-
PDF
- Split View
-
Views
-
Cite
Cite
Brendan R Jackson, Jeremy A W Gold, Pavithra Natarajan, John Rossow, Robyn Neblett Fanfair, Juliana da Silva, Karen K Wong, Sean D Browning, Sapna Bamrah Morris, Jessica Rogers-Brown, Alfonso C Hernandez-Romieu, Christine M Szablewski, Nadine Oosmanally, Melissa Tobin-D’Angelo, Cherie Drenzek, David J Murphy, Julie Hollberg, James M Blum, Robert Jansen, David W Wright, William M Sewell, Jack D Owens, Benjamin Lefkove, Frank W Brown, Deron C Burton, Timothy M Uyeki, Stephanie R Bialek, Priti R Patel, Beau B Bruce, Predictors at Admission of Mechanical Ventilation and Death in an Observational Cohort of Adults Hospitalized With Coronavirus Disease 2019, Clinical Infectious Diseases, Volume 73, Issue 11, 1 December 2021, Pages e4141–e4151, https://doi.org/10.1093/cid/ciaa1459
Close - Share Icon Share
Abstract
Coronavirus disease (COVID-19) can cause severe illness and death. Predictors of poor outcome collected on hospital admission may inform clinical and public health decisions.
We conducted a retrospective observational cohort investigation of 297 adults admitted to 8 academic and community hospitals in Georgia, United States, during March 2020. Using standardized medical record abstraction, we collected data on predictors including admission demographics, underlying medical conditions, outpatient antihypertensive medications, recorded symptoms, vital signs, radiographic findings, and laboratory values. We used random forest models to calculate adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for predictors of invasive mechanical ventilation (IMV) and death.
Compared with age <45 years, ages 65–74 years and ≥75 years were predictors of IMV (aORs, 3.12 [95% CI, 1.47–6.60] and 2.79 [95% CI, 1.23–6.33], respectively) and the strongest predictors for death (aORs, 12.92 [95% CI, 3.26–51.25] and 18.06 [95% CI, 4.43–73.63], respectively). Comorbidities associated with death (aORs, 2.4–3.8; P < .05) included end-stage renal disease, coronary artery disease, and neurologic disorders, but not pulmonary disease, immunocompromise, or hypertension. Prehospital use vs nonuse of angiotensin receptor blockers (aOR, 2.02 [95% CI, 1.03–3.96]) and dihydropyridine calcium channel blockers (aOR, 1.91 [95% CI, 1.03–3.55]) were associated with death.
After adjustment for patient and clinical characteristics, older age was the strongest predictor of death, exceeding comorbidities, abnormal vital signs, and laboratory test abnormalities. That coronary artery disease, but not chronic lung disease, was associated with death among hospitalized patients warrants further investigation, as do associations between certain antihypertensive medications and death.
(See the Editorial Commentary by McCauley and Ortega-Legaspi on pages e4152–3.)
Pandemic coronavirus disease 2019 (COVID-19) is causing severe illness and deaths across the United States and worldwide. Data from a variety of healthcare settings are needed on patient characteristics and clinical findings on admission to predict who is most likely to receive invasive mechanical ventilation (IMV) and die.
Analyses of medical records and administrative data have identified older age [1, 2] and several common chronic conditions as possible risk factors for severe illness and death from COVID-19, including cardiovascular disease [2–5], hypertension [3, 4, 6–8], diabetes [2], chronic obstructive pulmonary disease (COPD) [9], obesity [10–13], and cigarette smoking [14]. However, some associations are inconsistent across studies and have differed by patient population and outcome measure (eg, hospitalization, intensive care unit [ICU] admission, IMV, and death). Several studies did not adjust for age and other confounders or had incomplete patient outcomes [1, 8, 15]. An association between COVID-19 outcomes and antihypertensive medications, particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), is biologically plausible, given the role of the human ACE2 receptor in viral entry, but speculative [16]; recent studies have not identified associations between prehospital use of antihypertensive medication classes and diagnosed COVID-19 or composite adverse outcomes [17, 18].
Several studies examined predictors of adverse outcomes in COVID-19 and proposed predictive criteria based on specialized laboratory testing [19], but some of these studies examined laboratory values obtained days into patients’ hospital courses, making them less useful in predicting later outcomes than admission values [19–22]. Furthermore, predictors of IMV may be different from predictors of death, since many patients with IMV will recover and not all patients who die have received IMV. In this investigation, we gathered descriptive data available to most clinicians on patient hospital admission to examine predictors of IMV and death to inform clinical and public health practice.
METHODS
The Centers for Disease Control and Prevention (CDC) and the Georgia Department of Public Health (DPH) partnered with 3 hospital networks to abstract medical records of patients hospitalized with COVID-19 in 8 Georgia hospitals and assess the association between patient characteristics, underlying conditions, prehospital medications, and clinical findings on patient presentation with receipt of IMV or death. Seven hospitals were in metropolitan Atlanta, and 1 was in the southern region of Georgia; all provided tertiary care and included academic medical centers, a public teaching hospital, and community hospitals. The CDC and Georgia DPH determined this investigation to be a nonresearch public health response activity.
Patient Population
We collected data on patients hospitalized during March 2020 with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection—the virus that causes COVID-19—confirmed by reverse-transcription polymerase chain reaction; observation stays and deaths in the emergency department were also eligible for inclusion. Patients transferred between participating hospitals or admitted multiple times to the same hospital during March were analyzed as having a single hospitalization. Hospitals provided lists of patients with SARS-CoV-2 infection admitted during 1–30 March 2020 (N = 698). We abstracted data from medical records of 305 adult patients (≥18 years old) sequentially selected from these lists. For analysis, we included only patients with completed hospitalizations (ie, discharge or death, n = 297) as of 8 May 2020.
Data Collection
During 25 March–8 May 2020, investigators abstracted medical records using a secure REDCap form [23] that included elements on patient demographics, underlying medical conditions, prehospital medications, whether reason for admission was COVID-19 related, presenting signs and symptoms, laboratory testing, radiographic imaging, and outcomes. Records reviewed included clinician notes and first recorded vital signs, laboratory values, and imaging. The database was continually reviewed to correct missing data and implausible values. History of stroke was included under cardiovascular and not neurologic conditions. Hypoxia on admission was defined as oxygen saturation ≤94% on room air or use of supplemental oxygen. Prehospital antihypertensive medications were classified as ACE inhibitors, ARBs, β-blockers, dihydropyridine calcium channel blockers (dCCBs), thiazide diuretics, nonthiazide diuretics (eg, furosemide, spironolactone), other vasodilators (eg, hydralazine), and other medications (eg, clonidine, prazosin). Immunocompromise was defined as cancer with chemotherapy receipt within the previous year, history of solid organ or stem cell transplantation, human immunodeficiency virus infection, or current use of immunosuppressive medications. Outcomes were defined as IMV and death and were examined separately.
Data Analysis
To evaluate independent predictors of IMV and death at hospital admission, we used counterfactual random forest probability machines [24, 25] to adjust for all other variables reported, including number of comorbidities and whether reason for admission was COVID-19 related. In brief, separate random forest models were developed using patients with each value of the covariate under consideration. Estimated probabilities of the outcome were generated for all patients, including those who experienced a different value of exposure (ie, counterfactual). From the sum of the predicted probabilities, 2 × 2 tables were constructed and scaled to the data’s exposure margins. Odds ratios and standard errors were subsequently calculated by standard methods. Continuous variables (age, body mass index [BMI], vital signs, and laboratory values) were entered into random forests as continuous, but we reported associations for individual variables using quantiles or standard categories (for age and BMI) that roughly aligned with quintiles. Categories with the lowest values were used as reference groups. We used quantiles rather than laboratory reference ranges to provide finer detail than reference ranges allowed, as use of reference ranges only might obscure clinically meaningful differences.
R statistical software version 3.6.3 was used to conduct analyses. Random forests were generated using the randomForestSRC package (version 2.9.3) with default settings (1000 trees per forest, square root of the number of variables randomly selected as node splitting candidates, 10 random splits considered for each continuous variable). Missing data, ranging from 0% (for 64% [55/86] of variables, eg, age) to 29% for alkaline phosphatase, were imputed by a single random forest imputation (impute.rfsrc function).
To identify simple algorithms predictive of IMV and death independent of the random forest model, we developed machine-generated decision trees called fast-and-frugal trees (FFTs), which identify the variables and cut-points (for continuous variables) most predictive of outcome. Each decision point, or node, has 2 branches, 1 of which continues the tree and the other an exit. For the final node, both branches are exits. FFTs were generated using the ifan algorithm in the R FFTrees package (version 1.5.2) to best balance sensitivity and specificity. Final models rounded continuous variable cut-points to whole numbers to simplify use.
RESULTS
As of 8 May 2020, 297 of 305 (97.4%) patients had completed hospitalizations: 51 (17.2%) died and 246 (82.8%) were discharged alive (Supplementary Figure). Demographics and underlying medical conditions of this cohort have been previously described [26]. Of the 297, median age was 60 years (interquartile range [IQR], 45–69 years), 149 (50.2%) were female, 241 (81.1%) were non-Hispanic black, and 20 (6.7%) resided in a skilled nursing facility (SNF). Most (n = 277 [93.3%]) were hospitalized in metropolitan Atlanta. More than one-third (n = 112 [37.7%]) received ICU care, including 85 (28.6%) who received IMV. Of the 85 who received IMV, 38 (44.7%) died, whereas 13 died of the 212 (6.1%) who did not require/receive IMV. The median age of patients receiving IMV who died was older (70 years [IQR, 63–76 years]) than those who survived (61 years [IQR, 48.5–67 years]). Of the 13 who died without receiving IMV, median age was 81 years (IQR, 73–91 years).
Predictors of IMV and Death
In random forest models, increasing age was the strongest predictor of death, with age ≥75 years having an adjusted odds ratio (aOR) of 18.06 (95% CI, 4.43–73.63) and age 65–74 years having an aOR of 12.92 (95% CI, 3.26–51.25) compared with age <45 years (Table 1). For each older age stratum, aORs were 2–6 times higher for death compared with IMV. For IMV, elevated respiratory rate (aOR, 5.46 [95% CI, 2.41–12.34] for rates 20–22 vs <19), end-stage renal disease (ESRD) on dialysis (aOR, 4.05 [95% CI, 1.43–11.44]), and elevated aspartate aminotransferase (AST) (aOR, 3.5 [95% CI, 1.58–7.76] for highest vs lowest quintile) were stronger predictors than differences in age strata. Patients who resided in a SNF had higher odds of death (aOR, 4.15 [95% CI, 1.62–10.61]) but not IMV (aOR, 0.59 [95% CI, .19–1.80]) than those who resided elsewhere. Sex, insurance status, and non-Hispanic black race were not significantly associated with IMV receipt or death.
Demographic Characteristics, Underlying Conditions, and Prehospital Antihypertensive Medications as Potential Predictors in a Random Forest Model of Invasive Mechanical Ventilation and Death in a Cohort of 297 Patients With Completed Coronavirus Disease 2019 Hospitalizations in Georgia, United States
 |
 |
 |
 |
Demographic Characteristics, Underlying Conditions, and Prehospital Antihypertensive Medications as Potential Predictors in a Random Forest Model of Invasive Mechanical Ventilation and Death in a Cohort of 297 Patients With Completed Coronavirus Disease 2019 Hospitalizations in Georgia, United States
 |
 |
 |
 |
Among the underlying conditions, independent predictors of death due to COVID-19 included preexisting ESRD on dialysis (aOR, 3.84 [95% CI, 1.36–10.82]), neurologic disorders (aOR, 2.94 [95% CI, 1.39–6.23]), and coronary artery disease (CAD) (aOR, 2.37 [95% CI, 1.08–5.23]). More than half of patients with neurologic conditions had dementia or Parkinson disease. History of stroke had a nonsignificantly elevated odds ratio (aOR, 2.97 [95% CI, .93–9.47]) for death. Diabetes mellitus was associated with greater odds of receiving IMV (aOR, 1.90 [95% CI, 1.14–3.16]; P = .01) and with death (aOR, 1.77 [95% CI, .99–3.19]; P = .06). Hemoglobin A1c ≥8% was associated with IMV (aOR, 2.10 [95% CI, 1.01–4.35]) but not death. Elevated BMI, hypertension, heart failure, COPD, asthma, liver disease, chronic kidney disease without dialysis, and immunocompromising conditions were not significantly associated with higher odds of IMV or death after controlling for other variables in the model. Number of comorbidities was not significantly associated with death independently of their individual contribution, although having ≥3 comorbidities was associated with greater odds (aOR, 2.29 [95% CI, 1.01–5.16]) of IMV compared with having none.
History of hypertension or number of antihypertensive medications before admission was not associated with IMV or death controlling for other variables in the models, including age and comorbidities. However, prehospital use of ARBs or dCCBs, specifically, was associated with twice the odds of death (aORs, 2.02 [95% CI, 1.03–3.96] and 1.91 [95% CI, 1.03–3.55], respectively) compared with patients not taking either. Prehospital ARB use was also significantly associated with IMV (aOR, 1.84 [95% CI, 1.02–3.32]), but not dCCB use.
Among recorded admission signs and symptoms, altered mental status was significantly predictive of adverse outcomes, having 4.99 times the odds (95% CI, 2.07–12.01) of death compared with patients without this condition recorded (Table 2). Among presenting vital signs, hypoxia and elevated respiratory rate were significantly predictive of IMV. Admission systolic blood pressure <111 mm Hg (first quintile) vs 122–131 mm Hg (third quintile) and diastolic blood pressure <65 mm Hg (first quintile) vs 78–85 mm Hg (fourth quintile) were associated with significantly higher odds of IMV. For respiratory rate, the upper 3 quartiles of respiratory rate (≥19 breaths per minute) had elevated aORs for death compared with the referent lowest quartile, but respiratory rate was statistically significant only for the second quartile (aOR, 3.87 [95% CI, 1.62–9.28]).
Admission Symptoms and Vital Signs as Potential Predictors in a Random Forest Model of Invasive Mechanical Ventilation and Death in a Cohort of 297 Patients With Completed Coronavirus Disease 2019 Hospitalizations in Georgia, United States
 |
 |
 |
 |
Admission Symptoms and Vital Signs as Potential Predictors in a Random Forest Model of Invasive Mechanical Ventilation and Death in a Cohort of 297 Patients With Completed Coronavirus Disease 2019 Hospitalizations in Georgia, United States
 |
 |
 |
 |
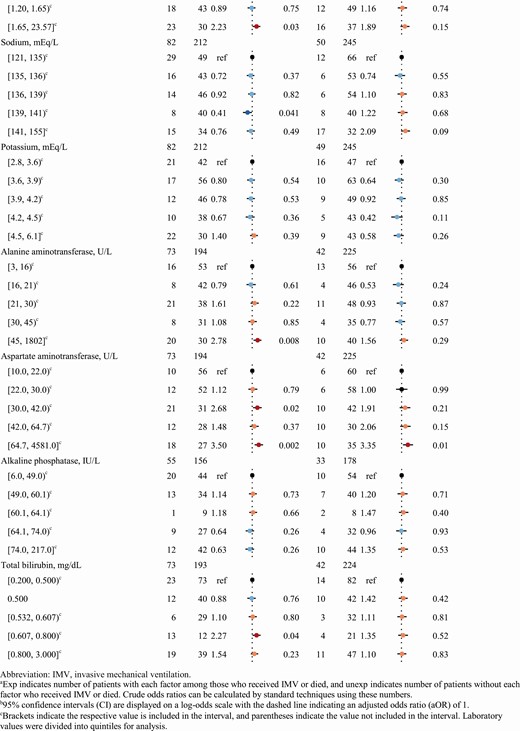
Laboratory tests associated with increased odds of death included thrombocytopenia (lowest quintile, platelets <142 cells/mm3) compared with other quintiles and the highest quintile of AST (≥63 IU/L) compared with the lowest (Table 3). Compared to those with values in the lowest quintiles, the highest quintile of absolute lymphocyte count (≥1.47 cells/mm3) was protective for death, the highest blood urea nitrogen (BUN) quintile (≥27 mg/dL) had greater odds of IMV and death, and the highest creatinine quintile (≥1.65 mg/dL) was also associated with IMV and was nonsignificantly associated with death (P = .15). Certain higher quintiles of alanine aminotransferase, AST, and total bilirubin were also associated with IMV compared with the lowest. Presence of a bilateral or multifocal infiltrate was significantly associated with death (aOR, 1.98 [95% CI, 1.05–3.76]); other abnormality or opacity on chest radiograph was not significantly associated with outcomes.
Radiographic Findings and Laboratory Values as Potential Predictors in a Random Forest Model of Invasive Mechanical Ventilation and Death in a Cohort of 297 Patients With Completed Coronavirus Disease 2019 Hospitalizations in Georgia, United States
 |
 |
 |
 |
Radiographic Findings and Laboratory Values as Potential Predictors in a Random Forest Model of Invasive Mechanical Ventilation and Death in a Cohort of 297 Patients With Completed Coronavirus Disease 2019 Hospitalizations in Georgia, United States
 |
 |
 |
 |
The selected FFT decision tree and selected cut-points for parsimoniously predicting IMV included respiratory rate (>20 breaths per minute), BUN (>21 mg/dL), hypoxia, and diastolic blood pressure (≤74 mm Hg), with overall accuracy of 70%, sensitivity of 60%, and specificity of 74% (Figure 1A). The selected FFT for death included age (≥63 years), BUN (>16 mg/dL), and AST (>37 U/L), with overall accuracy of 75%, sensitivity of 78%, and specificity of 74% (Figure 1B).
Predictive models of invasive mechanical ventilation (IMV) (A) and death (B) in a cohort of 297 patients with completed coronavirus disease 2019 hospitalizations in Georgia, United States, using machine-derived fast-frugal trees (FFTs) and cut-points. The IMV FFT had overall accuracy of 70%, sensitivity of 60%, and specificity of 74%, and the death FFT had overall accuracy of 75%, sensitivity of 78%, and specificity of 74%. Abbreviations: bpm, breaths per minute; IMV, invasive mechanical ventilation; O2, oxygen.
DISCUSSION
In this observational cohort of nearly 300 predominantly black adults hospitalized with COVID-19 early in the US epidemic, age was by far the strongest predictor of death, with the odds of death increasing markedly among older patients (>12 times greater odds for age 65–74 years and >18 times greater odds for age ≥75 vs those <45 years). By comparison, death was less strongly associated with underlying conditions (ie, ESRD, neurologic disorders, and CAD), SNF residence, and clinical findings (aORs, ≤4.2). The association between age and death persisted despite adjustment for a wide range of factors, including vital signs and laboratory results, which might be assumed to be more directly predictive of poor outcomes. Why older adults have a markedly higher risk of death merits further study but may relate to immune or vascular system changes that occur with aging.
Chronic lung disease [27], immunocompromise [27], tobacco use [28], and obesity [29] might be expected to be predictive of COVID-19–associated death based on data for influenza, another viral respiratory illness. However, in our analysis, these conditions were not associated with in-hospital death. Some earlier reports found associations between obesity and in-hospital death [13], in-ICU death [30], and severe illness [11] in COVID-19, although these studies also did not identify mortality associations for lung disease, immunocompromise, or smoking. However, various studies have linked all of these conditions to severe COVID-19 [31]. For example, a large United Kingdom study found obesity and chronic lung disease to be associated with death from COVID-19 among the general population [2]. Although our investigation’s relatively small sample size may have limited our power to detect associations, our results suggest that obesity without associated comorbidities was not a strong risk factor for in-hospital death. Further research is still needed to evaluate the associations between underlying conditions and risk of death in hospitalized COVID-19 patients.
That prehospital use of ARBs was associated with receiving IMV and in-hospital death, despite extensive adjustment for other factors, including black race, CAD, hypertension, and diabetes, is notable given that a link is biologically plausible and ARB use has been associated with renal dysfunction in COVID-19 [16, 32]. However, other studies found no association between ARB use and mortality [33] or a composite adverse outcome [18] in COVID-19 patients. Why dCCB use was also associated with death in our investigation is unclear, and relationships between antihypertensives and COVID-19 outcomes warrant further examination in larger well-controlled studies. Given that other studies have not linked prehospital antihypertensive use to death in COVID-19 and the limitations of our investigation, outpatients should continue on their prescribed antihypertensive regimens per existing guidance [34].
The simple FFT decision trees predicted outcomes with reasonable accuracy (70%–75%), which was lower than a recently proposed “rule-of-6” algorithm (~90%). However, this algorithm involved specialized laboratory testing (ie, lactate dehydrogenase, C-reactive protein, ferritin) collected up to 48 hours after admission [35], whereas the FFT involve tests routinely ordered in the emergency department. Only 3 variables—age, AST, and BUN—were 75% predictive of death in the FFT model; elevated AST and BUN on admission may be markers of multisystem inflammation, which has been associated with severe disease. Several other clinical factors were also predictive of death in the random forest model: altered mental status, thrombocytopenia, and lower lymphocyte counts. Although previous studies have reported associations between elevated admission AST and death [3] and lower lymphocyte counts and severe COVID-19 [22], and others have identified thrombocytopenia as a marker of poor outcomes [36], few studies have examined these factors on admission in a multivariable model. Notably, abnormal respiratory vital signs were less predictive of death, although they were strongly predictive of IMV in both the FFT and random forest models.
Our retrospective observational investigation has several notable limitations. Because data abstraction was limited to medical records, symptom data are less complete than those obtained by questionnaires. We were unable to evaluate certain specialized testing (eg, C-reactive protein, lactate dehydrogenase, D-dimer) [20, 22, 37] because they were infrequently ordered on admission. Second, the outcome IMV is highly influenced by clinical practice, and some clinicians may have pursued early IMV to minimize noninvasive ventilation and avoid emergency endotracheal intubation, given potential risks of viral transmission. As such, using death as an outcome, rather than solely relying on IMV or a composite outcome, allows examination of predictors less dependent on individual medical practices. Third, we used quantiles rather than vital and laboratory reference ranges, and some quantiles included a mix of normal and abnormal values, which could have biased those categories toward the null. Fourth, our analysis had limited power to detect weak associations, given the relatively small sample size and adjustment for many factors. However, random forest models allowed robust control for confounders, offering benefits over logistic regression, by allowing examination of more covariates, requiring fewer assumptions, and better accounting for interactions [24, 25]. Fifth, we examined nearly 100 admission factors. Although our approach may tend toward a bias to the null with this number of factors [24], we did not incorporate adjustments for multiple testing, and some associations might still have occurred by chance. However, the FFT yielded similar findings to our more well-controlled analyses; FFT also offer benefits over logistic regression because they rarely overfit data and are easy to interpret and use [38, 39]. Finally, although records were selected sequentially in the order in which hospitals identified cases, this cohort is ultimately a convenience sample, as it did not encompass all COVID-19 patients admitted to these hospitals during March 2020 [26]. While findings from this cohort, involving predominantly non-Hispanic black patients in a limited geographical area and time, may not be generalizable to other populations, our investigation provides valuable data on black patients with COVID-19, who have been disproportionately impacted by COVID-19 [40].
In summary, we provide simple decision trees that found the most important predictors for IMV were hypoxia, elevated respiratory rate, elevated BUN, and low diastolic blood pressure; for death the most important predictors were older age (≥63 years), elevated BUN, and elevated AST. These predictors were confirmed and augmented by several additional predictors from our multivariable model. Furthermore, the significant association between prehospital use of ARBs and IMV and death, and of dCCBs and death, warrants additional investigation.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease control and Prevention (CDC).
Financial support. This work was supported by the CDC.
Potential conflicts of interest. J. M. B. reports personal fees from Clew Medical, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
American College of Cardiology.




