-
PDF
- Split View
-
Views
-
Cite
Cite
Rebekah W Moehring, Elizabeth S Dodds Ashley, Angelina E Davis, April Pridgen Dyer, Alice Parish, Xinru Ren, Yuliya Lokhnygina, Lauri A Hicks, Arjun Srinivasan, Deverick J Anderson, Development of an Electronic Definition for De-escalation of Antibiotics in Hospitalized Patients, Clinical Infectious Diseases, Volume 73, Issue 11, 1 December 2021, Pages e4507–e4514, https://doi.org/10.1093/cid/ciaa932
Close - Share Icon Share
Abstract
Antimicrobial stewardship programs (ASPs) promote the principle of de-escalation: moving from broad- to narrow-spectrum agents and stopping antibiotics when no longer indicated. A standard, objective definition of de-escalation applied to electronic data could be useful for ASP assessments.
We derived an electronic definition of antibiotic de-escalation and performed a retrospective study among 5 hospitals. Antibiotics were ranked into 4 categories: narrow-spectrum, broad-spectrum, extended-spectrum, and agents targeted for protection. Eligible adult patients were cared for on inpatient units, had antibiotic therapy for at least 2 days, and were hospitalized for at least 3 days after starting antibiotics. Number of antibiotics and rank were assessed at 2 time points: day of antibiotic initiation and either day of discharge or day 5. De-escalation was defined as reduction in either the number of antibiotics or rank. Escalation was an increase in either number or rank. Unchanged was either no change or discordant directions of change. We summarized outcomes among hospitals, units, and diagnoses.
Among 39 226 eligible admissions, de-escalation occurred in 14 138 (36%), escalation in 5129 (13%), and antibiotics were unchanged in 19 959 (51%). De-escalation varied among hospitals (median, 37%; range, 31–39%, P < .001). Diagnoses with lower de-escalation rates included intra-abdominal (23%) and skin and soft tissue (28%) infections. Critical care had higher rates of both de-escalation and escalation compared with wards.
Our electronic de-escalation metric demonstrated variation among hospitals, units, and diagnoses. This metric may be useful for assessing stewardship opportunities and impact.
Hospital-based antimicrobial stewardship programs (ASPs) aim to improve antimicrobial use through ensuring optimal selection and administration of antimicrobials when they are needed and stopping or avoiding antimicrobials when they are not needed. Accreditation standards in the United States now require many hospitals to track antimicrobial use (AU) and the impact of ASP initiatives [1, 2]. The best combination of metrics to use for ASP assessments, however, remains a debate among experts [3–6]. Although days of therapy is the recommended AU metric in national guidelines [4], aggregate facility- or unit-level volume of AU does not assess decisions about antimicrobials for individual patients or demonstrate changes between agents [7]. Careful and nuanced treatment decisions for individual patients include daily reassessments and interim decision making based on dynamic clinical information.
De-escalation is a core principle of antimicrobial stewardship, defined as moving from empiric, broad-spectrum therapy to targeted, narrow-spectrum therapy as clinical data return, or stopping antibiotics when infection has been ruled out. Clinicians apply this principle to reduce antibiotic exposures, both in antibiotic spectrum and in days of antibiotics, and to avoid unintended negative consequences for their patients. However, no standard definition of de-escalation exists. Subjective judgment of de-escalation by clinical reviewers results in heterogeneity in measurement, limiting its use as a process metric for ASP assessments. Further, subjective review by a local antimicrobial stewardship expert requires time and personnel investment when ASPs are already underresourced [8, 9]. An electronic definition of de-escalation could avoid the resource burden required for individual review and offer an objective, reproducible assessment of the entire hospitalized population without introducing subjective biases. This process metric could then be used to identify opportunities for ASP intervention and to track the impact of ASP initiatives on antimicrobial decision making.
The aim of the Patient Safety Outcome Measures and Measurement Tools for Antibiotic Stewardship Programs project was to identify both relevant and feasible metrics for use by acute-care hospital ASPs with input from an expert panel and experience from 5 pilot hospitals [3, 10]. The Structured Taskforce of Experts Working At Reliable Standards for Stewardship (STEWARDS) expert panel achieved consensus in deeming de-escalation events as a metric useful for ASPs and tightly linked to antimicrobial prescribing and improved patient care. Feasibility of measurement of de-escalation events from electronic health records (EHRs), however, remained uncertain among panel members [3]. The aims of this study were to derive an electronic definition for antibiotic de-escalation using available data from the EHR and then to describe its distribution among a sample of hospitalized patients.
METHODS
We performed a retrospective cross-sectional study of de-escalation events among 5 hospitals from the Duke Health System and the Duke Antimicrobial Stewardship Outreach Network, including 4 community hospitals and 1 academic, tertiary care hospital. Admission-level inpatient data from 2015 and 2016 were extracted into a limited data set from pilot hospitals’ EHR data produced from routine clinical care. Extracted information included electronic medication administration record (eMAR) date and time for anti-infectives, admission and discharge dates, demographics, and in-hospital death. Complete capture of anti-infective agents was confirmed using a manual validation of the extract file that included review of agents captured, their frequency of administration, and a 30-patient random sample for in-depth review of all individual anti-infective administrations to confirm administration times and unit location.
Admissions included in the de-escalation analysis were further defined as a population of patients who would be eligible for de-escalation decisions. De-escalation decisions typically occur in the inpatient setting after 1 to 3 days of empiric therapy, when microbiology and/or diagnostic test results return and clinical response to antibiotic initiation can be assessed. Inclusion criteria for patient admissions were as follows: adults aged 18 or older, cared for on inpatient hospital units excluding behavioral health and procedural units (eg, cardiac catheterization or operating room), received at least 2 days of antibiotic therapy, had a length of stay of 3 days or more after the first calendar day of antibiotic exposure on an inpatient unit, and did not die within the first 5 days after initiation of antibiotics. Antibiotic administrations eligible for analysis included those used for acute infection treatment as defined by the National Healthcare Safety Network’s Antimicrobial Use (AU) Option agent category of “antibacterial” (Appendix B, version January 2017) [11]. Included routes were those intended for systemic absorption (intravenous, intramuscular, or digestive), excluding topical or inhaled administrations.
We ranked antibiotic agents into a 4-point scale based on agents’ spectrum of activity against bacterial pathogens (ie, “broadness”) as well as interest in protecting or conserving the agent from a program perspective (Table 1). The ranking schema was drafted by 2 authors (R. W. M. and E. S. D.), and then presented at multiple in-person and phone meetings for discussion, refinement, and feedback. Groups that reviewed and provided verbal feedback on the ranking schema included pharmacists, physicians, epidemiologists, and statisticians from our research group; ASP teams participating in pilot hospitals; as well as the authors. Final decisions on the schema were made by R. W. M. and E. D. A. after review of group feedback. In our experience, rank 4 agents are “last resort” agents typically targeted for prior authorization strategies or infrequently used in acute-care hospitals due to risks of toxicity and safety, concern about development of acquired drug resistance, or high cost. Route of antibiotic (ie, intravenous vs oral) was not considered in determination of rank. We then calculated an antibiotic rank and number of antibiotic agents received on each calendar day. When more than 1 agent was given on the same day, the highest individual agent’s rank was used (Table 2). Finally, we compared the direction in change of both antibiotic rank and number of antibiotics between 2 time points. Day 1 was defined as the first calendar day of antibiotic exposure on an inpatient unit. Day D was defined as day 5 after initiation of antibiotics or day of discharge if the patient was discharged on day 3 or 4. Each eligible admission was then assigned to 1 of 3 exclusive outcome categories. De-escalation was defined as reduction in either the number of antibiotics or rank measured on day D as compared with day 1. Escalation was an increase in either number or rank of agents. Unchanged was either no change or discordant directions of change in number and rank. For all 3 categories, the outcome examined was percentage among qualifying admissions. Descriptive statistics were used to describe admission characteristics, hospitals, unit type, and infection diagnoses by outcome category. Characteristics of eligible hospital admissions were compared between the de-escalation outcome categories using Kruskal-Wallis test for continuous variables and chi-square test for categorical variables. Infection diagnoses were defined using the Agency for Healthcare Research and Quality Clinical Classification Software, which groups similar International Classification of Diseases, 10th revision, codes into larger categories for streamlined analyses (Supplementary Table 1) [12]. Eligible admissions were assigned to a unit on day D, as defined above.
| Narrow-spectrum . | Broad-spectrum . | Extended-spectrum, Including MDROs and Pseudomonas . | Protected . |
|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . |
| First- and second-generation cephalosporins | Ceftriaxone | Anti-pseudomonal penicillins | Anti-pseudomonal carbapenem |
| Amoxicillin, Ampicillin | Third-generation oral Cephalosporins | Fluoroquinolones | Colistin |
| TMP/SMX | Azithromycin | Aminoglycosides | Tigecycline |
| Nafcillin, oxacillin | Clarithromycin | Vancomycin | Linezolid, tedizolid |
| Metronidazole | Amoxicillin/clavulanate | Cefepime, ceftazidime | Daptomycin |
| Doxycycline | Ampicillin/sulbactam | Ertapenem | Ceftaroline |
| Nitrofurantoin | Clindamycin | Aztreonam | Ceftazidime/avibactam |
| Penicillin | Ceftolozane/tazobactam |
| Narrow-spectrum . | Broad-spectrum . | Extended-spectrum, Including MDROs and Pseudomonas . | Protected . |
|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . |
| First- and second-generation cephalosporins | Ceftriaxone | Anti-pseudomonal penicillins | Anti-pseudomonal carbapenem |
| Amoxicillin, Ampicillin | Third-generation oral Cephalosporins | Fluoroquinolones | Colistin |
| TMP/SMX | Azithromycin | Aminoglycosides | Tigecycline |
| Nafcillin, oxacillin | Clarithromycin | Vancomycin | Linezolid, tedizolid |
| Metronidazole | Amoxicillin/clavulanate | Cefepime, ceftazidime | Daptomycin |
| Doxycycline | Ampicillin/sulbactam | Ertapenem | Ceftaroline |
| Nitrofurantoin | Clindamycin | Aztreonam | Ceftazidime/avibactam |
| Penicillin | Ceftolozane/tazobactam |
Abbreviations: MDRO, multidrug-resistant organism; TMP/SMX, Trimethoprim-sulfamethoxazole.
| Narrow-spectrum . | Broad-spectrum . | Extended-spectrum, Including MDROs and Pseudomonas . | Protected . |
|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . |
| First- and second-generation cephalosporins | Ceftriaxone | Anti-pseudomonal penicillins | Anti-pseudomonal carbapenem |
| Amoxicillin, Ampicillin | Third-generation oral Cephalosporins | Fluoroquinolones | Colistin |
| TMP/SMX | Azithromycin | Aminoglycosides | Tigecycline |
| Nafcillin, oxacillin | Clarithromycin | Vancomycin | Linezolid, tedizolid |
| Metronidazole | Amoxicillin/clavulanate | Cefepime, ceftazidime | Daptomycin |
| Doxycycline | Ampicillin/sulbactam | Ertapenem | Ceftaroline |
| Nitrofurantoin | Clindamycin | Aztreonam | Ceftazidime/avibactam |
| Penicillin | Ceftolozane/tazobactam |
| Narrow-spectrum . | Broad-spectrum . | Extended-spectrum, Including MDROs and Pseudomonas . | Protected . |
|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . |
| First- and second-generation cephalosporins | Ceftriaxone | Anti-pseudomonal penicillins | Anti-pseudomonal carbapenem |
| Amoxicillin, Ampicillin | Third-generation oral Cephalosporins | Fluoroquinolones | Colistin |
| TMP/SMX | Azithromycin | Aminoglycosides | Tigecycline |
| Nafcillin, oxacillin | Clarithromycin | Vancomycin | Linezolid, tedizolid |
| Metronidazole | Amoxicillin/clavulanate | Cefepime, ceftazidime | Daptomycin |
| Doxycycline | Ampicillin/sulbactam | Ertapenem | Ceftaroline |
| Nitrofurantoin | Clindamycin | Aztreonam | Ceftazidime/avibactam |
| Penicillin | Ceftolozane/tazobactam |
Abbreviations: MDRO, multidrug-resistant organism; TMP/SMX, Trimethoprim-sulfamethoxazole.
| Term . | Definition . | Examples . |
|---|---|---|
| Day 1 | First day of antibiotic exposure on an inpatient unit during hospitalization, using a calendar-day definition (12 am to 11:59 pm) | … |
| Day D | Day of discharge or day 5 of antibiotic exposure, whichever comes first | If the patient was discharged on day 4 after initiation of antibiotics, day D = 4; if the patient was discharged on day 8 after initiation of antibiotics, day D = 5 |
| Antibiotic rank | Highest individual agent rank for all agents given on the same calendar day; rank was measured on day 1 and again at day D | On day 1, the patient receives ceftriaxone + vancomycin; day 1 rank = 3 because the highest individual agent rank is 3 (vancomycin) |
| Number of antibiotics | Number of different antibiotic agents administered in a calendar day, measured on day 1 and day D | On day 1, the patient receives ceftriaxone + vancomycin; number of antibiotics = 2 |
| Term . | Definition . | Examples . |
|---|---|---|
| Day 1 | First day of antibiotic exposure on an inpatient unit during hospitalization, using a calendar-day definition (12 am to 11:59 pm) | … |
| Day D | Day of discharge or day 5 of antibiotic exposure, whichever comes first | If the patient was discharged on day 4 after initiation of antibiotics, day D = 4; if the patient was discharged on day 8 after initiation of antibiotics, day D = 5 |
| Antibiotic rank | Highest individual agent rank for all agents given on the same calendar day; rank was measured on day 1 and again at day D | On day 1, the patient receives ceftriaxone + vancomycin; day 1 rank = 3 because the highest individual agent rank is 3 (vancomycin) |
| Number of antibiotics | Number of different antibiotic agents administered in a calendar day, measured on day 1 and day D | On day 1, the patient receives ceftriaxone + vancomycin; number of antibiotics = 2 |
| Term . | Definition . | Examples . |
|---|---|---|
| Day 1 | First day of antibiotic exposure on an inpatient unit during hospitalization, using a calendar-day definition (12 am to 11:59 pm) | … |
| Day D | Day of discharge or day 5 of antibiotic exposure, whichever comes first | If the patient was discharged on day 4 after initiation of antibiotics, day D = 4; if the patient was discharged on day 8 after initiation of antibiotics, day D = 5 |
| Antibiotic rank | Highest individual agent rank for all agents given on the same calendar day; rank was measured on day 1 and again at day D | On day 1, the patient receives ceftriaxone + vancomycin; day 1 rank = 3 because the highest individual agent rank is 3 (vancomycin) |
| Number of antibiotics | Number of different antibiotic agents administered in a calendar day, measured on day 1 and day D | On day 1, the patient receives ceftriaxone + vancomycin; number of antibiotics = 2 |
| Term . | Definition . | Examples . |
|---|---|---|
| Day 1 | First day of antibiotic exposure on an inpatient unit during hospitalization, using a calendar-day definition (12 am to 11:59 pm) | … |
| Day D | Day of discharge or day 5 of antibiotic exposure, whichever comes first | If the patient was discharged on day 4 after initiation of antibiotics, day D = 4; if the patient was discharged on day 8 after initiation of antibiotics, day D = 5 |
| Antibiotic rank | Highest individual agent rank for all agents given on the same calendar day; rank was measured on day 1 and again at day D | On day 1, the patient receives ceftriaxone + vancomycin; day 1 rank = 3 because the highest individual agent rank is 3 (vancomycin) |
| Number of antibiotics | Number of different antibiotic agents administered in a calendar day, measured on day 1 and day D | On day 1, the patient receives ceftriaxone + vancomycin; number of antibiotics = 2 |
A manual case review to compare the electronic de-escalation metric with the clinical judgment of frontline stewards was completed retrospectively at the 5 pilot sites. A random sample of 250 patients were divided and assessed by 3 physician and 5 pharmacist antimicrobial stewards not familiar with the derivation of the electronic definition. Assessors received patient identifiers and day 1 and day D dates and were asked to adjudicate each case as “escalation,” “de-escalation,” or “unchanged” after a manual review of the EHR. Reviewers were asked to limit their judgement of de-escalation between the dates provided and were blinded to the electronic definition designation. A subset of 120 cases were re-reviewed by separate human reviewers to compare interrater reliability between human assessors. κ Statistics were used to evaluate concordance between reviewers and the electronic definition and between the human reviewers.
All analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC) and figures were prepared using R (R Foundation for Statistical Computing). This feasibility study was deemed exempt research by Duke University and participating hospitals’ institutional review boards.
RESULTS
A total of 39 226 inpatient admissions were included in the 2-year study after applying inclusion criteria. De-escalation occurred in 14 138 (36%) admissions, escalation in 5129 (13%), and antibiotics were unchanged in 19 959 (51%) admissions (Table 3). Among those in the unchanged category, less than 1% had discordant movements in rank and number of antibiotics. Outcome distributions were significantly different among hospitals (de-escalation median, 37%; range, 31–39%; P < .001) (Figure 1).
| . | No. of Antibiotics, n (%) . | ||
|---|---|---|---|
| Rank . | Lower . | Same . | Higher . |
| Lower | 10 551 (27)a | 1269 (3)a | 146 (<1)b |
| Same | 2318 (6)a | 19 703 (50)b | 3048 (8)c |
| Higher | 110 (<1)b | 732 (2)c | 1349 (3)c |
| . | No. of Antibiotics, n (%) . | ||
|---|---|---|---|
| Rank . | Lower . | Same . | Higher . |
| Lower | 10 551 (27)a | 1269 (3)a | 146 (<1)b |
| Same | 2318 (6)a | 19 703 (50)b | 3048 (8)c |
| Higher | 110 (<1)b | 732 (2)c | 1349 (3)c |
aDe-escalation.
bUnchanged.
cEscalation.
| . | No. of Antibiotics, n (%) . | ||
|---|---|---|---|
| Rank . | Lower . | Same . | Higher . |
| Lower | 10 551 (27)a | 1269 (3)a | 146 (<1)b |
| Same | 2318 (6)a | 19 703 (50)b | 3048 (8)c |
| Higher | 110 (<1)b | 732 (2)c | 1349 (3)c |
| . | No. of Antibiotics, n (%) . | ||
|---|---|---|---|
| Rank . | Lower . | Same . | Higher . |
| Lower | 10 551 (27)a | 1269 (3)a | 146 (<1)b |
| Same | 2318 (6)a | 19 703 (50)b | 3048 (8)c |
| Higher | 110 (<1)b | 732 (2)c | 1349 (3)c |
aDe-escalation.
bUnchanged.
cEscalation.
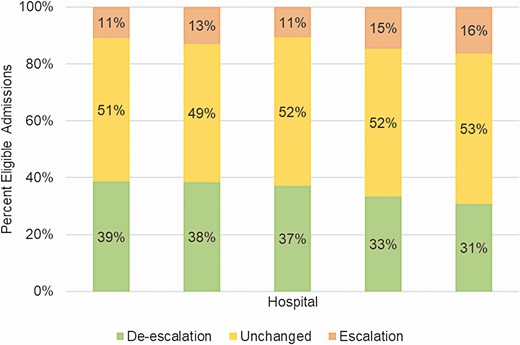
Distribution of de-escalation outcomes among 5 pilot hospitals. The academic medical center is represented by the second bar; the remaining pilot hospitals are community hospitals.
Demographic characteristics also differed by outcome category. While mean age was similar among admissions in each category, admissions with antibiotic escalations had higher proportions of patients who were male and white (Table 4). Admissions with escalations also had higher comorbidity scores, longer hospital lengths of stay, and lower numbers of antibiotics started on day 1 of hospitalization. Similar proportions of admissions in de-escalation and escalation categories had antibiotics started in the intensive care unit (ICU) (16%) as compared with a lower proportion of admissions with unchanged antibiotics (11%). Infectious diagnoses with lower rates of de-escalation included gastrointestinal (19%), intra-abdominal (23%), skin and soft tissue (28%), and ENT (ear, nose, throat)/upper respiratory tract infection (28%) (Figure 2, Supplementary Table 1). Intensive care units had higher rates of both de-escalation and escalation (43% and 16%) when compared with non-ICU wards (35% and 13%, P < .001) (Supplementary Figures 1 and 2, Supplementary Table 2). Distributions of the de-escalation outcome among differing unit types revealed larger variability among surgical wards compared with medical wards. The highest rate of the unchanged outcome (80%) occurred in a rehabilitation unit (Supplementary Figure 2).
Characteristics of Eligible Hospital Admissions by De-escalation Outcome Category
| Characteristic . | De-escalation (n = 14 138) . | Unchanged (n = 19 959) . | Escalation (n = 5129) . | Total (n = 39 226) . | P . |
|---|---|---|---|---|---|
| Age, mean (SD), years | 61.6 (18.1) | 61.6 (17.5) | 61.4 (17.2) | 61.5 (17.7) | .4 |
| Male, n (%) | 6138 (43) | 9110 (46) | 2491 (49) | 17 739 (45) | <.001 |
| Race, n (%) | <.001 | ||||
| White/Caucasian | 7776 (55) | 11 474 (58) | 3027 (59) | 22 277 (57) | |
| Black/African American | 4603 (33) | 6102 (31) | 1570 (31) | 12 275 (31) | |
| Native American | 1253 (9) | 1700 (9) | 365 (7) | 3318 (9) | |
| Asian | 114 (<1) | 132 (<1) | 31 (<1) | 277 (<1) | |
| Other | 135 (<1) | 200 (<1) | 35 (<1) | 370 (<1) | |
| Unknown | 187 (1) | 245 (1) | 66 (1) | 498 (1) | |
| Elixhauser score, mean (SD) | 3.6 (2.2) | 3.4 (2.2) | 4.1 (2.3) | 3.6 (2.2) | <.001 |
| Total length of stay, mean (SD), days | 8.4 (8.1) | 8.5 (8.5) | 11.7 (11.0) | 8.9 (8.8) | <.001 |
| Length of stay prior to day 1, n (%) | <.001 | ||||
| ≤1 day | 5190 (37) | 7801 (39) | 2810 (55) | 15 801 (40) | |
| 2–5 days | 8361 (59) | 11 253 (56) | 2126 (45) | 21 740 (55) | |
| >5 days | 586 (4) | 904 (5) | 193 (4) | 1683 (4) | |
| ICU on day 1, n (%) | <.001 | ||||
| ICU | 2264 (16) | 2224 (11) | 831 (16) | 5319 (14) | |
| Non-ICU | 11 874 (84) | 17 735 (89) | 4298 (84) | 33 907 (87) | |
| Antibiotic rank on day 1, n (%) | <.001 | ||||
| 1 | 1646 (12) | 3755 (19) | 1056 (21) | 6457 (17) | |
| 2 | 3440 (24) | 4746 (24) | 1044 (20) | 9230 (24) | |
| 3 | 8726 (62) | 10 948 (55) | 2915 (57) | 22 589 (58) | |
| 4 | 326 (2) | 510 (3) | 114 (2) | 950 (2) | |
| Number of antibiotics on day 1, n (%) | <.001 | ||||
| 1 | 8813 (63) | 15 265 (77) | 4450 (87) | 28 528 (73) | |
| 2 | 4609 (33) | 4373 (22) | 634 (12) | 9616 (25) | |
| 3 | 668 (5) | 311 (2) | 41 (<1) | 1020 (3) | |
| 4 | 48 (<1) | 10 (<1) | 4 (<1) | 62 (<1) | |
| De-escalation day (day D) , n (%) | <.001 | ||||
| 3 | 1816 (13) | 5524 (28) | 198 (4) | 7538 (19) | |
| 4 | 3465 (25) | 3255 (16) | 419 (8) | 7139 (18) | |
| 5 | 8857 (66) | 11 180 (56) | 4512 (88) | 24 549 (63) |
| Characteristic . | De-escalation (n = 14 138) . | Unchanged (n = 19 959) . | Escalation (n = 5129) . | Total (n = 39 226) . | P . |
|---|---|---|---|---|---|
| Age, mean (SD), years | 61.6 (18.1) | 61.6 (17.5) | 61.4 (17.2) | 61.5 (17.7) | .4 |
| Male, n (%) | 6138 (43) | 9110 (46) | 2491 (49) | 17 739 (45) | <.001 |
| Race, n (%) | <.001 | ||||
| White/Caucasian | 7776 (55) | 11 474 (58) | 3027 (59) | 22 277 (57) | |
| Black/African American | 4603 (33) | 6102 (31) | 1570 (31) | 12 275 (31) | |
| Native American | 1253 (9) | 1700 (9) | 365 (7) | 3318 (9) | |
| Asian | 114 (<1) | 132 (<1) | 31 (<1) | 277 (<1) | |
| Other | 135 (<1) | 200 (<1) | 35 (<1) | 370 (<1) | |
| Unknown | 187 (1) | 245 (1) | 66 (1) | 498 (1) | |
| Elixhauser score, mean (SD) | 3.6 (2.2) | 3.4 (2.2) | 4.1 (2.3) | 3.6 (2.2) | <.001 |
| Total length of stay, mean (SD), days | 8.4 (8.1) | 8.5 (8.5) | 11.7 (11.0) | 8.9 (8.8) | <.001 |
| Length of stay prior to day 1, n (%) | <.001 | ||||
| ≤1 day | 5190 (37) | 7801 (39) | 2810 (55) | 15 801 (40) | |
| 2–5 days | 8361 (59) | 11 253 (56) | 2126 (45) | 21 740 (55) | |
| >5 days | 586 (4) | 904 (5) | 193 (4) | 1683 (4) | |
| ICU on day 1, n (%) | <.001 | ||||
| ICU | 2264 (16) | 2224 (11) | 831 (16) | 5319 (14) | |
| Non-ICU | 11 874 (84) | 17 735 (89) | 4298 (84) | 33 907 (87) | |
| Antibiotic rank on day 1, n (%) | <.001 | ||||
| 1 | 1646 (12) | 3755 (19) | 1056 (21) | 6457 (17) | |
| 2 | 3440 (24) | 4746 (24) | 1044 (20) | 9230 (24) | |
| 3 | 8726 (62) | 10 948 (55) | 2915 (57) | 22 589 (58) | |
| 4 | 326 (2) | 510 (3) | 114 (2) | 950 (2) | |
| Number of antibiotics on day 1, n (%) | <.001 | ||||
| 1 | 8813 (63) | 15 265 (77) | 4450 (87) | 28 528 (73) | |
| 2 | 4609 (33) | 4373 (22) | 634 (12) | 9616 (25) | |
| 3 | 668 (5) | 311 (2) | 41 (<1) | 1020 (3) | |
| 4 | 48 (<1) | 10 (<1) | 4 (<1) | 62 (<1) | |
| De-escalation day (day D) , n (%) | <.001 | ||||
| 3 | 1816 (13) | 5524 (28) | 198 (4) | 7538 (19) | |
| 4 | 3465 (25) | 3255 (16) | 419 (8) | 7139 (18) | |
| 5 | 8857 (66) | 11 180 (56) | 4512 (88) | 24 549 (63) |
Abbreviations: ICU, intensive care unit; SD, standard deviation.
Characteristics of Eligible Hospital Admissions by De-escalation Outcome Category
| Characteristic . | De-escalation (n = 14 138) . | Unchanged (n = 19 959) . | Escalation (n = 5129) . | Total (n = 39 226) . | P . |
|---|---|---|---|---|---|
| Age, mean (SD), years | 61.6 (18.1) | 61.6 (17.5) | 61.4 (17.2) | 61.5 (17.7) | .4 |
| Male, n (%) | 6138 (43) | 9110 (46) | 2491 (49) | 17 739 (45) | <.001 |
| Race, n (%) | <.001 | ||||
| White/Caucasian | 7776 (55) | 11 474 (58) | 3027 (59) | 22 277 (57) | |
| Black/African American | 4603 (33) | 6102 (31) | 1570 (31) | 12 275 (31) | |
| Native American | 1253 (9) | 1700 (9) | 365 (7) | 3318 (9) | |
| Asian | 114 (<1) | 132 (<1) | 31 (<1) | 277 (<1) | |
| Other | 135 (<1) | 200 (<1) | 35 (<1) | 370 (<1) | |
| Unknown | 187 (1) | 245 (1) | 66 (1) | 498 (1) | |
| Elixhauser score, mean (SD) | 3.6 (2.2) | 3.4 (2.2) | 4.1 (2.3) | 3.6 (2.2) | <.001 |
| Total length of stay, mean (SD), days | 8.4 (8.1) | 8.5 (8.5) | 11.7 (11.0) | 8.9 (8.8) | <.001 |
| Length of stay prior to day 1, n (%) | <.001 | ||||
| ≤1 day | 5190 (37) | 7801 (39) | 2810 (55) | 15 801 (40) | |
| 2–5 days | 8361 (59) | 11 253 (56) | 2126 (45) | 21 740 (55) | |
| >5 days | 586 (4) | 904 (5) | 193 (4) | 1683 (4) | |
| ICU on day 1, n (%) | <.001 | ||||
| ICU | 2264 (16) | 2224 (11) | 831 (16) | 5319 (14) | |
| Non-ICU | 11 874 (84) | 17 735 (89) | 4298 (84) | 33 907 (87) | |
| Antibiotic rank on day 1, n (%) | <.001 | ||||
| 1 | 1646 (12) | 3755 (19) | 1056 (21) | 6457 (17) | |
| 2 | 3440 (24) | 4746 (24) | 1044 (20) | 9230 (24) | |
| 3 | 8726 (62) | 10 948 (55) | 2915 (57) | 22 589 (58) | |
| 4 | 326 (2) | 510 (3) | 114 (2) | 950 (2) | |
| Number of antibiotics on day 1, n (%) | <.001 | ||||
| 1 | 8813 (63) | 15 265 (77) | 4450 (87) | 28 528 (73) | |
| 2 | 4609 (33) | 4373 (22) | 634 (12) | 9616 (25) | |
| 3 | 668 (5) | 311 (2) | 41 (<1) | 1020 (3) | |
| 4 | 48 (<1) | 10 (<1) | 4 (<1) | 62 (<1) | |
| De-escalation day (day D) , n (%) | <.001 | ||||
| 3 | 1816 (13) | 5524 (28) | 198 (4) | 7538 (19) | |
| 4 | 3465 (25) | 3255 (16) | 419 (8) | 7139 (18) | |
| 5 | 8857 (66) | 11 180 (56) | 4512 (88) | 24 549 (63) |
| Characteristic . | De-escalation (n = 14 138) . | Unchanged (n = 19 959) . | Escalation (n = 5129) . | Total (n = 39 226) . | P . |
|---|---|---|---|---|---|
| Age, mean (SD), years | 61.6 (18.1) | 61.6 (17.5) | 61.4 (17.2) | 61.5 (17.7) | .4 |
| Male, n (%) | 6138 (43) | 9110 (46) | 2491 (49) | 17 739 (45) | <.001 |
| Race, n (%) | <.001 | ||||
| White/Caucasian | 7776 (55) | 11 474 (58) | 3027 (59) | 22 277 (57) | |
| Black/African American | 4603 (33) | 6102 (31) | 1570 (31) | 12 275 (31) | |
| Native American | 1253 (9) | 1700 (9) | 365 (7) | 3318 (9) | |
| Asian | 114 (<1) | 132 (<1) | 31 (<1) | 277 (<1) | |
| Other | 135 (<1) | 200 (<1) | 35 (<1) | 370 (<1) | |
| Unknown | 187 (1) | 245 (1) | 66 (1) | 498 (1) | |
| Elixhauser score, mean (SD) | 3.6 (2.2) | 3.4 (2.2) | 4.1 (2.3) | 3.6 (2.2) | <.001 |
| Total length of stay, mean (SD), days | 8.4 (8.1) | 8.5 (8.5) | 11.7 (11.0) | 8.9 (8.8) | <.001 |
| Length of stay prior to day 1, n (%) | <.001 | ||||
| ≤1 day | 5190 (37) | 7801 (39) | 2810 (55) | 15 801 (40) | |
| 2–5 days | 8361 (59) | 11 253 (56) | 2126 (45) | 21 740 (55) | |
| >5 days | 586 (4) | 904 (5) | 193 (4) | 1683 (4) | |
| ICU on day 1, n (%) | <.001 | ||||
| ICU | 2264 (16) | 2224 (11) | 831 (16) | 5319 (14) | |
| Non-ICU | 11 874 (84) | 17 735 (89) | 4298 (84) | 33 907 (87) | |
| Antibiotic rank on day 1, n (%) | <.001 | ||||
| 1 | 1646 (12) | 3755 (19) | 1056 (21) | 6457 (17) | |
| 2 | 3440 (24) | 4746 (24) | 1044 (20) | 9230 (24) | |
| 3 | 8726 (62) | 10 948 (55) | 2915 (57) | 22 589 (58) | |
| 4 | 326 (2) | 510 (3) | 114 (2) | 950 (2) | |
| Number of antibiotics on day 1, n (%) | <.001 | ||||
| 1 | 8813 (63) | 15 265 (77) | 4450 (87) | 28 528 (73) | |
| 2 | 4609 (33) | 4373 (22) | 634 (12) | 9616 (25) | |
| 3 | 668 (5) | 311 (2) | 41 (<1) | 1020 (3) | |
| 4 | 48 (<1) | 10 (<1) | 4 (<1) | 62 (<1) | |
| De-escalation day (day D) , n (%) | <.001 | ||||
| 3 | 1816 (13) | 5524 (28) | 198 (4) | 7538 (19) | |
| 4 | 3465 (25) | 3255 (16) | 419 (8) | 7139 (18) | |
| 5 | 8857 (66) | 11 180 (56) | 4512 (88) | 24 549 (63) |
Abbreviations: ICU, intensive care unit; SD, standard deviation.

Distribution of de-escalation outcomes by diagnosis. Each vertex of the ternary plot indicates one of the 3 possible outcomes: de-escalation, escalation, unchanged. Admissions assigned to each diagnosis category were plotted based on the proportions of admissions in each outcome category. Points clustered in the lower right corner because >50% of admissions had antibiotics unchanged. See Supplementary Table 1 for the percentages plotted above and AHRQ codes used to define infection diagnosis. Abbreviations: AHRQ, Agency for Healthcare Research and Quality; Bac, bacterial infection, unspecified site; Bone, bone and joint; BSI, bloodstream/septicemia; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; ENT, ear, nose, throat, and upper respiratory tract; GI, gastrointestinal tract; IAB, intra-abdominal infection; Miss, Missing ICD-10 (International Classification of Diseases, 10th revision) data; Oth, no infection diagnosis; PNA, pneumonia; Skin, skin and soft tissue; STI, sexually transmitted infection (not human immunodeficiency virus or hepatitis); UTI, urinary tract; Vas, vascular; >1 Dx, >1 infection diagnosis.
Overall, the agreement between the electronic definition and human assessors was slight to fair (κ, .22; 95% confidence interval [CI], .11–.32) (Table 5). There were several clinical scenarios where the electronic definition did not agree with human assessors. Some human assessors considered a transition to oral therapy (eg, move from intravenous [IV] ceftriaxone to oral fluoroquinolone) to be de-escalation. Oral switch may have been considered unchanged or escalation (eg, if moving from a single IV agent to combination oral therapy) using the electronic definition. More commonly, disagreements occurred because the electronic definition used calendar day (12 am—11:59 pm) time points, and administrations data rather than orders data. Human assessors were able to identify scenarios where empiric antibiotic regimens of more than 1 agent had administrations of the multiple agents split over the midnight time point. Also, human assessors considered when antibiotic orders were stopped partially through day D, while the electronic definition would have counted the full day even if only 1 administration occurred early on day D. Human assessors also evaluated postdischarge antibiotics, which were not captured with the electronic definition. Finally, human assessors considered additional antibiotic changes occurring between day 1 and day D, while the electronic definition only assessed exposures at 2 calendar-day time points. Among the 120 cases reviewed by 2 different human reviewers, concordance was also fair: human–human κ of .29 (95% CI, .15–.43) and human–electronic κ of .28 (95% CI, .13–.43).
Concordance of Human Retrospective Case Review With Proposed Electronic Definition for De-escalation
| . | Electronic . | |||
|---|---|---|---|---|
| Human . | De-escalation . | Unchanged . | Escalation . | Total . |
| De-escalation | 55 | 38 | 12 | 105 |
| Unchanged | 40 | 66 | 4 | 110 |
| Escalation | 7 | 17 | 11 | 35 |
| Total | 102 | 121 | 27 | 250 |
| . | Electronic . | |||
|---|---|---|---|---|
| Human . | De-escalation . | Unchanged . | Escalation . | Total . |
| De-escalation | 55 | 38 | 12 | 105 |
| Unchanged | 40 | 66 | 4 | 110 |
| Escalation | 7 | 17 | 11 | 35 |
| Total | 102 | 121 | 27 | 250 |
Concordance of Human Retrospective Case Review With Proposed Electronic Definition for De-escalation
| . | Electronic . | |||
|---|---|---|---|---|
| Human . | De-escalation . | Unchanged . | Escalation . | Total . |
| De-escalation | 55 | 38 | 12 | 105 |
| Unchanged | 40 | 66 | 4 | 110 |
| Escalation | 7 | 17 | 11 | 35 |
| Total | 102 | 121 | 27 | 250 |
| . | Electronic . | |||
|---|---|---|---|---|
| Human . | De-escalation . | Unchanged . | Escalation . | Total . |
| De-escalation | 55 | 38 | 12 | 105 |
| Unchanged | 40 | 66 | 4 | 110 |
| Escalation | 7 | 17 | 11 | 35 |
| Total | 102 | 121 | 27 | 250 |
DISCUSSION
We derived an electronic definition for antibiotic de-escalation using available data from the EHR from adult patients hospitalized at least 3 days. De-escalation occurred in approximately one-third of eligible admissions. We observed variability in de-escalation and escalation rates among hospitals, inpatient units, and infectious diagnoses. The strengths of the proposed definition are that it is fully electronic and objective, requiring no expert review or personnel resource to assess a large number of hospitalized patients. Further, the estimates were derived from patient-level datasets aggregated to calendar days, which also could be used to calculate day-of-therapy metrics from inpatient eMAR data. These types of datasets are already accessible by many ASPs who are capturing days of therapy data for program assessments. Previous work in objective assessments of de-escalation has focused on particular patient populations, such as patients with pneumonia or cared for in the ICU, rather than the hospitalized population as a whole [13–16]. The proposed de-escalation metric definition was applied across patient populations in order to promote the principle of de-escalation widely to any patient who receives antibiotics in the hospital.
The de-escalation rates observed among different unit types and infectious diagnoses had face validity. Infections originating from polymicrobial sources (eg, intra-abdominal, gastrointestinal) and infections where cultures are not usually obtained (eg, skin and soft tissue infections) had lower de-escalation rates, compared with diagnoses where clinicians can make a microbiologic diagnosis and have susceptibility data to inform a targeted antibiotic choice (eg, urinary tract infection and bloodstream infection). We also observed higher rates of both antibiotic de-escalation and escalation in ICUs as compared with non-ICU wards. This finding was consistent with the dynamic nature of clinical decision making in critically ill patients and suggests that ICU providers frequently re-assess antibiotic therapy. Importantly, over half of eligible admissions were assigned to the unchanged category, which suggests there is likely an opportunity to encourage and improve de-escalation rates among patients. However, we did not directly assess the patients in this pilot study for missed de-escalation opportunities, which should be confirmed in future investigations. Stewardship interventions such as prospective audit and feedback, handshake stewardship rounds, antibiotic time outs, or auto-stop dates requiring justification for continued therapy, may be strategies to push de-escalation rates higher. These improvements may not be evident when evaluating overall days of therapy rates, but still could represent improvements in the processes of antibiotic decision making.
Antibiotic-ranking schemas have been previously proposed by other researchers and need further debate and refinement [15]. Although we proposed a rank schema in Table 1, we believe that antibiotic-ranking systems should be a topic for consensus-building exercises and standardization among researchers. Our rank table is a simple, ordinal scale that accounts for both antibiotic spectrum of activity as well as ASP-focused criteria. Simple scales translate easily for frontline clinicians who make decisions quickly at the bedside. Similarly, simplicity allows stewards to easily explain the metric to hospital leaders who review quality data. Our ranking schema is similar to that of other investigators who have used ordinal scales and a limited number of categories [17–19]. Other researchers have developed more complex numeric scoring systems based on antibiotic spectrum, or the number of targeted pathogens against which an agent is active [16, 20]. Complex numeric scores for particular agents may be hard to remember at the point of care. From an ASP perspective, additional considerations outside of spectrum can weigh into de-escalation decision making. For example, daptomycin, while a relatively narrow-spectrum gram-positive agent, is often targeted by inpatient ASPs to preserve for infections with multidrug-resistant pathogens. Although the ultimate aim for such scores is to predict effects on microbiome disruption and other clinical outcomes such as Clostridioides difficile and acquired drug resistance, most antibiotics have not yet been tested and compared for these effects. The proposed antibiotic-ranking schema will need adjustment as ASP practice changes, microbiome research progresses, and as we gain a more complete understanding of the effects of specific agents on clinical outcomes.
When compared with human assessments, the electronic definition had specific areas of disagreement. These disagreements were largely due to the type of data and time points used in the electronic definition as well as varied interpretation of de-escalation among human assessors. The overall agreement among human reviewers and the electronic definition was only fairly concordant, similar to other investigations [16]. Human–human concordance was similarly only fair. We believe this finding illustrates the difficulty with the subjective, and highly variable, nature of expert assessments of de-escalation. Reviewing discordant events, however, did identify important limitations in the electronic definition.
Limitations of the proposed electronic definition are important to consider in interpreting these data for ASP assessments. First, antibiotics selected on day 1 impact the ability to de-escalate. For example, patients who start on rank 1 agent monotherapy do not have “room” to de-escalate further unless antibiotics are fully stopped. In contrast, patients who start on multiple, high-rank agents have an opportunity to move to several other lower-rank agents in addition to stopping agents and therefore are more likely to have de-escalation opportunities. Therefore, when assessing de-escalation rates, we must also consider day 1 decisions as opportunities for stewardship intervention. Assessment of day 1 choices in addition to de-escalation rates can help determine if empiric therapies may be overly aggressive. Second, the proposed definition did not consider IV to oral transitions as a factor used in determining the de-escalation outcome. We consider this a separate aim and principle of stewardship. Further, addition of route would have added complexity to a definition that already requires multiple steps. Third, the proposed definition was based on calendar days of antibiotic administrations in the inpatient setting. Capture of decisions made upon hospital discharge would have required an alternate data source in addition to eMAR data, such as electronic prescriptions data. Ideally, future editions of this metric should link inpatient and postdischarge days to consider in assessments of de-escalation. Our definition only assessed adult inpatients with at least 3 days of inpatient stay, and thus did not include assessments of patients with short lengths of stay, or children. A similar de-escalation metric definition could be derived for pediatric patients, although the antibiotic-ranking schema may need modification for that population. Finally, billed diagnosis codes may not adequately capture indication for antibiotics or infection diagnosis. As this is a pilot feasibility study that aimed to develop the definition, we have not yet studied associations with important clinical outcomes such as C. difficile or acquired drug resistance. This study did not assess the impact of current stewardship activities on the de-escalation rates in the participating hospitals. In the future, we plan to test the responsiveness of the electronic de-escalation metric to ASP interventions.
In conclusion, we developed a fully electronic definition for antibiotic de-escalation which could be used in ASP assessments. This is a metric that stewardship experts have indicated is important. Calculation of the de-escalation metric was feasible, used patient-level datasets derived for the purposes of days of therapy calculations, and thus may be accessible to ASPs already using these data for AU measurement. It offers additional, distinct information compared with volume of antimicrobial use and may assist in identifying opportunities for ASP intervention or demonstrating ASP impact.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the pilot hospitals and antimicrobial stewards in this project who provided essential feedback and input into the development of the de-escalation metric: Duke University Medical Center, Duke Regional Hospital, Piedmont Fayette Hospital, Piedmont Newnan Hospital, Southeastern Regional Medical Center. The authors thank antimicrobial stewards who assisted with case reviews: John Boreyko, Melissa Johnson, Travis Jones, Christina Sarubbi, Michael J. Smith, S. Shaefer Spires, Michael Wolcott, Michael Yarrington. The authors also thank Elizabeth Hermsen, Merck and Co, Inc, Kenilworth, New Jersey, for her support for this project and for input into development of the de-escalation metric.
Disclaimer. The findings and conclusions in this paper are those of the authors and may not reflect those of the Centers for Disease Control and Prevention. There was no writing assistance from the funding source.
Financial support. This work was supported by a grant from the CDC Foundation and the Agency for Healthcare Research and Quality (grant number K08 HS023866) to R. W. M. The source of this information is the Patient Tools for Antibiotic Stewardship Programs, a joint project made possible by a partnership between the CDC Foundation and Merck and Co, Inc, Kenilworth, New Jersey.
Potential conflicts of interest. D. J. A. reports grants from the Centers for Disease Control and Prevention (CDC), grants from the National Institutes of Health/National Institute of Allergy and Infectious Diseases, grants from the Agency for Healthcare Research and Quality, and personal fees from UpToDate, outside the submitted work. E. D. A. reports personal fees from Joint Commission Resources, outside the submitted work. R. W. M. reports grants from the CDC, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Agency for Healthcare Research and Quality.




