-
PDF
- Split View
-
Views
-
Cite
Cite
Emilio Jiménez-Martínez, Guillermo Cuervo, Jordi Carratalà, Ana Hornero, Pilar Ciercoles, Andres Gabarrós, Carmen Cabellos, Ivan Pelegrin, Maria Angeles Dominguez Luzón, Dolores García-Somoza, Jordi Càmara, Cristian Tebé, Jordi Adamuz, Miquel Pujol, A Care Bundle Intervention to Prevent Surgical Site Infections After a Craniotomy, Clinical Infectious Diseases, Volume 73, Issue 11, 1 December 2021, Pages e3921–e3928, https://doi.org/10.1093/cid/ciaa884
Close - Share Icon Share
Abstract
Although surgical site infections after a craniotomy (SSI-CRANs) are a serious problem that involves significant morbidity and costs, information on their prevention is scarce. We aimed to determine whether the implementation of a care bundle was effective in preventing SSI-CRANs.
A historical control study was used to evaluate the care bundle, which included a preoperative shower with 4% chlorhexidine soap, appropriate hair removal, adequate preoperative systemic antibiotic prophylaxis, the administration of 1 g of vancomycin powder into the subgaleal space before closing, and a postoperative dressing of the incisional surgical wound with a sterile absorbent cover. Patients were divided into 2 groups: preintervention (January 2013 to December 2015) and intervention (January 2016 to December 2017). The primary study end point was the incidence of SSI-CRANs within 1 year postsurgery. Propensity score matching was performed, and differences between the 2 study periods were assessed using Cox regression models.
A total of 595 and 422 patients were included in the preintervention and intervention periods, respectively. The incidence of SSI-CRANs was lower in the intervention period (15.3% vs 3.5%; P < .001). Using a propensity score model, 421 pairs of patients were matched. The care bundle intervention was independently associated with a reduced incidence of SSI-CRANs (adjusted odds ratio, 0.23; 95% confidence interval, .13–.40; P < .001).
The care bundle intervention was effective in reducing SSI-CRAN rates. The implementation of this multimodal preventive strategy should be considered in centers with high SSI-CRAN incidences.
Craniotomy is becoming an increasingly used procedure to treat a variety of serious conditions such as parenchymal and meningeal tumors, vascular diseases, and epilepsy [1]. A recent meta-analysis reported that the incidence of surgical site infections after a craniotomy (SSI-CRANs) ranges from 2.2% to 19.8% [2]. The consequences of developing an SSI-CRAN are devastating for both the patient and the healthcare system, given the high morbidity, mortality, and costs involved [3]. However, despite the frequency and seriousness of SSI-CRANs, they are often overlooked and there is little information regarding their prevention [4].
To date, antibiotic prophylaxis, administered either intravenously or topically, has been considered one of the main preventive measures against surgical site infections [5–7]. Although care bundles have been found to be useful in preventing surgical site infections mainly after cardiac or orthopedic surgeries [8], no study has yet analyzed the effectiveness of a care bundle intervention, which considers the main steps of the surgical process, in preventing SSI-CRANs. In this historical control pre–post intervention study, we aimed to determine whether the implementation of a care bundle was effective in preventing SSI-CRANs.
METHODS
Study Design, Setting, and Patients
A historical control study was performed in a 700-bed university hospital for adults in Barcelona, Spain. All consecutive adult patients without any active infection undergoing a clean open craniotomy, according to the criteria of the Centers for Disease Control and Prevention (CDC), were included [9]. The study was divided into 2 periods: preintervention (1 January 2013 to 31 December 2015) and intervention (1 January 2016 to 31 December 2017).
SSI-CRAN Surveillance
Data collection and follow-up during the 2 study periods were performed by members of the infection control team who had received specific training in surveillance methodology to ensure the collection of homogeneous and accurate data. Active mandatory postdischarge surveillance was carried out up to 1 year postsurgery, applying a multimodal approach that included the following: reviewing electronic clinical charts (primary and secondary care), checking readmissions, evaluating emergency visits, and reviewing microbiological and radiological data during the period of the postdischarge surveillance.
Intervention
In the preintervention period, standard surgical recommendations were followed in all craniotomy procedures. In December 2015, after noticing high rates of SSI-CRANs, a multidisciplinary team that included infection control members, a microbiologist, neurosurgeons, and skilled neurosurgery nurses was created to discuss how to decrease the high rate of SSI-CRANs and identify the measures that could be included in a preventive care bundle. The components of the care bundle were chosen by taking into account the risk factors for and causative agents of SSI-CRANs that were found in a previous study by our group [10], as well as considering the most up-to-date clinical practice guidelines regarding preventive measures for surgical site care [11–14]. In addition to the preventive measures applied in the preintervention period, the main care bundle measures included a preoperative shower with 4% chlorhexidine soap, appropriate hair removal, adequate preoperative systemic antibiotic prophylaxis, the administration of 1 g of vancomycin powder into the subgaleal space before closing, and a postoperative dressing of the incisional surgical wound with a sterile absorbent drape. Table 1 summarizes the measures included during the preintervention and intervention periods for all 3 surgical phases, also indicating the level of evidence. The members of the infection control team, neurosurgeons, and neurosurgery nurses were the same during the 2 study periods. During the intervention period, the neurosurgeons and nurses were periodically informed about SSI-CRAN rates to reinforce the implementation of the care bundle.
| Period . | Preoperative . | Intraoperative . | Postoperative . |
|---|---|---|---|
| Preintervention period | Showering (with 4% chlorhexidine gluconate solution) on the day before and on the morning of the operation, head included. | Preoperative skin preparation that includes the standardized application of at least 3 swabs soaked in povidone-iodine solution. | Surgical wound covered with a sterile impermeable drape for the first 24 hours postsurgery. |
| Hair cut with a sterile electric clipper in the surgical area. | Antibiotic prophylaxis with intravenous cefuroxime (1500 mg) 30–60 minutes prior to incision and readministration every 3 hours. | Head washed every 12 hours with povidone-iodine solution and surgical wound care performed every 24 hours with tap water in the first 48 hours. | |
| Skin closed using a skin stapler and the head washed with povidone-iodine solution. | |||
| Intervention period | Preoperative showering with 4% chlorhexidine soap on the day before and on the morning of the operation, head included (1A). Appropriate hair removal with single-use clippers if hair removal is necessary (1A). | Skin preparation that includes the standardized application of at least 3 swabs soaked in povidone-iodine solution (1A). Preoperative antibiotic prophylaxis involving intravenous cefuroxime (1500 mg) 30–60 minutes prior to incision and readministration every 3 hours during the operation (1A). Bone graft care: a) Kept inside a sterile container during the operation and immersed in a solution containing physiological serum and vancomycin (1 g/500 mL) (unresolved issue). After washing the subgaleal space and before closure of the scalp, 1 g of vancomycin powder was added into the subgaleal space in a saline solution before closing the skin (unresolved issue). Skin closed with a skin stapler (unresolved issue). Postsurgery head washing with povidone-iodine solution and saline solution (unresolved issue). | Surgical wound care: a) Surgical wound covered with a sterile absorbent drape for the first 48 hours postsurgery (1A). b) Start surgical wound care and stop drainage in the first 12 hours after surgery. If the wound is visibly stained with blood, wound care must be performed immediately (unresolved issue). c) Surgical wound care must be performed aseptically for the first 48 hours (unresolved issue). Head washing: a) Started within the first 12 hours when surgical wound care is being performed with povidone-iodine and saline solutions in the first 48 hours (unresolved issue). b) Performed every 24 hours from the third day until the removal of the staples. Later, performed every 48 hours with conventional soap until healing (unresolved issue). |
| Period . | Preoperative . | Intraoperative . | Postoperative . |
|---|---|---|---|
| Preintervention period | Showering (with 4% chlorhexidine gluconate solution) on the day before and on the morning of the operation, head included. | Preoperative skin preparation that includes the standardized application of at least 3 swabs soaked in povidone-iodine solution. | Surgical wound covered with a sterile impermeable drape for the first 24 hours postsurgery. |
| Hair cut with a sterile electric clipper in the surgical area. | Antibiotic prophylaxis with intravenous cefuroxime (1500 mg) 30–60 minutes prior to incision and readministration every 3 hours. | Head washed every 12 hours with povidone-iodine solution and surgical wound care performed every 24 hours with tap water in the first 48 hours. | |
| Skin closed using a skin stapler and the head washed with povidone-iodine solution. | |||
| Intervention period | Preoperative showering with 4% chlorhexidine soap on the day before and on the morning of the operation, head included (1A). Appropriate hair removal with single-use clippers if hair removal is necessary (1A). | Skin preparation that includes the standardized application of at least 3 swabs soaked in povidone-iodine solution (1A). Preoperative antibiotic prophylaxis involving intravenous cefuroxime (1500 mg) 30–60 minutes prior to incision and readministration every 3 hours during the operation (1A). Bone graft care: a) Kept inside a sterile container during the operation and immersed in a solution containing physiological serum and vancomycin (1 g/500 mL) (unresolved issue). After washing the subgaleal space and before closure of the scalp, 1 g of vancomycin powder was added into the subgaleal space in a saline solution before closing the skin (unresolved issue). Skin closed with a skin stapler (unresolved issue). Postsurgery head washing with povidone-iodine solution and saline solution (unresolved issue). | Surgical wound care: a) Surgical wound covered with a sterile absorbent drape for the first 48 hours postsurgery (1A). b) Start surgical wound care and stop drainage in the first 12 hours after surgery. If the wound is visibly stained with blood, wound care must be performed immediately (unresolved issue). c) Surgical wound care must be performed aseptically for the first 48 hours (unresolved issue). Head washing: a) Started within the first 12 hours when surgical wound care is being performed with povidone-iodine and saline solutions in the first 48 hours (unresolved issue). b) Performed every 24 hours from the third day until the removal of the staples. Later, performed every 48 hours with conventional soap until healing (unresolved issue). |
Level of evidence indicated in parentheses.
| Period . | Preoperative . | Intraoperative . | Postoperative . |
|---|---|---|---|
| Preintervention period | Showering (with 4% chlorhexidine gluconate solution) on the day before and on the morning of the operation, head included. | Preoperative skin preparation that includes the standardized application of at least 3 swabs soaked in povidone-iodine solution. | Surgical wound covered with a sterile impermeable drape for the first 24 hours postsurgery. |
| Hair cut with a sterile electric clipper in the surgical area. | Antibiotic prophylaxis with intravenous cefuroxime (1500 mg) 30–60 minutes prior to incision and readministration every 3 hours. | Head washed every 12 hours with povidone-iodine solution and surgical wound care performed every 24 hours with tap water in the first 48 hours. | |
| Skin closed using a skin stapler and the head washed with povidone-iodine solution. | |||
| Intervention period | Preoperative showering with 4% chlorhexidine soap on the day before and on the morning of the operation, head included (1A). Appropriate hair removal with single-use clippers if hair removal is necessary (1A). | Skin preparation that includes the standardized application of at least 3 swabs soaked in povidone-iodine solution (1A). Preoperative antibiotic prophylaxis involving intravenous cefuroxime (1500 mg) 30–60 minutes prior to incision and readministration every 3 hours during the operation (1A). Bone graft care: a) Kept inside a sterile container during the operation and immersed in a solution containing physiological serum and vancomycin (1 g/500 mL) (unresolved issue). After washing the subgaleal space and before closure of the scalp, 1 g of vancomycin powder was added into the subgaleal space in a saline solution before closing the skin (unresolved issue). Skin closed with a skin stapler (unresolved issue). Postsurgery head washing with povidone-iodine solution and saline solution (unresolved issue). | Surgical wound care: a) Surgical wound covered with a sterile absorbent drape for the first 48 hours postsurgery (1A). b) Start surgical wound care and stop drainage in the first 12 hours after surgery. If the wound is visibly stained with blood, wound care must be performed immediately (unresolved issue). c) Surgical wound care must be performed aseptically for the first 48 hours (unresolved issue). Head washing: a) Started within the first 12 hours when surgical wound care is being performed with povidone-iodine and saline solutions in the first 48 hours (unresolved issue). b) Performed every 24 hours from the third day until the removal of the staples. Later, performed every 48 hours with conventional soap until healing (unresolved issue). |
| Period . | Preoperative . | Intraoperative . | Postoperative . |
|---|---|---|---|
| Preintervention period | Showering (with 4% chlorhexidine gluconate solution) on the day before and on the morning of the operation, head included. | Preoperative skin preparation that includes the standardized application of at least 3 swabs soaked in povidone-iodine solution. | Surgical wound covered with a sterile impermeable drape for the first 24 hours postsurgery. |
| Hair cut with a sterile electric clipper in the surgical area. | Antibiotic prophylaxis with intravenous cefuroxime (1500 mg) 30–60 minutes prior to incision and readministration every 3 hours. | Head washed every 12 hours with povidone-iodine solution and surgical wound care performed every 24 hours with tap water in the first 48 hours. | |
| Skin closed using a skin stapler and the head washed with povidone-iodine solution. | |||
| Intervention period | Preoperative showering with 4% chlorhexidine soap on the day before and on the morning of the operation, head included (1A). Appropriate hair removal with single-use clippers if hair removal is necessary (1A). | Skin preparation that includes the standardized application of at least 3 swabs soaked in povidone-iodine solution (1A). Preoperative antibiotic prophylaxis involving intravenous cefuroxime (1500 mg) 30–60 minutes prior to incision and readministration every 3 hours during the operation (1A). Bone graft care: a) Kept inside a sterile container during the operation and immersed in a solution containing physiological serum and vancomycin (1 g/500 mL) (unresolved issue). After washing the subgaleal space and before closure of the scalp, 1 g of vancomycin powder was added into the subgaleal space in a saline solution before closing the skin (unresolved issue). Skin closed with a skin stapler (unresolved issue). Postsurgery head washing with povidone-iodine solution and saline solution (unresolved issue). | Surgical wound care: a) Surgical wound covered with a sterile absorbent drape for the first 48 hours postsurgery (1A). b) Start surgical wound care and stop drainage in the first 12 hours after surgery. If the wound is visibly stained with blood, wound care must be performed immediately (unresolved issue). c) Surgical wound care must be performed aseptically for the first 48 hours (unresolved issue). Head washing: a) Started within the first 12 hours when surgical wound care is being performed with povidone-iodine and saline solutions in the first 48 hours (unresolved issue). b) Performed every 24 hours from the third day until the removal of the staples. Later, performed every 48 hours with conventional soap until healing (unresolved issue). |
Level of evidence indicated in parentheses.
Study Outcomes, Variables, and Data Source
The primary study end point was the incidence of SSI-CRANs within 1 year postsurgery. Secondary outcomes included reintervention, readmission, postoperative 30-day mortality, length of hospital stay, and 1-year length of hospital stay. Basic demographic data were recorded, along with the following information on patient comorbidities and surgical procedures: the Charlson comorbidity score [15]; information on surgical procedures, including the American Society of Anesthesiologists (ASA) classification, whether the surgery was elective or emergency, reason for surgery (intrinsic tumor, extrinsic tumor, epilepsy, vascular diseases, trauma, or other), operative site (supratentorial, infratentorial, or retromastoid), whether antibiotic prophylaxis was administered according to hospital guidelines, duration of surgery, use of intracranial pressure monitors, reintervention, cerebrospinal fluid (CSF) leak, and metal plates; characteristics of the infection using CDC classification and bacterial etiology; and in-hospital outcome data (pre- and postsurgery in-hospital stay). Care bundle compliance data were collected for each individual intervention. The clinical characteristics of the patients who developed SSI-CRANs were compared with those of patients who did not develop SSI-CRANs in both periods.
Definitions
An SSI-CRAN was defined, according to CDC criteria [9], as follows: purulent drainage from a surgical incision; identification of microorganisms by culture using fluid or tissue obtained aseptically; incision that dehisces spontaneously or is deliberately opened by a surgeon or physician, localized pain or tenderness, localized inflammation (heat, erythema, and swelling), and/or fever (>38°C); and evidence of an abscess on images or during surgical revision. An SSI-CRAN was also classified, according to CDC criteria, as a superficial incisional, deep incisional, or organ-space infection.
The reason for surgery was defined by the patient’s underlying disease, which included an intrinsic tumor in the central nervous system parenchyma, an extrinsic tumor in the structures of the central nervous system in the skull and meninges, epilepsy, vascular diseases, traumatic injuries, and other.
The Charlson comorbidity score was used to predict 10-year mortality, depending on the age of the patient and comorbidities [15]. Intravenous antibiotic prophylaxis was considered adequate when all of the following criteria were met: antibiotics were administered according to local protocol, the infusion ended within 60 minutes of the surgical incision, and perioperative redosing was performed according to duration if indicated. Thirty-day mortality was defined as death occurring from any cause within 30 days of the surgical intervention. The length of hospital stay was recorded as the duration of the hospitalization for the surgical intervention, while the 1-year length of hospital stay was defined as the total number of days of hospitalization in the year following the neurosurgical intervention due to infections or other neurosurgical complications.
Microbiological Studies
In most patients with a suspected SSI-CRAN, microbiological samples from wounds and/or CSF or abscesses were taken for culture. Blood cultures were also obtained when indicated by the attending physician. Antibiotic susceptibility testing was performed using the microdilution method, following the guidelines of the Clinical and Laboratory Standards Institute (CLSI). The antimicrobial susceptibility of isolates was interpreted according to current CLSI criteria [16].
Statistical Methods
Categorical variables are shown as the number of cases and percentages, while continuous variables are presented as the mean and standard deviation or median and interquartile range (IQR). Continuous variables were compared using the Student t test or Mann-Whitney U test, where appropriate, while categorical variables were compared using the Fisher exact test or Pearson χ 2 test. Time until an SSI-CRAN was assessed using the Kaplan-Meier survival curve. A propensity score was created to match 1:1 patients with a similar covariable profile treated during the preintervention and intervention periods. Each patient from the preintervention period was matched to a patient from the intervention period using the nearest neighbor matching algorithm, with the maximum tolerance distance between the matched patients having a standard deviation of 0.1. We identified the variables that caused an imbalance between the groups after propensity score matching by calculating the standardized mean difference. Time until the onset of an infection was studied using Cox models, while Akaike information criterion was used for automated variable selection. Statistical significance was set at an arbitrary probability level of <0.05. R version 3.5.0 for Windows was used for the statistical analyses.
Ethical Issues
The need for informed consent and the provision of an information sheet were waived because data were routinely collected as part of hospital surveillance and quality improvement. Anonymity and data confidentiality (access to records, data encryption, and archiving of information) were maintained throughout the research process. Patients’ confidential information was protected in accordance with European regulations. The Clinical Research Ethics Committee of Bellvitge University Hospital approved the study.
RESULTS
The study included 1017 consecutive patients, 595 during the preintervention period and 422 during the intervention period. An average of 203 procedures were performed each year over the entire study duration. Baseline and clinical characteristics are summarized in Table 2. Significant differences in the baseline characteristics of the participants between the 2 periods were found for sex, ASA classification, type of surgery, duration of procedure, and the need for reintervention. Baseline and clinical characteristics of the patients during the unadjusted and propensity score-matched periods are summarized in Table 2.
| . | Before Propensity Score Matching (n = 1017) . | . | . | . | . | After Propensity Score Matching (n = 842) . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Pre-CB (n = 595) . | . | CB (n = 422) . | . | P Value . | Pre-CB (n = 421) . | . | CB (n = 421) . | . | P Value . |
| Male, n (%) | 274 | (46.1) | 228 | (54) | .015 | 208 | (49.4) | 227 | (53.9) | .214 |
| Median age, years (IQR) | 54 | (43–64) | 55 | (44.2–66) | .143 | 55 | (43–65) | 55 | (44–66) | .372 |
| Urgent surgery, n (%) | 79 | (13.3) | 54 | (12.8) | .709 | 55 | (13.1) | 53 | (12.6) | .956 |
| Reason for procedure | <.001 | .773 | ||||||||
| Intrinsic tumor, n (%) | 234 | (39.3) | 181 | (42.9) | 182 | (43.2) | 181 | (43) | ||
| Extrinsic tumor, n (%) | 123 | (20.7) | 105 | (24.9) | 99 | (23.5) | 105 | (24.9) | ||
| Epilepsy, n (%) | 23 | (3.9) | 8 | (1.9) | 6 | (1.4) | 8 | (1.9) | ||
| Vascular, n (%) | 152 | (25.5) | 100 | (23.7) | 113 | (26.8) | 99 | (23.5) | ||
| Trauma, n (%) | 17 | (2.9) | 20 | (4.7) | 16 | (3.8) | 20 | (4.7) | ||
| Other, n (%) | 46 | (7.7) | 8 | (1.9) | 5 | (1.2) | 8 | (1.9) | ||
| Surgical site | <.001 | .574 | ||||||||
| Supratentorial, n (%) | 449 | (75.5) | 363 | (86) | 351 | (83.4) | 362 | (86) | ||
| Infratentorial, n (%) | 62 | (10.4) | 29 | (6.9) | 34 | (8.1) | 29 | (6.9) | ||
| Retromastoid, n (%) | 84 | (14.1) | 30 | (7.1) | 36 | (8.5) | 30 | (7.1) | ||
| Duration of surgery, minutes (IQR) | 243 | (185–314) | 260 | (199–335) | .003 | 253 | (193–325) | 260 | (200–335) | .315 |
| Charlson score, median (IQR) | 3 | (0–13) | 3 | (0–12) | 3 | (2–4) | 3 | (2–4) | .178 | |
| American Society of Anesthesiologists classification | < .001 | .907 | ||||||||
| 1, n (%) | 62 | (10.4) | 54 | (12.8) | 52 | (12.4) | 54 | (12.8) | ||
| 2, n (%) | 310 | (52.1) | 271 | (64.2) | 260 | (61.8) | 270 | (64.1) | ||
| 3, n (%) | 202 | (33.9) | 85 | (20.1) | 96 | (22.8) | 85 | (20.2) | ||
| 4, n (%) | 20 | (3.4) | 11 | (2.6) | 12 | (2.8) | 11 | (2.6) | ||
| 5, n (%) | 1 | (0.2) | 1 | (0.2) | 1 | (0.2) | 1 | (0.2) | ||
| Intracranial pressure sensor, n (%) | 36 | (6) | 23 | (5.4) | .789 | 28 | (6.6) | 23 | (5.5) | .563 |
| Cerebrospinal fluid leak, n (%) | 11 | (1.8) | 3 | (0.7) | .207 | 3 | (0.7) | 3 | (0.7) | 1.000 |
| Metal plates, n (%) | 543 | (91.3) | 411 | (97.4) | <.001 | 407 | (96.7) | 410 | (97.4) | .685 |
| . | Before Propensity Score Matching (n = 1017) . | . | . | . | . | After Propensity Score Matching (n = 842) . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Pre-CB (n = 595) . | . | CB (n = 422) . | . | P Value . | Pre-CB (n = 421) . | . | CB (n = 421) . | . | P Value . |
| Male, n (%) | 274 | (46.1) | 228 | (54) | .015 | 208 | (49.4) | 227 | (53.9) | .214 |
| Median age, years (IQR) | 54 | (43–64) | 55 | (44.2–66) | .143 | 55 | (43–65) | 55 | (44–66) | .372 |
| Urgent surgery, n (%) | 79 | (13.3) | 54 | (12.8) | .709 | 55 | (13.1) | 53 | (12.6) | .956 |
| Reason for procedure | <.001 | .773 | ||||||||
| Intrinsic tumor, n (%) | 234 | (39.3) | 181 | (42.9) | 182 | (43.2) | 181 | (43) | ||
| Extrinsic tumor, n (%) | 123 | (20.7) | 105 | (24.9) | 99 | (23.5) | 105 | (24.9) | ||
| Epilepsy, n (%) | 23 | (3.9) | 8 | (1.9) | 6 | (1.4) | 8 | (1.9) | ||
| Vascular, n (%) | 152 | (25.5) | 100 | (23.7) | 113 | (26.8) | 99 | (23.5) | ||
| Trauma, n (%) | 17 | (2.9) | 20 | (4.7) | 16 | (3.8) | 20 | (4.7) | ||
| Other, n (%) | 46 | (7.7) | 8 | (1.9) | 5 | (1.2) | 8 | (1.9) | ||
| Surgical site | <.001 | .574 | ||||||||
| Supratentorial, n (%) | 449 | (75.5) | 363 | (86) | 351 | (83.4) | 362 | (86) | ||
| Infratentorial, n (%) | 62 | (10.4) | 29 | (6.9) | 34 | (8.1) | 29 | (6.9) | ||
| Retromastoid, n (%) | 84 | (14.1) | 30 | (7.1) | 36 | (8.5) | 30 | (7.1) | ||
| Duration of surgery, minutes (IQR) | 243 | (185–314) | 260 | (199–335) | .003 | 253 | (193–325) | 260 | (200–335) | .315 |
| Charlson score, median (IQR) | 3 | (0–13) | 3 | (0–12) | 3 | (2–4) | 3 | (2–4) | .178 | |
| American Society of Anesthesiologists classification | < .001 | .907 | ||||||||
| 1, n (%) | 62 | (10.4) | 54 | (12.8) | 52 | (12.4) | 54 | (12.8) | ||
| 2, n (%) | 310 | (52.1) | 271 | (64.2) | 260 | (61.8) | 270 | (64.1) | ||
| 3, n (%) | 202 | (33.9) | 85 | (20.1) | 96 | (22.8) | 85 | (20.2) | ||
| 4, n (%) | 20 | (3.4) | 11 | (2.6) | 12 | (2.8) | 11 | (2.6) | ||
| 5, n (%) | 1 | (0.2) | 1 | (0.2) | 1 | (0.2) | 1 | (0.2) | ||
| Intracranial pressure sensor, n (%) | 36 | (6) | 23 | (5.4) | .789 | 28 | (6.6) | 23 | (5.5) | .563 |
| Cerebrospinal fluid leak, n (%) | 11 | (1.8) | 3 | (0.7) | .207 | 3 | (0.7) | 3 | (0.7) | 1.000 |
| Metal plates, n (%) | 543 | (91.3) | 411 | (97.4) | <.001 | 407 | (96.7) | 410 | (97.4) | .685 |
Abbreviation: CB, care bundle. Significant variables are marked bold.
| . | Before Propensity Score Matching (n = 1017) . | . | . | . | . | After Propensity Score Matching (n = 842) . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Pre-CB (n = 595) . | . | CB (n = 422) . | . | P Value . | Pre-CB (n = 421) . | . | CB (n = 421) . | . | P Value . |
| Male, n (%) | 274 | (46.1) | 228 | (54) | .015 | 208 | (49.4) | 227 | (53.9) | .214 |
| Median age, years (IQR) | 54 | (43–64) | 55 | (44.2–66) | .143 | 55 | (43–65) | 55 | (44–66) | .372 |
| Urgent surgery, n (%) | 79 | (13.3) | 54 | (12.8) | .709 | 55 | (13.1) | 53 | (12.6) | .956 |
| Reason for procedure | <.001 | .773 | ||||||||
| Intrinsic tumor, n (%) | 234 | (39.3) | 181 | (42.9) | 182 | (43.2) | 181 | (43) | ||
| Extrinsic tumor, n (%) | 123 | (20.7) | 105 | (24.9) | 99 | (23.5) | 105 | (24.9) | ||
| Epilepsy, n (%) | 23 | (3.9) | 8 | (1.9) | 6 | (1.4) | 8 | (1.9) | ||
| Vascular, n (%) | 152 | (25.5) | 100 | (23.7) | 113 | (26.8) | 99 | (23.5) | ||
| Trauma, n (%) | 17 | (2.9) | 20 | (4.7) | 16 | (3.8) | 20 | (4.7) | ||
| Other, n (%) | 46 | (7.7) | 8 | (1.9) | 5 | (1.2) | 8 | (1.9) | ||
| Surgical site | <.001 | .574 | ||||||||
| Supratentorial, n (%) | 449 | (75.5) | 363 | (86) | 351 | (83.4) | 362 | (86) | ||
| Infratentorial, n (%) | 62 | (10.4) | 29 | (6.9) | 34 | (8.1) | 29 | (6.9) | ||
| Retromastoid, n (%) | 84 | (14.1) | 30 | (7.1) | 36 | (8.5) | 30 | (7.1) | ||
| Duration of surgery, minutes (IQR) | 243 | (185–314) | 260 | (199–335) | .003 | 253 | (193–325) | 260 | (200–335) | .315 |
| Charlson score, median (IQR) | 3 | (0–13) | 3 | (0–12) | 3 | (2–4) | 3 | (2–4) | .178 | |
| American Society of Anesthesiologists classification | < .001 | .907 | ||||||||
| 1, n (%) | 62 | (10.4) | 54 | (12.8) | 52 | (12.4) | 54 | (12.8) | ||
| 2, n (%) | 310 | (52.1) | 271 | (64.2) | 260 | (61.8) | 270 | (64.1) | ||
| 3, n (%) | 202 | (33.9) | 85 | (20.1) | 96 | (22.8) | 85 | (20.2) | ||
| 4, n (%) | 20 | (3.4) | 11 | (2.6) | 12 | (2.8) | 11 | (2.6) | ||
| 5, n (%) | 1 | (0.2) | 1 | (0.2) | 1 | (0.2) | 1 | (0.2) | ||
| Intracranial pressure sensor, n (%) | 36 | (6) | 23 | (5.4) | .789 | 28 | (6.6) | 23 | (5.5) | .563 |
| Cerebrospinal fluid leak, n (%) | 11 | (1.8) | 3 | (0.7) | .207 | 3 | (0.7) | 3 | (0.7) | 1.000 |
| Metal plates, n (%) | 543 | (91.3) | 411 | (97.4) | <.001 | 407 | (96.7) | 410 | (97.4) | .685 |
| . | Before Propensity Score Matching (n = 1017) . | . | . | . | . | After Propensity Score Matching (n = 842) . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| . | Pre-CB (n = 595) . | . | CB (n = 422) . | . | P Value . | Pre-CB (n = 421) . | . | CB (n = 421) . | . | P Value . |
| Male, n (%) | 274 | (46.1) | 228 | (54) | .015 | 208 | (49.4) | 227 | (53.9) | .214 |
| Median age, years (IQR) | 54 | (43–64) | 55 | (44.2–66) | .143 | 55 | (43–65) | 55 | (44–66) | .372 |
| Urgent surgery, n (%) | 79 | (13.3) | 54 | (12.8) | .709 | 55 | (13.1) | 53 | (12.6) | .956 |
| Reason for procedure | <.001 | .773 | ||||||||
| Intrinsic tumor, n (%) | 234 | (39.3) | 181 | (42.9) | 182 | (43.2) | 181 | (43) | ||
| Extrinsic tumor, n (%) | 123 | (20.7) | 105 | (24.9) | 99 | (23.5) | 105 | (24.9) | ||
| Epilepsy, n (%) | 23 | (3.9) | 8 | (1.9) | 6 | (1.4) | 8 | (1.9) | ||
| Vascular, n (%) | 152 | (25.5) | 100 | (23.7) | 113 | (26.8) | 99 | (23.5) | ||
| Trauma, n (%) | 17 | (2.9) | 20 | (4.7) | 16 | (3.8) | 20 | (4.7) | ||
| Other, n (%) | 46 | (7.7) | 8 | (1.9) | 5 | (1.2) | 8 | (1.9) | ||
| Surgical site | <.001 | .574 | ||||||||
| Supratentorial, n (%) | 449 | (75.5) | 363 | (86) | 351 | (83.4) | 362 | (86) | ||
| Infratentorial, n (%) | 62 | (10.4) | 29 | (6.9) | 34 | (8.1) | 29 | (6.9) | ||
| Retromastoid, n (%) | 84 | (14.1) | 30 | (7.1) | 36 | (8.5) | 30 | (7.1) | ||
| Duration of surgery, minutes (IQR) | 243 | (185–314) | 260 | (199–335) | .003 | 253 | (193–325) | 260 | (200–335) | .315 |
| Charlson score, median (IQR) | 3 | (0–13) | 3 | (0–12) | 3 | (2–4) | 3 | (2–4) | .178 | |
| American Society of Anesthesiologists classification | < .001 | .907 | ||||||||
| 1, n (%) | 62 | (10.4) | 54 | (12.8) | 52 | (12.4) | 54 | (12.8) | ||
| 2, n (%) | 310 | (52.1) | 271 | (64.2) | 260 | (61.8) | 270 | (64.1) | ||
| 3, n (%) | 202 | (33.9) | 85 | (20.1) | 96 | (22.8) | 85 | (20.2) | ||
| 4, n (%) | 20 | (3.4) | 11 | (2.6) | 12 | (2.8) | 11 | (2.6) | ||
| 5, n (%) | 1 | (0.2) | 1 | (0.2) | 1 | (0.2) | 1 | (0.2) | ||
| Intracranial pressure sensor, n (%) | 36 | (6) | 23 | (5.4) | .789 | 28 | (6.6) | 23 | (5.5) | .563 |
| Cerebrospinal fluid leak, n (%) | 11 | (1.8) | 3 | (0.7) | .207 | 3 | (0.7) | 3 | (0.7) | 1.000 |
| Metal plates, n (%) | 543 | (91.3) | 411 | (97.4) | <.001 | 407 | (96.7) | 410 | (97.4) | .685 |
Abbreviation: CB, care bundle. Significant variables are marked bold.
During the 2 years of follow-up for the intervention period, full compliance with the care bundle (all the measures implemented) was achieved in 238 of the 422 (56.4%) cases. Regarding the reasons for noncompliance, 106 of the 422 (25.1%) patients did not receive an appropriate antibiotic prophylaxis prior to incision, while the use of vancomycin powder was not recorded in the surgical chart sheet for 184 of the 422 (43.6%) cases. Compliance with all other preoperative and postoperative care bundle measures was high, with appropriateness greater than 90%. The rates of SSI-CRANs according to bundle compliance are shown in Figure 1.
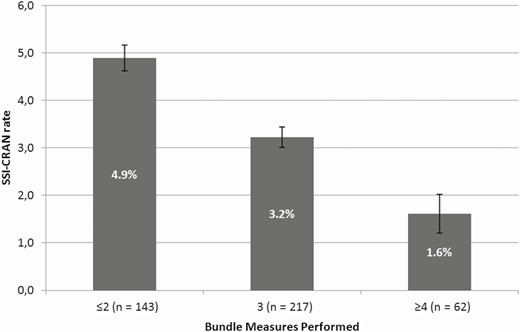
Rates of infections according to compliance with care bundle implementation. Abbreviation: SSI-CRAN, surgical site infection after a craniotomy.
Regarding the primary study end point, the incidence of SSI-CRANs was significantly lower in the intervention period (15.3% vs 3.5%; P < .001). Data concerning primary and secondary outcomes are summarized in Table 3. Figure 2 shows the secular trends of SSI-CRAN incidence during the study period. The SSI-CRAN rates in relation to the reason for surgery are shown in Supplementary Material 1. The median number of days from surgery to diagnosis of an SSI-CRAN were similar in both groups (21, IQR: 14–43 vs 19, IQR: 17.5–39.5), as well as detection. Most of the cases of SSI-CRANs were detected during the postdischarge surveillance period, which included readmission (60/91 [65.9%] vs 10/15 [66.7%]; P = .469). Supplementary Material 2 shows the days from surgery to SSI diagnosis plotted in a Kaplan-Meier curve. Analyzing the risk of SSI-CRAN depending on the adequacy of preoperative antibiotic prophylaxis, no significant differences were found between patients who received adequate antibiotic prophylaxis and those who did not (92/106 [11.1%] vs 14/106 [7.4%], respectively; P = .148). There was no significant difference regarding SSI-CRAN when the application of vancomycin powder was independently analyzed (7/15 [3.8%] patients did not receive vancomycin powder and developed an SSI-CRAN vs 8/15 [3.4%] who received vancomycin powder and developed an SSI-CRAN; P = .798).
| Outcome . | . | . | . | . | . |
|---|---|---|---|---|---|
| . | Preintervention period (n = 595) . | . | Intervention period (n = 422) . | . | P Value . |
| Primary | |||||
| SSI-CRAN, n (%) | 91 | (15.3) | 15 | (3.5) | <.001 |
| Occurrence of SSI-CRAN, days (IQR) | 21 | (14–43) | 19 | (17.5–39.5) | .703 |
| Detection | .469 | ||||
| During hospital admission, n (%) | 31 | (34.1) | 5 | (33.3) | |
| Postdischarge surveillance, n (%) | 60 | (65.9) | 10 | (66.7) | |
| SSI-CRAN classification | .724 | ||||
| Superficial incisional, n (%) | 8 | (8.8) | 2 | (13.3) | |
| Deep incisional, n (%) | 16 | (17.6) | 3 | (20) | |
| Organ-space, n (%) | 67 | (73.6) | 10 | (66.7) | |
| Secondary | |||||
| Reintervention, n (%) | 158 | (26.6) | 81 | (19.2) | .008 |
| Readmission | 54 | (6.9) | 5 | (2.1) | .004 |
| 30-day mortality postsurgery, n (%) | 33 | (5.5) | 14 | (3.3) | .129 |
| LOS, median (IQR) | 7 | (5–14.5) | 7 | (5–14) | .808 |
| 1-year LOS, median (IQR) | 9 | (5–24) | 10 | (5–19.25) | .384 |
| Outcome . | . | . | . | . | . |
|---|---|---|---|---|---|
| . | Preintervention period (n = 595) . | . | Intervention period (n = 422) . | . | P Value . |
| Primary | |||||
| SSI-CRAN, n (%) | 91 | (15.3) | 15 | (3.5) | <.001 |
| Occurrence of SSI-CRAN, days (IQR) | 21 | (14–43) | 19 | (17.5–39.5) | .703 |
| Detection | .469 | ||||
| During hospital admission, n (%) | 31 | (34.1) | 5 | (33.3) | |
| Postdischarge surveillance, n (%) | 60 | (65.9) | 10 | (66.7) | |
| SSI-CRAN classification | .724 | ||||
| Superficial incisional, n (%) | 8 | (8.8) | 2 | (13.3) | |
| Deep incisional, n (%) | 16 | (17.6) | 3 | (20) | |
| Organ-space, n (%) | 67 | (73.6) | 10 | (66.7) | |
| Secondary | |||||
| Reintervention, n (%) | 158 | (26.6) | 81 | (19.2) | .008 |
| Readmission | 54 | (6.9) | 5 | (2.1) | .004 |
| 30-day mortality postsurgery, n (%) | 33 | (5.5) | 14 | (3.3) | .129 |
| LOS, median (IQR) | 7 | (5–14.5) | 7 | (5–14) | .808 |
| 1-year LOS, median (IQR) | 9 | (5–24) | 10 | (5–19.25) | .384 |
Abbreviations: SSI-CRAN, surgical site infection after a craniotomy; IQR, interquartile range; LOS, length of in-hospital stay. Significant variables are marked bold.
| Outcome . | . | . | . | . | . |
|---|---|---|---|---|---|
| . | Preintervention period (n = 595) . | . | Intervention period (n = 422) . | . | P Value . |
| Primary | |||||
| SSI-CRAN, n (%) | 91 | (15.3) | 15 | (3.5) | <.001 |
| Occurrence of SSI-CRAN, days (IQR) | 21 | (14–43) | 19 | (17.5–39.5) | .703 |
| Detection | .469 | ||||
| During hospital admission, n (%) | 31 | (34.1) | 5 | (33.3) | |
| Postdischarge surveillance, n (%) | 60 | (65.9) | 10 | (66.7) | |
| SSI-CRAN classification | .724 | ||||
| Superficial incisional, n (%) | 8 | (8.8) | 2 | (13.3) | |
| Deep incisional, n (%) | 16 | (17.6) | 3 | (20) | |
| Organ-space, n (%) | 67 | (73.6) | 10 | (66.7) | |
| Secondary | |||||
| Reintervention, n (%) | 158 | (26.6) | 81 | (19.2) | .008 |
| Readmission | 54 | (6.9) | 5 | (2.1) | .004 |
| 30-day mortality postsurgery, n (%) | 33 | (5.5) | 14 | (3.3) | .129 |
| LOS, median (IQR) | 7 | (5–14.5) | 7 | (5–14) | .808 |
| 1-year LOS, median (IQR) | 9 | (5–24) | 10 | (5–19.25) | .384 |
| Outcome . | . | . | . | . | . |
|---|---|---|---|---|---|
| . | Preintervention period (n = 595) . | . | Intervention period (n = 422) . | . | P Value . |
| Primary | |||||
| SSI-CRAN, n (%) | 91 | (15.3) | 15 | (3.5) | <.001 |
| Occurrence of SSI-CRAN, days (IQR) | 21 | (14–43) | 19 | (17.5–39.5) | .703 |
| Detection | .469 | ||||
| During hospital admission, n (%) | 31 | (34.1) | 5 | (33.3) | |
| Postdischarge surveillance, n (%) | 60 | (65.9) | 10 | (66.7) | |
| SSI-CRAN classification | .724 | ||||
| Superficial incisional, n (%) | 8 | (8.8) | 2 | (13.3) | |
| Deep incisional, n (%) | 16 | (17.6) | 3 | (20) | |
| Organ-space, n (%) | 67 | (73.6) | 10 | (66.7) | |
| Secondary | |||||
| Reintervention, n (%) | 158 | (26.6) | 81 | (19.2) | .008 |
| Readmission | 54 | (6.9) | 5 | (2.1) | .004 |
| 30-day mortality postsurgery, n (%) | 33 | (5.5) | 14 | (3.3) | .129 |
| LOS, median (IQR) | 7 | (5–14.5) | 7 | (5–14) | .808 |
| 1-year LOS, median (IQR) | 9 | (5–24) | 10 | (5–19.25) | .384 |
Abbreviations: SSI-CRAN, surgical site infection after a craniotomy; IQR, interquartile range; LOS, length of in-hospital stay. Significant variables are marked bold.
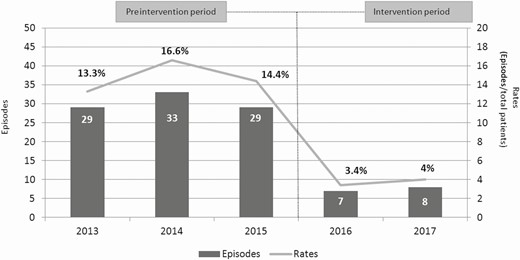
Episodes and rates of surgical site infections after a craniotomy in the preintervention and intervention periods.
As shown in Table 4, the causative agents of SSI-CRANs varied between the study periods, with Cutibacterium acnes and Staphylococcus epidermidis being the most common microorganisms isolated in the preintervention period, and methicillin-susceptible Staphylococcus aureus (MSSA) being the most prevalent in the intervention period.
Most Prevalent Causative Agents of Surgical Site Infection After a Craniotomy and Outcomes in Both Periods
| Causative Agent . | Preintervention Period (n = 91) . | . | Intervention Period (n = 15) . | . |
|---|---|---|---|---|
| Cutibacterium acnes | 21 | (23.1) | 2 | (13.3) |
| Staphylococcus epidermidis | 21 | (23.1) | 1 | (6.6) |
| Methicillin-susceptible Staphylococcus aureus | 18 | (19.8) | 5 | (33.3) |
| Enterobacter cloacae | 11 | (12.1) | 2 | (13.3) |
| Acinetobacter baumannii | 4 | (4.4) | 0 | (0) |
| Causative Agent . | Preintervention Period (n = 91) . | . | Intervention Period (n = 15) . | . |
|---|---|---|---|---|
| Cutibacterium acnes | 21 | (23.1) | 2 | (13.3) |
| Staphylococcus epidermidis | 21 | (23.1) | 1 | (6.6) |
| Methicillin-susceptible Staphylococcus aureus | 18 | (19.8) | 5 | (33.3) |
| Enterobacter cloacae | 11 | (12.1) | 2 | (13.3) |
| Acinetobacter baumannii | 4 | (4.4) | 0 | (0) |
Most Prevalent Causative Agents of Surgical Site Infection After a Craniotomy and Outcomes in Both Periods
| Causative Agent . | Preintervention Period (n = 91) . | . | Intervention Period (n = 15) . | . |
|---|---|---|---|---|
| Cutibacterium acnes | 21 | (23.1) | 2 | (13.3) |
| Staphylococcus epidermidis | 21 | (23.1) | 1 | (6.6) |
| Methicillin-susceptible Staphylococcus aureus | 18 | (19.8) | 5 | (33.3) |
| Enterobacter cloacae | 11 | (12.1) | 2 | (13.3) |
| Acinetobacter baumannii | 4 | (4.4) | 0 | (0) |
| Causative Agent . | Preintervention Period (n = 91) . | . | Intervention Period (n = 15) . | . |
|---|---|---|---|---|
| Cutibacterium acnes | 21 | (23.1) | 2 | (13.3) |
| Staphylococcus epidermidis | 21 | (23.1) | 1 | (6.6) |
| Methicillin-susceptible Staphylococcus aureus | 18 | (19.8) | 5 | (33.3) |
| Enterobacter cloacae | 11 | (12.1) | 2 | (13.3) |
| Acinetobacter baumannii | 4 | (4.4) | 0 | (0) |
After propensity score matching, 421 patients from the preintervention period were matched 1:1 with patients from the intervention period. There were no significant differences between the 2 matched groups. Table 2 shows the characteristics and significant differences between the unmatched and matched groups. Across 11 covariates, the standardized differences were ≥0.1, demonstrating that all variables were sufficiently balanced between the 2 matched groups (Supplementary Material 3).
Multivariate logistic regression analysis showed that the only independent factors associated with SSI-CRANs were care bundle implementation (adjusted odds ratio [AOR], 0.23; 95% confidence interval [CI], .13–.40; P < .001) and CSF leak (AOR, 3.93; 95% CI, 1.11–12.68; P = .025). The results of the multivariate logistic regression analysis are summarized in Table 5. After propensity score matching, care bundle intervention was independently associated with a reduced incidence of SSI-CRANs (AOR, 0.21; 95% CI, .12–.37; Table 5).
Multivariate Analysis of Factors Associated With Surgical Site Infection After a Craniotomy
| . | Before Propensity Score Matching . | . | . | . | After Propensity Score Matching . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | AOR . | 95% CI . | . | . | AOR . | 95% CI . | . | . |
| . | . | Lower . | Upper . | P Value . | . | Lower . | Upper . | P Value . |
| Care bundle implementation | 0.23 | .13 | .40 | <.001 | 0.21 | .12 | .37 | <.001 |
| Female | 0.90 | .59 | 1.36 | .605 | ||||
| Age | 1 | .98 | 1.02 | .645 | ||||
| Urgent surgery | 1 | .49 | 1.93 | .989 | ||||
| Charlson score | 0.96 | .85 | 1.08 | .547 | ||||
| American Society of Anesthesiologists classification | ||||||||
| 2 | 0.75 | .38 | 1.53 | .408 | ||||
| 3 | 1.17 | .56 | 2.51 | .687 | ||||
| 4/5 | 7.74 | .51 | 5.51 | .353 | ||||
| Surgical site | ||||||||
| Infratentorial | 1.74 | .90 | 3.22 | .085 | ||||
| Retromastoid | 1.13 | .57 | 2.11 | .717 | ||||
| Metal plates | 0.65 | .33 | 1.39 | .248 | ||||
| Intracranial pressure sensor | 0.88 | .32 | 2.13 | .783 | ||||
| Cerebrospinal fluid leak | 3.93 | 1.11 | 12.68 | .025 |
| . | Before Propensity Score Matching . | . | . | . | After Propensity Score Matching . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | AOR . | 95% CI . | . | . | AOR . | 95% CI . | . | . |
| . | . | Lower . | Upper . | P Value . | . | Lower . | Upper . | P Value . |
| Care bundle implementation | 0.23 | .13 | .40 | <.001 | 0.21 | .12 | .37 | <.001 |
| Female | 0.90 | .59 | 1.36 | .605 | ||||
| Age | 1 | .98 | 1.02 | .645 | ||||
| Urgent surgery | 1 | .49 | 1.93 | .989 | ||||
| Charlson score | 0.96 | .85 | 1.08 | .547 | ||||
| American Society of Anesthesiologists classification | ||||||||
| 2 | 0.75 | .38 | 1.53 | .408 | ||||
| 3 | 1.17 | .56 | 2.51 | .687 | ||||
| 4/5 | 7.74 | .51 | 5.51 | .353 | ||||
| Surgical site | ||||||||
| Infratentorial | 1.74 | .90 | 3.22 | .085 | ||||
| Retromastoid | 1.13 | .57 | 2.11 | .717 | ||||
| Metal plates | 0.65 | .33 | 1.39 | .248 | ||||
| Intracranial pressure sensor | 0.88 | .32 | 2.13 | .783 | ||||
| Cerebrospinal fluid leak | 3.93 | 1.11 | 12.68 | .025 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval. Significant variables are marked bold.
Multivariate Analysis of Factors Associated With Surgical Site Infection After a Craniotomy
| . | Before Propensity Score Matching . | . | . | . | After Propensity Score Matching . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | AOR . | 95% CI . | . | . | AOR . | 95% CI . | . | . |
| . | . | Lower . | Upper . | P Value . | . | Lower . | Upper . | P Value . |
| Care bundle implementation | 0.23 | .13 | .40 | <.001 | 0.21 | .12 | .37 | <.001 |
| Female | 0.90 | .59 | 1.36 | .605 | ||||
| Age | 1 | .98 | 1.02 | .645 | ||||
| Urgent surgery | 1 | .49 | 1.93 | .989 | ||||
| Charlson score | 0.96 | .85 | 1.08 | .547 | ||||
| American Society of Anesthesiologists classification | ||||||||
| 2 | 0.75 | .38 | 1.53 | .408 | ||||
| 3 | 1.17 | .56 | 2.51 | .687 | ||||
| 4/5 | 7.74 | .51 | 5.51 | .353 | ||||
| Surgical site | ||||||||
| Infratentorial | 1.74 | .90 | 3.22 | .085 | ||||
| Retromastoid | 1.13 | .57 | 2.11 | .717 | ||||
| Metal plates | 0.65 | .33 | 1.39 | .248 | ||||
| Intracranial pressure sensor | 0.88 | .32 | 2.13 | .783 | ||||
| Cerebrospinal fluid leak | 3.93 | 1.11 | 12.68 | .025 |
| . | Before Propensity Score Matching . | . | . | . | After Propensity Score Matching . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | AOR . | 95% CI . | . | . | AOR . | 95% CI . | . | . |
| . | . | Lower . | Upper . | P Value . | . | Lower . | Upper . | P Value . |
| Care bundle implementation | 0.23 | .13 | .40 | <.001 | 0.21 | .12 | .37 | <.001 |
| Female | 0.90 | .59 | 1.36 | .605 | ||||
| Age | 1 | .98 | 1.02 | .645 | ||||
| Urgent surgery | 1 | .49 | 1.93 | .989 | ||||
| Charlson score | 0.96 | .85 | 1.08 | .547 | ||||
| American Society of Anesthesiologists classification | ||||||||
| 2 | 0.75 | .38 | 1.53 | .408 | ||||
| 3 | 1.17 | .56 | 2.51 | .687 | ||||
| 4/5 | 7.74 | .51 | 5.51 | .353 | ||||
| Surgical site | ||||||||
| Infratentorial | 1.74 | .90 | 3.22 | .085 | ||||
| Retromastoid | 1.13 | .57 | 2.11 | .717 | ||||
| Metal plates | 0.65 | .33 | 1.39 | .248 | ||||
| Intracranial pressure sensor | 0.88 | .32 | 2.13 | .783 | ||||
| Cerebrospinal fluid leak | 3.93 | 1.11 | 12.68 | .025 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval. Significant variables are marked bold.
DISCUSSION
In this study, conducted over a long period of time and involving a large number of patients who were carefully followed up, we found that the implementation of a specific care bundle was effective in preventing SSI-CRANs.
Only a few studies have evaluated care bundles in craniotomies, with varying results [17–19]. These studies have some limitations that should be noted, with those demonstrating effectiveness having a small sample size [19], and those showing a lack of effectiveness only focusing on the intraoperative phase [17, 18]. Furthermore, these studies varied substantially in the inclusion criteria and in the patients’ baseline characteristics.
Regarding the measures implemented in the care bundle, bath or shower prior to surgery and adequate removal of hair by clipping are already recommended in clinical guidelines [6, 7, 13, 20, 21]. The main causative microorganisms of SSI-CRANs in the preintervention period were gram-positive cocci, which are common bacterial colonizers of the skin [10]. For this reason, even though the World Health Organization suggests a shower prior to surgery either with normal or antiseptic soap [14], we decided to use an antiseptic soap to eliminate a greater number of skin pathogens, both in the preintervention and intervention periods.
The use of intrawound vancomycin powder was a major change in our clinical practice. Several recent studies [22–29] have demonstrated the benefits of using vancomycin, suggesting that the use of topical vancomycin is safe and effective and does not lead to emerging resistance, being a cost-saving measure that can prevent SSI-CRANs. In our study, when we analyzed etiological changes between the 2 periods in depth, we observed a significant decrease in SSI-CRANs caused by gram-positive bacteria. Although this reduction could be attributed to the effects of intrawound vancomycin powder, randomized, controlled trials are needed to show the effectiveness of intrawound vancomycin powder in decreasing the incidence of SSI-CRANs. Regarding MSSA, SSI-CRANs were still observed in the intervention period. However, it should be noted that the relative increase from 20% to 33% is only apparent as a consequence of the significant reduction in other microorganisms. In fact, the incidence of MSSA infection was significantly reduced (from 3 to 1.2/100 interventions in the intervention period, P = .051).
In the postoperative phase, another crucial measure of the care bundle was surgical wound care. A high rate of SSI-CRANs caused by gram-negative bacilli, particularly Enterobacter cloacae, was observed in the preintervention period. We hypothesized that the type of surgical wound care performed in the preintervention period could have favored the proliferation of gram-negative bacilli because of the wet environment created in the incisional wound [30–32]. The use of a sterile absorbent dressing for the first 48 hours postsurgery and saline solution to wash the head during the intervention period ensured that the surgical wound was kept clean and wet, avoiding exogenous contamination and infection by gram-negative pathogens. Moreover, we assume that it is not only a temporal association since this microorganism is resistant to the antibiotics used in prophylaxis (both cefuroxime and vancomycin).
It has been stated that the effectiveness of a bundle is proportional to its compliance [33]. Despite the compliance rate in our study, the SSI-CRAN incidence was significantly reduced. Therefore, greater compliance with the implementation of the care bundle could achieve even greater reductions in the SSI-CRAN rate. However, we hypothesized that there was underreporting of the package measures. Surveillance in our study was based on review of electronic medical records, and compliance was based on professional records. Future work should be focused on how to improve adherence to these documents.
Importantly, constant feedback from the infection control team to front-line staff is essential to ensure the success of the care bundle. It should be noted that the more bundle measures that were applied, the lower the SSI-CRAN rates. For this reason, based on the result of this study and our experience, all measures need to be implemented for the care bundle to be effective. More studies are needed to assess the weight of each measure and determine if one of them led to a great impact in the prevention of SSI-CRAN.
Our study had some limitations that should be noted. First, it was carried out at a single center and, although the characteristics of the SSI-CRANs were similar to those observed in other hospitals, the frequency of infections and the impact of measures may vary between hospitals. Second, it had limitations that are inherent to studies that use nonrandomized samples. To overcome this, a rigorous statistical analysis was carried out in which propensity score matching was used to homogenize the sample and obtain the most reliable results. A well-designed randomized, controlled trial is needed to change guideline recommendations regarding this issue. Finally, the simultaneous application of a diverse number of measures made it difficult to identify the ones that were the most effective.
In summary, the care bundle intervention evaluated in our study was effective in reducing the incidence of SSI-CRANs. This multimodal preventive strategy, which addresses the main steps of the surgical process, should be considered for implementation in centers with high rates of SSI-CRANs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank CERCA Program/Generalitat de Catalunya for institutional support and IDIBELL.
Disclaimer. The study’s funders were not involved in the study design, data interpretation, analysis, or revision of the final manuscript. The corresponding author had full access to all the study data and had the final responsibility of submitting the manuscript for publication.
Financial support. This study was supported by the Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0005), Instituto de Salud Carlos III, the Spanish Ministry of Economy, Industry and Competitiveness, and the Research Committee of Bellvitge University Hospital.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
Author notes
E. J. M. and C. G. contributed equally to this work.




