocardiography | ||||||||||
| Transthoracic alone c | 36 (25.7) | 10 (21.2) | 2 (11.8) | 2 (33.3) | 14 (20.0) | 14 (29.2) | 3 (33.3) | 5 (38.5) | 22 (31.4) | .175 |
| At least transoesophageal | 104 (74.3) | 37 (78.7) | 15 (88.2) | 4 (66.7) | 56 (80.0) | 34 (70.8) | 6 (66.7) | 8 (61.5) | 48 (68.6) | .175 |
| Vegetation | 71 (50.7) | 18 (38.3) | 6 (35.3) | 4 (66.7) | 28 (40.0) | 35 (72.9) | 3 (33.3) | 5 (38.5) | 43 (61.4) | .018 |
| Peri annular complication | 16 (11.4) | 8 (17.0) | 1 (5.9) | 0 (0) | 9 (12.9) | 7 (14.6) | 0 (0) | 0 (0) | 7 (10.0) | .791 |
| New partial dehiscence | 13 (9.3) | 9 (19.2) | 2 (11.8) | 2 (33.3) | 13 (18.6) | NA | NA | NA | NA | |
| New valvular regurgitation | 34 (24.3) | 7 (14.9) | 2 (11.8) | 2 (33.3) | 11 (15.7) | 20 (41.7) | 3 (33.3) | 0 (0) | 23 (32.9) | .029 |
| Noncontributive echocardiography | 53 (37.9) | 19 (40.4) | 9 (52.9) | 1 (16.7) | 29 (41.4) | 11 (22.9) | 5 (55.6) | 8 (61.5) | 24 (34.3) | .486 |
Values are mean (interquartile range) or n (%).
Abbreviations: CRP, C-reactive protein; HACEK, Haemophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IE, infective endocarditis; NA, not applicable; NV, native valve; PV, prosthetic valve; TOE, transoesophageal echocardiography; TTE, transthoracic echocardiography.
aStatistical comparisons between PV and NV patients.
bUnderlying disease affecting the vital prognostic or fatal at 5 years; values are (%) except for age (mean ± standard deviation).
cAmong the 14 prosthetic valve patients without TOE before 18F-FDG-PET/CT, 5 had vegetation on TTE, 6 had TOE after 18F-FDG-PET/CT, and 2 had contraindication to TOE.
Baseline Characteristics of the 140 Patients According to the Nature of the Cardiac Valve and to the Final Duke-Li IE Classification (Gold Standard) at 6 Months
| . | All Patients N=140 . | PV Patients N=70 . | . | . | . | NV Patients N=70 . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | . | DefiniteN=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | P-valuea . |
| Age (years) | 67 | 65.3 (15.7) | 69.2 (14.1) | 60.7 (24.5) | 65.83 (16.12) | 62.1 (13.8) | 67.56 (12.01) | 71.31 (13.8) | 64.5 (13.9) | .295 |
| Male | 140 (74.3) | 37 (78.7) | 11 (64.71) | 2 (33.3) | 50 (71.4) | 40 (83.3) | 8 (88.9) | 6 (46.2) | 54 (77.1) | .562 |
| Diabetes | 29 (20.7) | 9 (19.1) | 2 (11.8) | 1 (16.8) | 12 (17.1) | 6 (12.5) | 4 (44.4) | 7 (53.9) | 17 (24.3) | .404 |
| History of cancer | 23 (16.4) | 6 (12.8) | 4 (23.5) | 1 (16.8) | 11 (15.7) | 6 (12.5) | 2 (22.2) | 4 (30.8) | 12 (17.1) | >.999 |
| Severe comorbidityb | 31 (22.1) | 10 (21.3) | 5 (29.4) | 2 (33.3) | 17 (24.3) | 7 (14.6) | 2 (22.2) | 5 (38.5) | 14 (20.0) | .684 |
| Bioprosthetic valve | 39 (27.9) | 25 (53.2) | 9 (52.9) | 5 (83.3) | 39 (55.7) | NA | NA | NA | NA | - |
| Mechanical valve | 31 (22.1) | 22 (46.8) | 7 (41.2) | 2 (33.3) | 31 (44.3) | NA | NA | NA | NA | - |
| CRP >= 40 mg/L | 93 (67.9) | 31 (67.4) | 9 (52.9) | 2 (33.3) | 42 (60.9) | 36 (78.3) | 6 (66.7) | 9 (69.2) | 51 (75) | .100 |
| Causative microorganisms | ||||||||||
| Staphylococcus aureus | 26 (18.6) | 7 (14.9) | 1 (5.9) | 0 (0) | 8 (11.4) | 9 (18.8) | 2 (22.2) | 7 (53.9) | 18 (25.7) | .248 |
| Coagulase-negative staphylococci | 17 (12.1) | 5 (10.6) | 1 (5.9) | 2 (33.3) | 8 (11.4) | 5 (10.4) | 1 (11.1) | 3 (23.1) | 9 (12.9) | |
| Oral streptococci | 25 (17.9) | 11 (23.4) | 2 (11.8) | 0 (0) | 13 (18.6) | 11 (22.9) | 1 (11.1) | 0 (0) | 12 (17.1) | |
| Streptococcus bovis | 11 (7.9) | 6 (12.8) | 0 (0) | 0 (0) | 6 (8.6) | 4 (8.3) | 0 (0) | 1 (7.7) | 5 (7.1) | |
| Enterococcus | 12 (8.6) | 4 (8.5) | 2 (11.8) | 0 (0) | 6 (8.6) | 5 (10.4) | 1 (11.1) | 0 (0) | 6 (8.6) | |
| HACEK | 5 (3.6) | 4 (8.5) | 0 (0) | 0 (0) | 4 (5.7) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1.4) | |
| Other | 23 (16.4) | 8 (17.1) | 2 (11.8) | 1 (16.7) | 11 (15.7) | 10 (20.8) | 2 (22.2) | 0 (0) | 12 (17.1) | |
| Negative blood cultures | 21 (15.0) | 2 (4.7) | 9 (52.9) | 3 (33.3) | 14 (20.0) | 3 (6.3) | 2 (22.2) | 2 (15.4) | 7 (10.0) | |
| Echocardiography | ||||||||||
| Transthoracic alone c | 36 (25.7) | 10 (21.2) | 2 (11.8) | 2 (33.3) | 14 (20.0) | 14 (29.2) | 3 (33.3) | 5 (38.5) | 22 (31.4) | .175 |
| At least transoesophageal | 104 (74.3) | 37 (78.7) | 15 (88.2) | 4 (66.7) | 56 (80.0) | 34 (70.8) | 6 (66.7) | 8 (61.5) | 48 (68.6) | .175 |
| Vegetation | 71 (50.7) | 18 (38.3) | 6 (35.3) | 4 (66.7) | 28 (40.0) | 35 (72.9) | 3 (33.3) | 5 (38.5) | 43 (61.4) | .018 |
| Peri annular complication | 16 (11.4) | 8 (17.0) | 1 (5.9) | 0 (0) | 9 (12.9) | 7 (14.6) | 0 (0) | 0 (0) | 7 (10.0) | .791 |
| New partial dehiscence | 13 (9.3) | 9 (19.2) | 2 (11.8) | 2 (33.3) | 13 (18.6) | NA | NA | NA | NA | |
| New valvular regurgitation | 34 (24.3) | 7 (14.9) | 2 (11.8) | 2 (33.3) | 11 (15.7) | 20 (41.7) | 3 (33.3) | 0 (0) | 23 (32.9) | .029 |
| Noncontributive echocardiography | 53 (37.9) | 19 (40.4) | 9 (52.9) | 1 (16.7) | 29 (41.4) | 11 (22.9) | 5 (55.6) | 8 (61.5) | 24 (34.3) | .486 |
| . | All Patients N=140 . | PV Patients N=70 . | . | . | . | NV Patients N=70 . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | . | DefiniteN=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | P-valuea . |
| Age (years) | 67 | 65.3 (15.7) | 69.2 (14.1) | 60.7 (24.5) | 65.83 (16.12) | 62.1 (13.8) | 67.56 (12.01) | 71.31 (13.8) | 64.5 (13.9) | .295 |
| Male | 140 (74.3) | 37 (78.7) | 11 (64.71) | 2 (33.3) | 50 (71.4) | 40 (83.3) | 8 (88.9) | 6 (46.2) | 54 (77.1) | .562 |
| Diabetes | 29 (20.7) | 9 (19.1) | 2 (11.8) | 1 (16.8) | 12 (17.1) | 6 (12.5) | 4 (44.4) | 7 (53.9) | 17 (24.3) | .404 |
| History of cancer | 23 (16.4) | 6 (12.8) | 4 (23.5) | 1 (16.8) | 11 (15.7) | 6 (12.5) | 2 (22.2) | 4 (30.8) | 12 (17.1) | >.999 |
| Severe comorbidityb | 31 (22.1) | 10 (21.3) | 5 (29.4) | 2 (33.3) | 17 (24.3) | 7 (14.6) | 2 (22.2) | 5 (38.5) | 14 (20.0) | .684 |
| Bioprosthetic valve | 39 (27.9) | 25 (53.2) | 9 (52.9) | 5 (83.3) | 39 (55.7) | NA | NA | NA | NA | - |
| Mechanical valve | 31 (22.1) | 22 (46.8) | 7 (41.2) | 2 (33.3) | 31 (44.3) | NA | NA | NA | NA | - |
| CRP >= 40 mg/L | 93 (67.9) | 31 (67.4) | 9 (52.9) | 2 (33.3) | 42 (60.9) | 36 (78.3) | 6 (66.7) | 9 (69.2) | 51 (75) | .100 |
| Causative microorganisms | ||||||||||
| Staphylococcus aureus | 26 (18.6) | 7 (14.9) | 1 (5.9) | 0 (0) | 8 (11.4) | 9 (18.8) | 2 (22.2) | 7 (53.9) | 18 (25.7) | .248 |
| Coagulase-negative staphylococci | 17 (12.1) | 5 (10.6) | 1 (5.9) | 2 (33.3) | 8 (11.4) | 5 (10.4) | 1 (11.1) | 3 (23.1) | 9 (12.9) | |
| Oral streptococci | 25 (17.9) | 11 (23.4) | 2 (11.8) | 0 (0) | 13 (18.6) | 11 (22.9) | 1 (11.1) | 0 (0) | 12 (17.1) | |
| Streptococcus bovis | 11 (7.9) | 6 (12.8) | 0 (0) | 0 (0) | 6 (8.6) | 4 (8.3) | 0 (0) | 1 (7.7) | 5 (7.1) | |
| Enterococcus | 12 (8.6) | 4 (8.5) | 2 (11.8) | 0 (0) | 6 (8.6) | 5 (10.4) | 1 (11.1) | 0 (0) | 6 (8.6) | |
| HACEK | 5 (3.6) | 4 (8.5) | 0 (0) | 0 (0) | 4 (5.7) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1.4) | |
| Other | 23 (16.4) | 8 (17.1) | 2 (11.8) | 1 (16.7) | 11 (15.7) | 10 (20.8) | 2 (22.2) | 0 (0) | 12 (17.1) | |
| Negative blood cultures | 21 (15.0) | 2 (4.7) | 9 (52.9) | 3 (33.3) | 14 (20.0) | 3 (6.3) | 2 (22.2) | 2 (15.4) | 7 (10.0) | |
| Echocardiography | ||||||||||
| Transthoracic alone c | 36 (25.7) | 10 (21.2) | 2 (11.8) | 2 (33.3) | 14 (20.0) | 14 (29.2) | 3 (33.3) | 5 (38.5) | 22 (31.4) | .175 |
| At least transoesophageal | 104 (74.3) | 37 (78.7) | 15 (88.2) | 4 (66.7) | 56 (80.0) | 34 (70.8) | 6 (66.7) | 8 (61.5) | 48 (68.6) | .175 |
| Vegetation | 71 (50.7) | 18 (38.3) | 6 (35.3) | 4 (66.7) | 28 (40.0) | 35 (72.9) | 3 (33.3) | 5 (38.5) | 43 (61.4) | .018 |
| Peri annular complication | 16 (11.4) | 8 (17.0) | 1 (5.9) | 0 (0) | 9 (12.9) | 7 (14.6) | 0 (0) | 0 (0) | 7 (10.0) | .791 |
| New partial dehiscence | 13 (9.3) | 9 (19.2) | 2 (11.8) | 2 (33.3) | 13 (18.6) | NA | NA | NA | NA | |
| New valvular regurgitation | 34 (24.3) | 7 (14.9) | 2 (11.8) | 2 (33.3) | 11 (15.7) | 20 (41.7) | 3 (33.3) | 0 (0) | 23 (32.9) | .029 |
| Noncontributive echocardiography | 53 (37.9) | 19 (40.4) | 9 (52.9) | 1 (16.7) | 29 (41.4) | 11 (22.9) | 5 (55.6) | 8 (61.5) | 24 (34.3) | .486 |
Values are mean (interquartile range) or n (%).
Abbreviations: CRP, C-reactive protein; HACEK, Haemophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IE, infective endocarditis; NA, not applicable; NV, native valve; PV, prosthetic valve; TOE, transoesophageal echocardiography; TTE, transthoracic echocardiography.
aStatistical comparisons between PV and NV patients.
bUnderlying disease affecting the vital prognostic or fatal at 5 years; values are (%) except for age (mean ± standard deviation).
cAmong the 14 prosthetic valve patients without TOE before 18F-FDG-PET/CT, 5 had vegetation on TTE, 6 had TOE after 18F-FDG-PET/CT, and 2 had contraindication to TOE.
Follow-up
At M6, the final IE classification (ie, gold standard) was definite in 95 (67.9%) patients (47 definite PVIE and 48 definite NVIE), possible in 26 (18.6%) patients and excluded in 19 (13.6%) patients (Figure 1; Tables 1 and 2).
Diagnostic Value of the Duke-Li Criteria at Inclusion and After 18F-FDG-PET/CT According to the Final Duke-LI IE Classification in the 140 Patients
| . | . | Prosthetic Valve Patients N=70 . | . | . | . | Native Valve Patients N=70 . | . | . | . | P-valuea . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | Total N=140 . | Definite N=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | . |
| Duke-Li classification at inclusionb | ||||||||||
| Definite | 80 (57.1) | 32 (68.1) | 2 (11.8) | 0 (0) | 34 (48.6) | 42 (87.5) | 0 (0) | 4 (30.8) | 46 (65.7) | .095 |
| Possible | 56 (40.0) | 15 (31.9) | 13 (76.5) | 5 (83.3) | 33 (47.14 | 6 (12.5) | 9 (100) | 8 (61.5) | 23 (32.6) | |
| Excludedc | 4 (2.6) | 0 (0) | 2 (11.8) | 1 (16.7) | 3 (4.3) | 0 (0) | 0 (0) | 1 (7.7) | 1 (1.4) | |
| 18F-FDG-PET/CT results | ||||||||||
| Perivalvular uptake | ||||||||||
| Abnormal uptaked | 64 (45.7) | 38 (80.4) | 7 (41.2) | 2 (33.3) | 47 (67.1) | 16 (33.3) | 1 (11.1) | 0 (0) | 17 (24.3) | <.001 |
| Noninterpretable | 5 (3.6) | 2 (4.3) | 2 (11.8) | 0 (0) | 4 (5.1) | 1 (2.2) | 0 (0) | 0 (0) | 1 (1.4) | .282 |
| Extracardiac uptake | ||||||||||
| Peripheral IE complicatione | 69 (49.3) | 24 (51.1) | 7 (41.2) | 1 (16.7) | 32 (45.7) | 27 (56.3) | 3 (33.3) | 7 (53.9) | 37 (52.9) | .381 |
| Portal of entry | ||||||||||
| All | 33 (23.6) | 11 (23.4) | 4 (23.5) | 0 (0) | 15 (21.4) | 14 (29.2) | 1 (11.1) | 3 (23.1) | 18 (25.7) | .302 |
| Revealed | 12 (8.0) | 6 (12.8) | 2 (11.8) | 0 (0) | 8 (11.4) | 3 (6.3) | 0 (0) | 1 (7.7) | 4 (5.7) | .366 |
| Confirmed | 21 (15.0) | 5 (10.6) | 2 (11.8) | 0 (0) | 7 (10.0) | 11 (22.9) | 1 (11.1) | 2 (15.4) | 14 (20.0) | .154 |
| Modification of Duke-Li criteriaf,g | ||||||||||
| Modification of any Duke criterion | 43 (30.7) | 22 (46.8) | 3 (17.7) | 1 (16.7) | 26 (37.1) | 14 (29.2) | 1 (11.1) | 2 (15.4) | 17 (24.3) | .142 |
| Modification of a minor Duke criterion | 21 (15.0) | 6 (27.3) | 0 (0) | 1 (100) | 7 (10.0) | 11 (22.9) | 1(100) | 2 (100) | 14 (20.0) | <.001 |
| Modification of a major Duke criterion | 23 (16.4) | 17 (77.3) | 3 (100) | 0 (0) | 20 (28.6) | 3 (6.3) | 0 (0) | 0 (0) | 3 (4.3) | <.001 |
| Modification of Duke-Li classificationf,g | ||||||||||
| Any modification | 21 (15.0) | 13 (27.7) | 3 (17.7) | 1 (16.7) | 17 (24.3) | 3 (6.3) | 0 (0) | 1 (7.7) | 4 (5.7) | .003 |
| Modification due to a minor Duke criterion | 6 (4.3) | 3 (4.6) | 0 (0) | 1 (16.7) | 4 (5.7) | 1 (2.1) | 0 (0) | 1 (100) | 2 (2.9) | .544 |
| Modification due to a major Duke criterion | 16 (11.4) | 11 (84.6) | 3 (100) | 0 (0) | 14 (20.0) | 2 (4.2) | 0 (0) | 0 (0) | 2 (2.9) | .228 |
| m-ESC201518F-FDG-PET/CT classificationg | ||||||||||
| Definite | 95 (67.9) | 45 (95.7) | 1 (5.9) | 0 (0) | 46 (65.7) | 45 (93.8) | 0 (0) | 4 (30.8) | 49 (70.0) | .889 |
| Possible | 41 (29.3) | 2 (4.3) | 16 (94.1) | 4 (66.7) | 22 (31.4) | 3 (6.3) | 9 (100) | 7 (53.9) | 19 (27.1) | |
| Excluded | 4 (2.9) | 0 (0) | 0 (0) | 2 (33.3) | 2 (2.9) | 0 (0) | 0 (0) | 2 (15.4) | 2 (2.9) |
| . | . | Prosthetic Valve Patients N=70 . | . | . | . | Native Valve Patients N=70 . | . | . | . | P-valuea . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | Total N=140 . | Definite N=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | . |
| Duke-Li classification at inclusionb | ||||||||||
| Definite | 80 (57.1) | 32 (68.1) | 2 (11.8) | 0 (0) | 34 (48.6) | 42 (87.5) | 0 (0) | 4 (30.8) | 46 (65.7) | .095 |
| Possible | 56 (40.0) | 15 (31.9) | 13 (76.5) | 5 (83.3) | 33 (47.14 | 6 (12.5) | 9 (100) | 8 (61.5) | 23 (32.6) | |
| Excludedc | 4 (2.6) | 0 (0) | 2 (11.8) | 1 (16.7) | 3 (4.3) | 0 (0) | 0 (0) | 1 (7.7) | 1 (1.4) | |
| 18F-FDG-PET/CT results | ||||||||||
| Perivalvular uptake | ||||||||||
| Abnormal uptaked | 64 (45.7) | 38 (80.4) | 7 (41.2) | 2 (33.3) | 47 (67.1) | 16 (33.3) | 1 (11.1) | 0 (0) | 17 (24.3) | <.001 |
| Noninterpretable | 5 (3.6) | 2 (4.3) | 2 (11.8) | 0 (0) | 4 (5.1) | 1 (2.2) | 0 (0) | 0 (0) | 1 (1.4) | .282 |
| Extracardiac uptake | ||||||||||
| Peripheral IE complicatione | 69 (49.3) | 24 (51.1) | 7 (41.2) | 1 (16.7) | 32 (45.7) | 27 (56.3) | 3 (33.3) | 7 (53.9) | 37 (52.9) | .381 |
| Portal of entry | ||||||||||
| All | 33 (23.6) | 11 (23.4) | 4 (23.5) | 0 (0) | 15 (21.4) | 14 (29.2) | 1 (11.1) | 3 (23.1) | 18 (25.7) | .302 |
| Revealed | 12 (8.0) | 6 (12.8) | 2 (11.8) | 0 (0) | 8 (11.4) | 3 (6.3) | 0 (0) | 1 (7.7) | 4 (5.7) | .366 |
| Confirmed | 21 (15.0) | 5 (10.6) | 2 (11.8) | 0 (0) | 7 (10.0) | 11 (22.9) | 1 (11.1) | 2 (15.4) | 14 (20.0) | .154 |
| Modification of Duke-Li criteriaf,g | ||||||||||
| Modification of any Duke criterion | 43 (30.7) | 22 (46.8) | 3 (17.7) | 1 (16.7) | 26 (37.1) | 14 (29.2) | 1 (11.1) | 2 (15.4) | 17 (24.3) | .142 |
| Modification of a minor Duke criterion | 21 (15.0) | 6 (27.3) | 0 (0) | 1 (100) | 7 (10.0) | 11 (22.9) | 1(100) | 2 (100) | 14 (20.0) | <.001 |
| Modification of a major Duke criterion | 23 (16.4) | 17 (77.3) | 3 (100) | 0 (0) | 20 (28.6) | 3 (6.3) | 0 (0) | 0 (0) | 3 (4.3) | <.001 |
| Modification of Duke-Li classificationf,g | ||||||||||
| Any modification | 21 (15.0) | 13 (27.7) | 3 (17.7) | 1 (16.7) | 17 (24.3) | 3 (6.3) | 0 (0) | 1 (7.7) | 4 (5.7) | .003 |
| Modification due to a minor Duke criterion | 6 (4.3) | 3 (4.6) | 0 (0) | 1 (16.7) | 4 (5.7) | 1 (2.1) | 0 (0) | 1 (100) | 2 (2.9) | .544 |
| Modification due to a major Duke criterion | 16 (11.4) | 11 (84.6) | 3 (100) | 0 (0) | 14 (20.0) | 2 (4.2) | 0 (0) | 0 (0) | 2 (2.9) | .228 |
| m-ESC201518F-FDG-PET/CT classificationg | ||||||||||
| Definite | 95 (67.9) | 45 (95.7) | 1 (5.9) | 0 (0) | 46 (65.7) | 45 (93.8) | 0 (0) | 4 (30.8) | 49 (70.0) | .889 |
| Possible | 41 (29.3) | 2 (4.3) | 16 (94.1) | 4 (66.7) | 22 (31.4) | 3 (6.3) | 9 (100) | 7 (53.9) | 19 (27.1) | |
| Excluded | 4 (2.9) | 0 (0) | 0 (0) | 2 (33.3) | 2 (2.9) | 0 (0) | 0 (0) | 2 (15.4) | 2 (2.9) |
Values are n (%).
Abbreviations: FDG, fluorodeoxyglucose; IE, infective endocarditis; PET/CT, positron emission tomography/computed tomography.
aStatistical comparisons between PV and NV patients.
bDuke classification modified by Li [16].
cNot fulfilling the definite and possible IE Duke definition at inclusion.
dIn prosthetic valve patients, periprosthetic FDG uptake was considered abnormal when heterogeneous (either focal or diffuse with a focal enhancement) and/or extending beyond the peri-annular area; 10 additional prosthetic valve patients had valvular uptake considered as normal.
eExcluding sternum, prostate, colon, mouth, and skin uptake.
fModification of the criteria or the classification of the “Duke-Li classification at inclusion.”
gSee text for m-ESC 2015 18F-FDG-PET/CT classification definition.
Diagnostic Value of the Duke-Li Criteria at Inclusion and After 18F-FDG-PET/CT According to the Final Duke-LI IE Classification in the 140 Patients
| . | . | Prosthetic Valve Patients N=70 . | . | . | . | Native Valve Patients N=70 . | . | . | . | P-valuea . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | Total N=140 . | Definite N=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | . |
| Duke-Li classification at inclusionb | ||||||||||
| Definite | 80 (57.1) | 32 (68.1) | 2 (11.8) | 0 (0) | 34 (48.6) | 42 (87.5) | 0 (0) | 4 (30.8) | 46 (65.7) | .095 |
| Possible | 56 (40.0) | 15 (31.9) | 13 (76.5) | 5 (83.3) | 33 (47.14 | 6 (12.5) | 9 (100) | 8 (61.5) | 23 (32.6) | |
| Excludedc | 4 (2.6) | 0 (0) | 2 (11.8) | 1 (16.7) | 3 (4.3) | 0 (0) | 0 (0) | 1 (7.7) | 1 (1.4) | |
| 18F-FDG-PET/CT results | ||||||||||
| Perivalvular uptake | ||||||||||
| Abnormal uptaked | 64 (45.7) | 38 (80.4) | 7 (41.2) | 2 (33.3) | 47 (67.1) | 16 (33.3) | 1 (11.1) | 0 (0) | 17 (24.3) | <.001 |
| Noninterpretable | 5 (3.6) | 2 (4.3) | 2 (11.8) | 0 (0) | 4 (5.1) | 1 (2.2) | 0 (0) | 0 (0) | 1 (1.4) | .282 |
| Extracardiac uptake | ||||||||||
| Peripheral IE complicatione | 69 (49.3) | 24 (51.1) | 7 (41.2) | 1 (16.7) | 32 (45.7) | 27 (56.3) | 3 (33.3) | 7 (53.9) | 37 (52.9) | .381 |
| Portal of entry | ||||||||||
| All | 33 (23.6) | 11 (23.4) | 4 (23.5) | 0 (0) | 15 (21.4) | 14 (29.2) | 1 (11.1) | 3 (23.1) | 18 (25.7) | .302 |
| Revealed | 12 (8.0) | 6 (12.8) | 2 (11.8) | 0 (0) | 8 (11.4) | 3 (6.3) | 0 (0) | 1 (7.7) | 4 (5.7) | .366 |
| Confirmed | 21 (15.0) | 5 (10.6) | 2 (11.8) | 0 (0) | 7 (10.0) | 11 (22.9) | 1 (11.1) | 2 (15.4) | 14 (20.0) | .154 |
| Modification of Duke-Li criteriaf,g | ||||||||||
| Modification of any Duke criterion | 43 (30.7) | 22 (46.8) | 3 (17.7) | 1 (16.7) | 26 (37.1) | 14 (29.2) | 1 (11.1) | 2 (15.4) | 17 (24.3) | .142 |
| Modification of a minor Duke criterion | 21 (15.0) | 6 (27.3) | 0 (0) | 1 (100) | 7 (10.0) | 11 (22.9) | 1(100) | 2 (100) | 14 (20.0) | <.001 |
| Modification of a major Duke criterion | 23 (16.4) | 17 (77.3) | 3 (100) | 0 (0) | 20 (28.6) | 3 (6.3) | 0 (0) | 0 (0) | 3 (4.3) | <.001 |
| Modification of Duke-Li classificationf,g | ||||||||||
| Any modification | 21 (15.0) | 13 (27.7) | 3 (17.7) | 1 (16.7) | 17 (24.3) | 3 (6.3) | 0 (0) | 1 (7.7) | 4 (5.7) | .003 |
| Modification due to a minor Duke criterion | 6 (4.3) | 3 (4.6) | 0 (0) | 1 (16.7) | 4 (5.7) | 1 (2.1) | 0 (0) | 1 (100) | 2 (2.9) | .544 |
| Modification due to a major Duke criterion | 16 (11.4) | 11 (84.6) | 3 (100) | 0 (0) | 14 (20.0) | 2 (4.2) | 0 (0) | 0 (0) | 2 (2.9) | .228 |
| m-ESC201518F-FDG-PET/CT classificationg | ||||||||||
| Definite | 95 (67.9) | 45 (95.7) | 1 (5.9) | 0 (0) | 46 (65.7) | 45 (93.8) | 0 (0) | 4 (30.8) | 49 (70.0) | .889 |
| Possible | 41 (29.3) | 2 (4.3) | 16 (94.1) | 4 (66.7) | 22 (31.4) | 3 (6.3) | 9 (100) | 7 (53.9) | 19 (27.1) | |
| Excluded | 4 (2.9) | 0 (0) | 0 (0) | 2 (33.3) | 2 (2.9) | 0 (0) | 0 (0) | 2 (15.4) | 2 (2.9) |
| . | . | Prosthetic Valve Patients N=70 . | . | . | . | Native Valve Patients N=70 . | . | . | . | P-valuea . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | Total N=140 . | Definite N=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | . |
| Duke-Li classification at inclusionb | ||||||||||
| Definite | 80 (57.1) | 32 (68.1) | 2 (11.8) | 0 (0) | 34 (48.6) | 42 (87.5) | 0 (0) | 4 (30.8) | 46 (65.7) | .095 |
| Possible | 56 (40.0) | 15 (31.9) | 13 (76.5) | 5 (83.3) | 33 (47.14 | 6 (12.5) | 9 (100) | 8 (61.5) | 23 (32.6) | |
| Excludedc | 4 (2.6) | 0 (0) | 2 (11.8) | 1 (16.7) | 3 (4.3) | 0 (0) | 0 (0) | 1 (7.7) | 1 (1.4) | |
| 18F-FDG-PET/CT results | ||||||||||
| Perivalvular uptake | ||||||||||
| Abnormal uptaked | 64 (45.7) | 38 (80.4) | 7 (41.2) | 2 (33.3) | 47 (67.1) | 16 (33.3) | 1 (11.1) | 0 (0) | 17 (24.3) | <.001 |
| Noninterpretable | 5 (3.6) | 2 (4.3) | 2 (11.8) | 0 (0) | 4 (5.1) | 1 (2.2) | 0 (0) | 0 (0) | 1 (1.4) | .282 |
| Extracardiac uptake | ||||||||||
| Peripheral IE complicatione | 69 (49.3) | 24 (51.1) | 7 (41.2) | 1 (16.7) | 32 (45.7) | 27 (56.3) | 3 (33.3) | 7 (53.9) | 37 (52.9) | .381 |
| Portal of entry | ||||||||||
| All | 33 (23.6) | 11 (23.4) | 4 (23.5) | 0 (0) | 15 (21.4) | 14 (29.2) | 1 (11.1) | 3 (23.1) | 18 (25.7) | .302 |
| Revealed | 12 (8.0) | 6 (12.8) | 2 (11.8) | 0 (0) | 8 (11.4) | 3 (6.3) | 0 (0) | 1 (7.7) | 4 (5.7) | .366 |
| Confirmed | 21 (15.0) | 5 (10.6) | 2 (11.8) | 0 (0) | 7 (10.0) | 11 (22.9) | 1 (11.1) | 2 (15.4) | 14 (20.0) | .154 |
| Modification of Duke-Li criteriaf,g | ||||||||||
| Modification of any Duke criterion | 43 (30.7) | 22 (46.8) | 3 (17.7) | 1 (16.7) | 26 (37.1) | 14 (29.2) | 1 (11.1) | 2 (15.4) | 17 (24.3) | .142 |
| Modification of a minor Duke criterion | 21 (15.0) | 6 (27.3) | 0 (0) | 1 (100) | 7 (10.0) | 11 (22.9) | 1(100) | 2 (100) | 14 (20.0) | <.001 |
| Modification of a major Duke criterion | 23 (16.4) | 17 (77.3) | 3 (100) | 0 (0) | 20 (28.6) | 3 (6.3) | 0 (0) | 0 (0) | 3 (4.3) | <.001 |
| Modification of Duke-Li classificationf,g | ||||||||||
| Any modification | 21 (15.0) | 13 (27.7) | 3 (17.7) | 1 (16.7) | 17 (24.3) | 3 (6.3) | 0 (0) | 1 (7.7) | 4 (5.7) | .003 |
| Modification due to a minor Duke criterion | 6 (4.3) | 3 (4.6) | 0 (0) | 1 (16.7) | 4 (5.7) | 1 (2.1) | 0 (0) | 1 (100) | 2 (2.9) | .544 |
| Modification due to a major Duke criterion | 16 (11.4) | 11 (84.6) | 3 (100) | 0 (0) | 14 (20.0) | 2 (4.2) | 0 (0) | 0 (0) | 2 (2.9) | .228 |
| m-ESC201518F-FDG-PET/CT classificationg | ||||||||||
| Definite | 95 (67.9) | 45 (95.7) | 1 (5.9) | 0 (0) | 46 (65.7) | 45 (93.8) | 0 (0) | 4 (30.8) | 49 (70.0) | .889 |
| Possible | 41 (29.3) | 2 (4.3) | 16 (94.1) | 4 (66.7) | 22 (31.4) | 3 (6.3) | 9 (100) | 7 (53.9) | 19 (27.1) | |
| Excluded | 4 (2.9) | 0 (0) | 0 (0) | 2 (33.3) | 2 (2.9) | 0 (0) | 0 (0) | 2 (15.4) | 2 (2.9) |
Values are n (%).
Abbreviations: FDG, fluorodeoxyglucose; IE, infective endocarditis; PET/CT, positron emission tomography/computed tomography.
aStatistical comparisons between PV and NV patients.
bDuke classification modified by Li [16].
cNot fulfilling the definite and possible IE Duke definition at inclusion.
dIn prosthetic valve patients, periprosthetic FDG uptake was considered abnormal when heterogeneous (either focal or diffuse with a focal enhancement) and/or extending beyond the peri-annular area; 10 additional prosthetic valve patients had valvular uptake considered as normal.
eExcluding sternum, prostate, colon, mouth, and skin uptake.
fModification of the criteria or the classification of the “Duke-Li classification at inclusion.”
gSee text for m-ESC 2015 18F-FDG-PET/CT classification definition.
Diagnostic Value of Perivalvular 18F-FDG-PET/CT
18F-FDG-PET/CT was performed at a median time of 2 days after inclusion (IQR: 1–3.25), and of 7 days (IQR: 4–10) after antibiotic initiation.
Perivalvular/Valvular Uptake
Abnormal (peri)valvular uptake was present in 64 (45.7%) patients (47 [67.2%] in PV patients and 17 [24.3%] in NV patients) (P < .001) (Table 2). Nonspecific homogeneous perivalvular FDG uptake attributed to the presence of a PV was reported in 10 (14.3%) additional PV patients and not considered as a major criterion.
In PV patients, SUVmax of perivalvular uptake and mean SUVmax /blood-pool SUV ratio were not statistically different between patients with definite, possible, or excluded PVE according to the final diagnosis. The intensity of perivalvular uptake was independent of the time elapsed since the valve implantation and of the time span between initiation of IE antibiotic therapy and 18F-FDG-PET/CT scan.
Comparison of Perivalvular 18 F-FDG-PET/CT Uptake With Echocardiographic Findings
Table 1 summarizes the echocardiographic findings according to the final classification at 6 months. Vegetations were found in 71 patients (28 PV patients and 43 NV patients). In patients with vegetations at echocardiography, perivalvular 18F-FDG-PET/CT uptake was considered abnormal in 18 (64.2%) out of the 28 PV patients and in 13 (30.2%) out of the 43 NV patients (P = .29). In patients with noncontributive echocardiography, perivalvular 18F-FDG-PET/CT was considered as a criterion for IE in 22 of the 29 PV patients and in 4 of the 24 NV patients (P < .01).
Extracardiac Uptake (Emboli, Distant Infection and Portal of Entry)
Whole-body 18F-FDG-PET/CT identified extracardiac uptake in 69 (49.3%) patients (Table 2). Cerebral acquisitions were performed in 137 (97.9%) patients and were abnormal in 12 (8.8%) (6 patients with cerebral abscess and 6 others with ischemic stroke, which were further confirmed by cerebral imaging).
In addition to the 69 patients diagnosed with emboli and/or distant infection, a portal of entry was detected by 18F-FDG-PET/CT in 33 patients (23.6%), which was previously unknown in 12 (8.6%) patients (8 PV and 4 NV patients) (Table 2).
18F-FDG-PET/CT Impact on Diagnosis and Therapy
Diagnostic Impact
18F-FDG-PET/CT added at least one Duke criterion (major and/or minor) in 43 (30.7%) (95% CI: 23%–39%) patients. This was a major Duke criterion in 23 patients (20 in PV patients and 3 in NV patients) and/or a minor Duke criterion in 21 patients (7 in PV patients and 14 in NV patients) (Table S2). This addition of a Duke criterion led to the modification of the Duke-Li classification in 21 (15%) patients: 17 (24.3%) in PV patients and 4 (5.7%) in NV patients (P = .004). Duke-Li classification was upgraded in 18 patients (12.9%): 15 (17%) PV patients and in 3 (4%) NV patients. Duke-Li classification was downgraded in 3 patients (2.1%): 2 (3%) PV patients and in 1 (1.4%) NV patient (Table S2). As compared to final classification at 6 months, upgrading was confirmed as adequate in 16/18 patients (13/15 PV patients and 3/3 NV patients), and downgrading was confirmed as adequate in 1/3 patient (a PV patient) Absolute NRI was 12.1% (20.0% in PV patients and 4.3% in NV patients). The diagnostic performances are presented in Table S1.
Patients’ Management Modification
The therapeutic managment was modified following 18F-FDG-PET/CT scan in 37 of the 140 patients (26.4%; 95% CI: 19.1%–35.5%) corresponding to 15 (21.4%) of the PV patients and 22 (31.4%) of the NV patients (P = .25) (Tables 3). These modifications were related to antibiotic therapy (modification of duration and/or of type) in 22 patients, surgical management (surgery postponed, advanced, indicated, or canceled) in 7, both in 5, and other aspects in the 3 remaining patients (Table 3). These modifications were mainly due to the presence of a perivalvular uptake in 9 PV patients and due to the presence of an extra-cardiac uptake in 17 NV patients (Figures 2A and 2B).
Patients ‘Management Modification Following 18F-FDG-PET/CT Results According to the Final Duke-Li IE classification (Gold Standard) and Cardiac Surgery in the 140 Patients
| . | . | Prosthetic Valve Patients N=70 . | . | . | . | Native Valve Patients N=70 . | . | . | . | P-value a . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | TotalN=140 . | Definite N=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | . |
| Modification of patients’ management following18F-FDG PET/CTb | ||||||||||
| 37 (26.4) | 10 (21.3) | 4 (23.5) | 1 (16.7) | 15 (21.4) | 13(27.1) | 4 (44.4) | 5 (38.5) | 22 (31.4) | .25 | |
| Antibiotic treatment c | 18 (12.8) | 3 (6.3) | 3 (17.6 | 1 (16.7) | 7 (10.0) | 5 (10.4) | 3 (33.3) | 3 (23.1) | 11 (15.7) | .476 |
| Cardiac surgery | 6 (4.3) | 3 (6.3) | 0 (0) | 0 (0) | 3 (4.3) | 3 (6.2) | 0 (0) | 0 (0) | 3 (4.3) | |
| Anticoagulation | 1 (0.7) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Specific treatment of an IE abdominal localizationd | 1 (0.7) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 1 (0.3) | |
| Antibiotic and surgery | 5 (3.6) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1) | 3 (6.2) | 1 (11.1) | 0 (0) | 4 (5.7) | |
| Antibiotic treatment and anticoagulation | 1 (0.7) | 0 | 0 (0) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 1 (0.3) | .476 |
| Antibiotic treatment and specific treatment of an IE abdominal localization | 2 (1.4) | 1 (2.1) | 1 (2.1) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Surgery and specific treatment of an IE abdominal localization | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 1 (0.3) | |
| Antibiotic treatment, anticoagulation and specific treatment of an IE abdominal localization | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.8) | 1 (0.3) | |
| Cardiac surgery | ||||||||||
| Cardiac surgery during initial hospitalization | 41 (29.3) | 12 (25.5) | 4 (23.5) | 1 (16.7) | 17 (24.3) | 21 (43.7) | 3 (33.3) | 0 (0) | 24 (34.3) | .265 |
| Cardiac surgery during the 6 first months following inclusion | 53 (37.9) | 14 (29.8) | 5 (29.4) | 1 (16.7) | 20 (28.6) | 28 (58.3) | 4 (44.4) | 1 (7.7) | 33 (47.1) | .036 |
| . | . | Prosthetic Valve Patients N=70 . | . | . | . | Native Valve Patients N=70 . | . | . | . | P-value a . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | TotalN=140 . | Definite N=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | . |
| Modification of patients’ management following18F-FDG PET/CTb | ||||||||||
| 37 (26.4) | 10 (21.3) | 4 (23.5) | 1 (16.7) | 15 (21.4) | 13(27.1) | 4 (44.4) | 5 (38.5) | 22 (31.4) | .25 | |
| Antibiotic treatment c | 18 (12.8) | 3 (6.3) | 3 (17.6 | 1 (16.7) | 7 (10.0) | 5 (10.4) | 3 (33.3) | 3 (23.1) | 11 (15.7) | .476 |
| Cardiac surgery | 6 (4.3) | 3 (6.3) | 0 (0) | 0 (0) | 3 (4.3) | 3 (6.2) | 0 (0) | 0 (0) | 3 (4.3) | |
| Anticoagulation | 1 (0.7) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Specific treatment of an IE abdominal localizationd | 1 (0.7) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 1 (0.3) | |
| Antibiotic and surgery | 5 (3.6) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1) | 3 (6.2) | 1 (11.1) | 0 (0) | 4 (5.7) | |
| Antibiotic treatment and anticoagulation | 1 (0.7) | 0 | 0 (0) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 1 (0.3) | .476 |
| Antibiotic treatment and specific treatment of an IE abdominal localization | 2 (1.4) | 1 (2.1) | 1 (2.1) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Surgery and specific treatment of an IE abdominal localization | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 1 (0.3) | |
| Antibiotic treatment, anticoagulation and specific treatment of an IE abdominal localization | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.8) | 1 (0.3) | |
| Cardiac surgery | ||||||||||
| Cardiac surgery during initial hospitalization | 41 (29.3) | 12 (25.5) | 4 (23.5) | 1 (16.7) | 17 (24.3) | 21 (43.7) | 3 (33.3) | 0 (0) | 24 (34.3) | .265 |
| Cardiac surgery during the 6 first months following inclusion | 53 (37.9) | 14 (29.8) | 5 (29.4) | 1 (16.7) | 20 (28.6) | 28 (58.3) | 4 (44.4) | 1 (7.7) | 33 (47.1) | .036 |
Surgery modifications include surgery cancellation, surgery indication or modification of surgery timing, or valve substitute. Anticoagulation modifications include interruption of modification of anticoagulation level. Values are n (%).
Abbreviations: FDG, fluorodeoxyglucose; IE, infective endocarditis; PET/CT, positron emission tomography/computed tomography.
aStatistical comparisons between PV and NV patients.
bThe duration of antibiotic therapy was reduced in 6 patients due to the exclusion of IE diagnosis by 18F-FDG PET/CT which was in favor of an alternative diagnosis, prolonged in 4 patients. An antibiotic with a better diffusion in bone, joints or prostate gland was added in 11 patients and the dose of an antibiotic was reduced in one patient due to the exclusion of IE diagnosis.
cIncluding 2 patients with detection of IE portal of entry.
dIncluding 1 patient with detection of IE portal of entry.
Patients ‘Management Modification Following 18F-FDG-PET/CT Results According to the Final Duke-Li IE classification (Gold Standard) and Cardiac Surgery in the 140 Patients
| . | . | Prosthetic Valve Patients N=70 . | . | . | . | Native Valve Patients N=70 . | . | . | . | P-value a . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | TotalN=140 . | Definite N=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | . |
| Modification of patients’ management following18F-FDG PET/CTb | ||||||||||
| 37 (26.4) | 10 (21.3) | 4 (23.5) | 1 (16.7) | 15 (21.4) | 13(27.1) | 4 (44.4) | 5 (38.5) | 22 (31.4) | .25 | |
| Antibiotic treatment c | 18 (12.8) | 3 (6.3) | 3 (17.6 | 1 (16.7) | 7 (10.0) | 5 (10.4) | 3 (33.3) | 3 (23.1) | 11 (15.7) | .476 |
| Cardiac surgery | 6 (4.3) | 3 (6.3) | 0 (0) | 0 (0) | 3 (4.3) | 3 (6.2) | 0 (0) | 0 (0) | 3 (4.3) | |
| Anticoagulation | 1 (0.7) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Specific treatment of an IE abdominal localizationd | 1 (0.7) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 1 (0.3) | |
| Antibiotic and surgery | 5 (3.6) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1) | 3 (6.2) | 1 (11.1) | 0 (0) | 4 (5.7) | |
| Antibiotic treatment and anticoagulation | 1 (0.7) | 0 | 0 (0) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 1 (0.3) | .476 |
| Antibiotic treatment and specific treatment of an IE abdominal localization | 2 (1.4) | 1 (2.1) | 1 (2.1) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Surgery and specific treatment of an IE abdominal localization | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 1 (0.3) | |
| Antibiotic treatment, anticoagulation and specific treatment of an IE abdominal localization | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.8) | 1 (0.3) | |
| Cardiac surgery | ||||||||||
| Cardiac surgery during initial hospitalization | 41 (29.3) | 12 (25.5) | 4 (23.5) | 1 (16.7) | 17 (24.3) | 21 (43.7) | 3 (33.3) | 0 (0) | 24 (34.3) | .265 |
| Cardiac surgery during the 6 first months following inclusion | 53 (37.9) | 14 (29.8) | 5 (29.4) | 1 (16.7) | 20 (28.6) | 28 (58.3) | 4 (44.4) | 1 (7.7) | 33 (47.1) | .036 |
| . | . | Prosthetic Valve Patients N=70 . | . | . | . | Native Valve Patients N=70 . | . | . | . | P-value a . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Final IE Classification (Gold Standard) . | . | . | . | Final IE Classification (Gold Standard) . | . | . | . | . |
| . | TotalN=140 . | Definite N=47 . | Possible N=17 . | Excluded N=6 . | Total N=70 . | Definite N=48 . | Possible N=9 . | Excluded N=13 . | Total N=70 . | . |
| Modification of patients’ management following18F-FDG PET/CTb | ||||||||||
| 37 (26.4) | 10 (21.3) | 4 (23.5) | 1 (16.7) | 15 (21.4) | 13(27.1) | 4 (44.4) | 5 (38.5) | 22 (31.4) | .25 | |
| Antibiotic treatment c | 18 (12.8) | 3 (6.3) | 3 (17.6 | 1 (16.7) | 7 (10.0) | 5 (10.4) | 3 (33.3) | 3 (23.1) | 11 (15.7) | .476 |
| Cardiac surgery | 6 (4.3) | 3 (6.3) | 0 (0) | 0 (0) | 3 (4.3) | 3 (6.2) | 0 (0) | 0 (0) | 3 (4.3) | |
| Anticoagulation | 1 (0.7) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Specific treatment of an IE abdominal localizationd | 1 (0.7) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 1 (0.3) | |
| Antibiotic and surgery | 5 (3.6) | 1 (2.1) | 0 (0) | 0 (0) | 1 (1) | 3 (6.2) | 1 (11.1) | 0 (0) | 4 (5.7) | |
| Antibiotic treatment and anticoagulation | 1 (0.7) | 0 | 0 (0) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 1 (0.3) | .476 |
| Antibiotic treatment and specific treatment of an IE abdominal localization | 2 (1.4) | 1 (2.1) | 1 (2.1) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Surgery and specific treatment of an IE abdominal localization | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 1 (0.3) | |
| Antibiotic treatment, anticoagulation and specific treatment of an IE abdominal localization | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.8) | 1 (0.3) | |
| Cardiac surgery | ||||||||||
| Cardiac surgery during initial hospitalization | 41 (29.3) | 12 (25.5) | 4 (23.5) | 1 (16.7) | 17 (24.3) | 21 (43.7) | 3 (33.3) | 0 (0) | 24 (34.3) | .265 |
| Cardiac surgery during the 6 first months following inclusion | 53 (37.9) | 14 (29.8) | 5 (29.4) | 1 (16.7) | 20 (28.6) | 28 (58.3) | 4 (44.4) | 1 (7.7) | 33 (47.1) | .036 |
Surgery modifications include surgery cancellation, surgery indication or modification of surgery timing, or valve substitute. Anticoagulation modifications include interruption of modification of anticoagulation level. Values are n (%).
Abbreviations: FDG, fluorodeoxyglucose; IE, infective endocarditis; PET/CT, positron emission tomography/computed tomography.
aStatistical comparisons between PV and NV patients.
bThe duration of antibiotic therapy was reduced in 6 patients due to the exclusion of IE diagnosis by 18F-FDG PET/CT which was in favor of an alternative diagnosis, prolonged in 4 patients. An antibiotic with a better diffusion in bone, joints or prostate gland was added in 11 patients and the dose of an antibiotic was reduced in one patient due to the exclusion of IE diagnosis.
cIncluding 2 patients with detection of IE portal of entry.
dIncluding 1 patient with detection of IE portal of entry.
![A, IE of native mitral valve. At admission, the patient had 2 major criteria (vegetation and positive blood cultures [Rothia aeria]) and 2 minor criteria (fever and predisposing heart condition [mitral regurgitation]) (definite IE). The PET/CT scan showed an FDG uptake localized to the anterolateral portion of the mitral annulus (red arrows, panel B, oblique reoriented slices) and an large arterial septic aneurysm in the deep femoral artery (yellow arrows, panel C: axial and coronal slices) which was previously not identified. 18F-FDG-PET/CT added a minor Duke criterion (2 major criteria and 3 minor criteria) but did not modify Duke classification which remained definite. Arterial septic aneurysm was treated by an endovascular procedure.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/73/3/10.1093_cid_ciaa666/1/m_ciaa666f0002a.jpeg?Expires=1750231920&Signature=HdGoWp99L76kEYUW86urH8PMlMZAs1BlD3J8O3OVuc7zYq7oc5TIim5CjKqNp3GaQwbFfugn2thDy6tfAFecaAZ3e7oARYcRkG8-mn9qI-doBcNaya8DxySKZqLngbeEbuyIA0n0wkyf0yT2-spQ8aOz2wh5xu080Z4WNtxTFsqA3DjrK32mAqNrCXBbnrH6UTzWi-NHf5Ph7Wt-BYhC9HntLVwB~q-nR6d-4xt2rL1hMTWZGpX7PQ1nfiQI7DDtcUtwFzmmp7dsHRpb6uUMJSzJXXBlNze3brAtsTWDYOYfBSzaWp0-0aVrtx0yz3ayEv6kpQF75N2MNnJFVbN-zA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
A, IE of native mitral valve. At admission, the patient had 2 major criteria (vegetation and positive blood cultures [Rothia aeria]) and 2 minor criteria (fever and predisposing heart condition [mitral regurgitation]) (definite IE). The PET/CT scan showed an FDG uptake localized to the anterolateral portion of the mitral annulus (red arrows, panel B, oblique reoriented slices) and an large arterial septic aneurysm in the deep femoral artery (yellow arrows, panel C: axial and coronal slices) which was previously not identified. 18F-FDG-PET/CT added a minor Duke criterion (2 major criteria and 3 minor criteria) but did not modify Duke classification which remained definite. Arterial septic aneurysm was treated by an endovascular procedure.
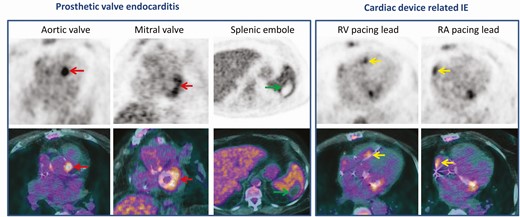
Infective endocarditis of aortic and mitral bioprosthetic valves in a patient with a pacemaker which has been implanted several years ago. At admission, the patient had 1 major criteria (vegetation on mitral valve on transoesophageal echocardiography without lesion on pacemaker leads) and 4 minor criteria (fever, predisposing heart condition, cerebral emboli, a positive blood culture for Staphylococcus epidermidis) (definite IE). The FDG PET/CT scan showed a focal FDG uptake in both aortic and mitral paravalvular areas (red arrows), and a septic emboli in the spleen (green arrows). Extraction of the pacemaker was not initially planned due to the normality of the TEO and the unique positive blood culture. However, an infection of the pacemaker has been suggested by focal FDG uptake located on both atrial and ventricular pacing leads (yellow arrows). Duke classification was not modified (definite IE) but the patient’s management modified and the pacing hardware was extracted. Abbreviations: FDG, fluorodeoxyglucose; IE, infective endocarditis; PET/CT, positron emission tomography/ computed tomography.
Characteristics of the Patients Who Benefited From 18F-FDG-PET/CT
Forty percent of the 140 patients (95% CI: 32%–48%) benefited from 18F-FDG-PET/CT as previously defined; they had more frequently noncontributing baseline echocardiography (P < .001) and/or were more frequently classified as possible IE at inclusion (P = .04; Table 4). The nature of the cardiac valve (bioprosthesis, mechanical valve, or native valve) was not a determinant of the benefit.
Comparison of the Characteristics of Patients According to Whether or Not They Benefited From 18F-FDG-PET/CT
| . | Patients Who Did Not Benefit From 18F-FDG-PET/CT . | Patients Who Benefit From 18F-FDG-PET/CT . | P-value . |
|---|---|---|---|
| . | n=84 . | n=56 . | . |
| Age, median (IQR) | 67 (56.75–76.25) | 66.5 (56.75–78.25) | .79 |
| Male, n (%) | 61 (72.6) | 43 (76.8) | .69 |
| Diabetes, n (%) | 14 (16.7) | 15 (26.8) | .20 |
| Nature of the cardiac valve | .63 | ||
| Native valve, n (%) | 43 (51.8) | 27 (50.0) | |
| Bioprosthesis valve, n (%) | 24 (28.9) | 13 (24.1) | |
| Mechanical valve, n (%) | 16 (19.3) | 14 (25.9) | |
| Causative microorganisms | .51 | ||
| Staphylococcus aureus | 16 (19.1) | 10 (17.9) | |
| Coagulase-negative staphylococci | 10 (11.9) | 7 (12.5) | |
| Oral streptococci | 12 (14.3) | 13 (23.2) | |
| Streptococcus bovis | 5 (5.9) | 6 (10.7) | |
| Enterococcus | 8 (9.5) | 4 (7.1) | |
| HACEK | 3 (3.6) | 2 (3.6) | |
| Other | 13 (15.5) | 10 (17.9) | |
| Negative blood cultures | 17 (20.2) | 4 (7.1) | |
| Echocardiography | |||
| Noncontributing echocardiography* | 22 (26.2) | 34 (60.7) | <.001 |
| Duke-Li classification at inclusiona | |||
| Definite | 55 (65.5) | 25 (44.6) | |
| Possible | 27 (32.1) | 29 (51.8) | .04b |
| Excludedc | 2 (2.4) | 2 (3.6) |
| . | Patients Who Did Not Benefit From 18F-FDG-PET/CT . | Patients Who Benefit From 18F-FDG-PET/CT . | P-value . |
|---|---|---|---|
| . | n=84 . | n=56 . | . |
| Age, median (IQR) | 67 (56.75–76.25) | 66.5 (56.75–78.25) | .79 |
| Male, n (%) | 61 (72.6) | 43 (76.8) | .69 |
| Diabetes, n (%) | 14 (16.7) | 15 (26.8) | .20 |
| Nature of the cardiac valve | .63 | ||
| Native valve, n (%) | 43 (51.8) | 27 (50.0) | |
| Bioprosthesis valve, n (%) | 24 (28.9) | 13 (24.1) | |
| Mechanical valve, n (%) | 16 (19.3) | 14 (25.9) | |
| Causative microorganisms | .51 | ||
| Staphylococcus aureus | 16 (19.1) | 10 (17.9) | |
| Coagulase-negative staphylococci | 10 (11.9) | 7 (12.5) | |
| Oral streptococci | 12 (14.3) | 13 (23.2) | |
| Streptococcus bovis | 5 (5.9) | 6 (10.7) | |
| Enterococcus | 8 (9.5) | 4 (7.1) | |
| HACEK | 3 (3.6) | 2 (3.6) | |
| Other | 13 (15.5) | 10 (17.9) | |
| Negative blood cultures | 17 (20.2) | 4 (7.1) | |
| Echocardiography | |||
| Noncontributing echocardiography* | 22 (26.2) | 34 (60.7) | <.001 |
| Duke-Li classification at inclusiona | |||
| Definite | 55 (65.5) | 25 (44.6) | |
| Possible | 27 (32.1) | 29 (51.8) | .04b |
| Excludedc | 2 (2.4) | 2 (3.6) |
Patients who benefit from 18F-FDG-PET/CT were those whose Duke-Li classification was correctly modified by 18-FDG-PET/CT, and/or portal of entry discovered and/or therapeutic plan modified.
Abbreviations: HACEK, Haemophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IQR, interquartile range.
*Trans-thoracic and trans-oesophageal echocardiography not fulfilling the definition for a major Duke criteria [16].
aDuke classification modified by Li [16].
b Comparison possible versus definite or excluded.
c Not fulfilling the definite and possible IE Duke-Li definition at inclusion.
Comparison of the Characteristics of Patients According to Whether or Not They Benefited From 18F-FDG-PET/CT
| . | Patients Who Did Not Benefit From 18F-FDG-PET/CT . | Patients Who Benefit From 18F-FDG-PET/CT . | P-value . |
|---|---|---|---|
| . | n=84 . | n=56 . | . |
| Age, median (IQR) | 67 (56.75–76.25) | 66.5 (56.75–78.25) | .79 |
| Male, n (%) | 61 (72.6) | 43 (76.8) | .69 |
| Diabetes, n (%) | 14 (16.7) | 15 (26.8) | .20 |
| Nature of the cardiac valve | .63 | ||
| Native valve, n (%) | 43 (51.8) | 27 (50.0) | |
| Bioprosthesis valve, n (%) | 24 (28.9) | 13 (24.1) | |
| Mechanical valve, n (%) | 16 (19.3) | 14 (25.9) | |
| Causative microorganisms | .51 | ||
| Staphylococcus aureus | 16 (19.1) | 10 (17.9) | |
| Coagulase-negative staphylococci | 10 (11.9) | 7 (12.5) | |
| Oral streptococci | 12 (14.3) | 13 (23.2) | |
| Streptococcus bovis | 5 (5.9) | 6 (10.7) | |
| Enterococcus | 8 (9.5) | 4 (7.1) | |
| HACEK | 3 (3.6) | 2 (3.6) | |
| Other | 13 (15.5) | 10 (17.9) | |
| Negative blood cultures | 17 (20.2) | 4 (7.1) | |
| Echocardiography | |||
| Noncontributing echocardiography* | 22 (26.2) | 34 (60.7) | <.001 |
| Duke-Li classification at inclusiona | |||
| Definite | 55 (65.5) | 25 (44.6) | |
| Possible | 27 (32.1) | 29 (51.8) | .04b |
| Excludedc | 2 (2.4) | 2 (3.6) |
| . | Patients Who Did Not Benefit From 18F-FDG-PET/CT . | Patients Who Benefit From 18F-FDG-PET/CT . | P-value . |
|---|---|---|---|
| . | n=84 . | n=56 . | . |
| Age, median (IQR) | 67 (56.75–76.25) | 66.5 (56.75–78.25) | .79 |
| Male, n (%) | 61 (72.6) | 43 (76.8) | .69 |
| Diabetes, n (%) | 14 (16.7) | 15 (26.8) | .20 |
| Nature of the cardiac valve | .63 | ||
| Native valve, n (%) | 43 (51.8) | 27 (50.0) | |
| Bioprosthesis valve, n (%) | 24 (28.9) | 13 (24.1) | |
| Mechanical valve, n (%) | 16 (19.3) | 14 (25.9) | |
| Causative microorganisms | .51 | ||
| Staphylococcus aureus | 16 (19.1) | 10 (17.9) | |
| Coagulase-negative staphylococci | 10 (11.9) | 7 (12.5) | |
| Oral streptococci | 12 (14.3) | 13 (23.2) | |
| Streptococcus bovis | 5 (5.9) | 6 (10.7) | |
| Enterococcus | 8 (9.5) | 4 (7.1) | |
| HACEK | 3 (3.6) | 2 (3.6) | |
| Other | 13 (15.5) | 10 (17.9) | |
| Negative blood cultures | 17 (20.2) | 4 (7.1) | |
| Echocardiography | |||
| Noncontributing echocardiography* | 22 (26.2) | 34 (60.7) | <.001 |
| Duke-Li classification at inclusiona | |||
| Definite | 55 (65.5) | 25 (44.6) | |
| Possible | 27 (32.1) | 29 (51.8) | .04b |
| Excludedc | 2 (2.4) | 2 (3.6) |
Patients who benefit from 18F-FDG-PET/CT were those whose Duke-Li classification was correctly modified by 18-FDG-PET/CT, and/or portal of entry discovered and/or therapeutic plan modified.
Abbreviations: HACEK, Haemophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae; IQR, interquartile range.
*Trans-thoracic and trans-oesophageal echocardiography not fulfilling the definition for a major Duke criteria [16].
aDuke classification modified by Li [16].
b Comparison possible versus definite or excluded.
c Not fulfilling the definite and possible IE Duke-Li definition at inclusion.
DISCUSSION
In this prospective multicenter study evaluating for the first time to our knowledge the diagnostic and patients’ management modifications induced by systematic whole body 18F-FDG-PET/CT in patients with a high level of suspicion of IE, we showed that a significant proportion of both PV and NV patients benefited from 18F-FDG-PET/CT.
In this multicenter study, we standardized patient preparation, acquisition protocols, and image interpretation through training sessions with specific attention to valve uptake patterns in order to homogenize the classification as nonspecific or infection-related [20]. This was all the more worthwhile because of the high prevalence of positive FDG uptake in the perivalvular area regardless of the time span since prosthetic valve implantation when the interpretation of perivalvular uptake was only considered as positive or negative and not combined with qualitative interpretation [14]. This qualitative interpretation led us to consider one-fifth of the PV patients with valvular uptake as non-IE related. Finally, the low number of PV patients with prior use of surgical adhesive does not explain in itself the false positive rates.
Perivalvular 18F-FDG-PET/CT was considered abnormal in some patients with nonconclusive echocardiography (most of them were TOE), but this was observed in a much larger proportion in PV patients. In patients with abnormal echocardiography, concordance with perivalvular 18F-FDG-PET/CT was higher in those with periannular complication than in those with vegetation, in relation with the low content in inflammatory cells of vegetations, the limited spatial resolution of 18F-FDG-PET/CT and the presence of motion artifacts.
As previously reported, peripheral localizations of IE detected by 18F-FDG-PET/CT were frequent and not previously identified in approximately one-third of patients [5–10]. The rate of peripheral localization was lower in PV patients than in NV patients, as reported in the literature [21]. The possibility for clinicians to identify extracardiac locations of IE with 18F-FDG-PET/CT can help them avoid the use of thoraco-abdomino-pelvic CT scan, which may favor renal failure [22]. As previously reported, 18F-FDG-PET/CT enabled the revelation of portal of entry in some patients. For the first time to our knowledge, we showed that cerebral 18F-FDG-PET/CT acquisition may identify asymptomatic lesions despite a high physiological uptake of 18F-FDG.
In the present study population, 18F-FDG-PET/CT improved IE diagnosis in PV and NV patients (up to approximately 1 out of 5 patients), although to a different extent (5 times more in PV patients than in NV patients based on net reclassification improvement). The higher impact in PV patients was due jointly to more frequent cardiac abnormal foci detected in these patients and to a lower proportion of patients with definite IE before the 18F-FDG-PET/CT scan as compared to NV patients, making the results of detected lesions by 18F-FDG-PET/CT more likely to impact diagnosis. Of note, the diagnostic impact of perivalvular/valvular uptake was higher than those of extracardiac uptake, as the first is a major criterion and the second is a minor criterion in the m-ESC-2015 18F-FDG-PET/CT classification. Furthermore, the diagnostic performance of the m-ESC2015 18F-FDG-PET/CT classification was not improved in the subpopulation of patients with CRP ≥ 40 mg/L conversely to what was reported by some authors [13], using as we did a qualitative interpretation of the images.
A low sensitivity of 18F-FDG-PET/CT in patients with NVIE has been reported previously, particularly in the study by de Camargo et al, which enrolled a substantial proportion of patients with NVIE [11]. Histological analysis of infected native valves suggests that extended fibrosis and a low content of polymorphonuclear cells accounted for low FDG uptake. Consequently, there is general agreement that FDG PET/CT is not recommended for the diagnosis of NVIE. However, because the sensitivity of echocardiography is higher in NV patients than in patients with prosthetic valves, the rate of definite IE at baseline as defined by the Duke classification was higher in NV patients than in those with prosthetic valve. Therefore, measuring the diagnostic impact of 18F-FDG-PET/CT through the modification of the Duke classification underestimates the diagnostic impact of 18F-FDG-PET/CT in NV patients.
The present study is the first to assess patients’ management modification. It shows that the 18F-FDG-PET/CT cardiac or extracardiac uptakes at the origin of the therapeutic impact was different between NV and PV patients, because more frequent primary or metastatic septic foci were detected by 18F-FDG-PET/CT in the former group, in agreement with previous reports [21]. This led to change both antibiotic and surgery plans. As a result, the overall impact of 18F-FDG-PET/CT in the study population, combining diagnosis and therapeutic management, was independent of the nature of the valve (native or prosthetic).
We must acknowledge several limitations to our study. First, we did not use iodine injection, which has been reported to increase 18F-FDG-PET/CT sensitivity in patients with PV [23]. However this was done intentionally to decrease renal toxicity. Second, we did not verify that perivalvular uptake disappeared after patients’ cure, which would have been the most accurate way to exclude false positives but would have made patients’ care more cumbersome. Third, our experimental design did not assess whether the 18F-FDG-PET/CT-related changes in diagnostic and therapeutic plans improved patient outcomes or led to unnecessary procedures and increased costs. Fourth, one-fifth of the PV patients did not have TOE before 18F-FDG-PET/CT. However, 43% of these patients had had a TTE, which revealed vegetation or a perivalvular abscess, giving them a major Duke criterion. Finally, because this study was conducted in reference centers, the proportion of patients with suspected IE on valvular prosthesis was overrepresented.
To conclude, this prospective evaluation of the diagnostic and therapeutic impacts of 18F-FDG-PET/CT support its implementation in patients with initial nondefinite NVIE, as well as PVIE or in case of nonconclusive echocardiography. Despite a lower diagnostic sensitivity in NVIE, therapeutic management is influenced by extracardiac findings of whole body staging of the disease. However, 18F-FDG-PET/CT scan must be qualitatively interpreted by trained specialists in order to differentiate abnormal perivalvular uptake related to IE from normal uptake related to prosthetic valve.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments
AEPEI TEPvENDO study group
Principal investigator. Xavier Duval.
Steering Committee. Bruno Hoen, Bernard Iung, Francois Rouzet, Sarah Tubiana.
Clinical centers. Besançon: Tubanur Albayrak, Yvette Bernard, Hatem Boulahdour, Florent Briand, Catherine Chirouze, Jean-François Faucher, Alexandre Guignier, Laurent Hustache-Mathieu, Gabriela Illes-Hajnal, Joséphine Moreau, Olivier Morel, Marie-France Seronde, Dijon: Niloufar Behechti, Mathieu Blot, Marielle Buisson, Alexandre Cochet, Jean-Christophe Eicher, Olivier Humbert, Julien Lecluse-Barth, Sophie Mahy, Lionel Piroth, Philippe Andre; Lyon: André s, François Delahaye, Armelle Delahaye, Bastien Grégoire; Montpellier: Aurélie Bourdon, Stéphane Cade, Marie-Laure Casanova, Diane Cerutti, Delphine De Verbizier, Vincent Le Moing, Angelina Martinez, David Morquin, Kamila Solecki; Nancy: Stéphanie Bonay, Elodie Chevalier, Marine Claudin, Wassila Djaballah, François Goehringer, Olivier Huttin, Eliette Jeanmaire, Pierre-Yves Marie, Véronique Roch, Christine Selton-Suty, Sandrine Vauthier, Clément Venner; Nantes: Nathalie Asseray, Charlotte Biron, David Boutoille, Julia Brochard-Libois, Morgane Cavellec, Caroline Cueff, Sandrine Delarue, Catherine Di Prizio, Levent Dinc, Imen Fellah, Damien Guijarro, Mathias Lachaud, Laurianne Le Gloan, Thierry Le Tourneau, Anne-Sophie Lecompte, Maeva Lefebvre, Adrien Luçon, Cédric Mathieu, Jérémie Orain, Amandine Pallardy, Nicolas Piriou, Maxime Poilane, Jérôme Sassier; Paris: Khadija Ben Ali, Eric Brochet, Charles Burdet, Bettia Celestin, Claire Cimadevilla, Xavier Duval, Fabien Hiafyl, Emila Ilic-Habensus, Bernard Iung, Marie Lachatre, Laurent Lepage, Xavier Lescure, François Rouzet, William Vindrios, Michel Wolff, Yazdan Yazdanpanah; Rennes: Anne Devillers, Erwan Donal, Adèle Lacroix, Bernard Lelong, Matthieu Revest, Pierre Tattevin, Elise Thebault.
Coordination and statistical analyses (Clinical trial unit, Hôpitaux Universitaires Paris Nord Val de Seine, AP-HP, Paris). Camille Couffignal, Marina Esposito-Farese, Cédric Laouenan, Sonia Maklouf, France Mentre, Margot Prevault, Ophélie Rogier.
Financial support. This work was supported by the French Ministry of Health (AOM 13549-P130938).
Sponsor. DRCI APHP.
Potential conflicts of interest. B. I. reports consulting fees from Edwards Lifesciences and travel fees from Boehringer Ingelheim, outside the submitted work. F. R. reports personal fees from GE Healthcare and grants from Spectrum Dynamics, outside the submitted work. X. D. reports grants from Pfizer Company and personal fees from Sanofi Pasteur company, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
C. L., B. I., and F. R. contributed equally.




