-
PDF
- Split View
-
Views
-
Cite
Cite
Derek J Bays, George R Thompson, Susan Reef, Linda Snyder, Alana J Freifeld, Milt Huppert, David Salkin, Machelle D Wilson, John N Galgiani, Natural History of Disseminated Coccidioidomycosis: Examination of the Veterans Affairs–Armed Forces Database, Clinical Infectious Diseases, Volume 73, Issue 11, 1 December 2021, Pages e3814–e3819, https://doi.org/10.1093/cid/ciaa1154
Close - Share Icon Share
Abstract
The natural history of non–central nervous system (non-CNS) disseminated coccidioidomycosis (DCM) has not been previously characterized. The historical Veterans Affairs (VA)–Armed Forces coccidioidomycosis patient group provides a unique cohort of patients not treated with standard antifungal therapy, allowing for characterization of the natural history of coccidioidomycosis.
We conducted a retrospective study of 531 VA–Armed Forces coccidioidomycosis patients diagnosed between 1955–1958 and followed to 1966. Groups were identified as non-DCM (462 patients), DCM (44 patients), and CNS (25 patients). The duration of the initial infection, fate of the primary infection, all-cause mortality, and mortality secondary to coccidioidomycosis were assessed and compared between groups.
Mortality due to coccidioidomycosis at the last known follow-up was significantly different across the groups: 0.65% in the non-DCM group, 25% in the DCM group, and 88% in the CNS group (P < .001). The primary fate of pulmonary infection demonstrated key differences, with pulmonary nodules observed in 39.61% of the non-DCM group, 13.64% of the DCM group, and 20% of the CNS group (P < .001). There were differences in cavity formation, with 34.20% in the non-DCM group, 9.09% in the DCM group, and 8% in the CNS group (P < .001). Dissemination was the presenting manifestation or was concurrent with the initial infection in 41% and 56% of patients in the non-CNS DCM and CNS groups, respectively.
This large, retrospective cohort study helps characterize the natural history of DCM, provides insight into the host immunologic response, and has direct clinical implications for the management and follow-up of patients.
Coccidioidomycosis is an endemic, dimorphic fungal infection caused by Coccidioides immitis and Coccidioides posadasii. It is estimated to affect 150 000 individuals a year, with varying clinical manifestations, ranging from asymptomatic infection to pneumonia and/or fatal dissemination to the central nervous system (CNS) [1, 2]. The natural history of nondisseminated coccidioidomycosis was originally described in 1938, when it was shown that 92% of patients recovered without complication and only 1% had fatal disease [3]. Disseminated coccidioidomycosis (DCM) involving the CNS was described previously and found to be almost uniformly fatal [4–6]. Although there have been descriptions of the scope of non-CNS DCM [7], the manifestations and outcomes of a large cohort without the benefit of antifungal therapy have not been quantitatively described.
In the 1950s, the Veterans Affairs (VA)–Armed Forces Cooperative Study of coccidioidomycosis was created [4], which provided a cohort to describe the natural history of coccidioidal infections, given the absence of effective, conventional antifungal therapy. This unique group of patients did not receive conventional antifungals (amphotericin B and/or triazoles), providing a group to study the natural history of coccidioidomycosis and to provide insight into the timing of complications and disease manifestations. The patients in this database were assessed in this study to characterize DCM for the first time and describe the natural history of this morbid infection.
METHODS
Patients treated for coccidioidomycosis between 1 January 1955 and 30 December 1958 were identified at the Veterans Administration and military hospitals (12 from the VA, 2 from the Army, 1 from the Air Force, and 1 from the Navy) and facilities in the nonendemic area (50 VA patients from Washington to New York and from Louisiana to Montana) [4, 8]. If the diagnosis of coccidioidal infection had been established previously at another medical facility, outside records were obtained to corroborate the diagnosis. The diagnosis was made by histologic, serologic, or culture positivity. Once each infection was identified, its course was traced for as long a period as possible. All medical records were reviewed and classified by a pulmonary specialist (D. S.) and a mycologist (M. H.). Data were abstracted onto standardized collection forms, coded on computer cards, transferred onto electronic media, and subsequently transitioned to an online database (REDCap, Vanderbilt University, Nashville, TN) for further analysis.
Patients were defined as having non-DCM if evidence of disease could not be found outside of the chest [4]. CNS infection was defined as evidence of dissemination to the central nervous system/meninges, as previously described [4]. DCM was defined as coccidioidal infections outside of the lung, but without evidence of meningitis or CNS involvement. Patients with evidence of both CNS and DCM were placed in the CNS group. These definitions are consistent with the updated European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) definitions [9].
Variables abstracted from patient records included basic demographic information and laboratory data, mostly from initial presentation, including complement fixation (CF) serology, coccidioidin antigen skin test, and complete blood count data. Qualitative laboratory data for complete blood count data were made available and defined as normal, leukocytosis, leukocytosis and eosinophilia, eosinophilia without leukocytosis, and unknown. Symptom severity was assessed with the initial infection and defined as asymptomatic, mild, moderate, and severe during an original chart review. We also recorded the duration of initial infection, fate of primary infection, all-cause mortality, and mortality secondary to coccidioidomycosis. Chi-square tests, Wilcoxon rank sum tests, and log-rank tests with Kaplan-Meier curves were used to compare all-cause mortality and mortality secondary to coccidioidomycosis between groups. All statistical analyses were conducted using SAS software version 9.4 for Windows (SAS Institute Inc., Cary, NC).
RESULTS
There were 699 records available for review. Of these, 104 were excluded because of incomplete data. Of the 595 patients, 64 patients were excluded for receiving some form of amphotericin B deoxycholate (local/topical or intravenous), which may have altered the natural history of disease. Surgical management was rarely implemented (51 patients in the non-DCM group, 1 in the DCM group, and 0 in the CNS group). It was often used for diagnostic purposes or limited to pulmonary procedures and not felt to alter the natural history of disseminated disease. The remaining 531 cases were categorized as non-DCM (462 patients), DCM (44 patients), and CNS (25 patients) infections. The distribution of dissemination sites can be found in SupplementaryTables 1 and 2.
Results of Comparisons of Baseline Characteristics for a Sample of 531 Coccidioidomycosis Patients
| Variable . | . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Valuea . |
|---|---|---|---|---|---|
| Sex, n (%) | Male | 449 (97) | 44 (100) | 25 (100) | .37 |
| Female | 13 (3) | 0 | 0 | ||
| Ethnicity, n (%) | White | 374 (80.9) | 16 (36.4) | 13 (52) | <.0001 |
| Hispanic | 23 (4.9) | 1 (2.3) | 3 (12) | ||
| Black or African American | 47 (10.2) | 20 (45.5) | 8 (32) | ||
| Filipino | 12 (2.6) | 6 (13.6) | 1 (4) | ||
| Other | 6 (1.3) | 1 (2.3) | 0 | ||
| Comorbid conditions, n (%) | None | 404 (87.5) | 35 (79.6) | 20 (80) | .55 |
| Diabetes | 20 (4.3) | 3 (6.8) | 1 (4) | ||
| Peptic ulcer disease | 4 (.9) | 1 (2.3) | 1 (4) | ||
| Pulmonary disease | 83 (18) | 8 (18.2) | 4 (16) | ||
| Other | 34 (7.4) | 5 (11.4) | 3 (12) | ||
| Presenting symptoms, n (%) | None | 201 (43.5) | 10 (22.7) | 8 (32) | .005 |
| Mild | 27 (5.8) | 2 (4.6) | 1 (4) | ||
| Moderate | 76 (16.5) | 2 (4.6) | 4 (16) | ||
| Severe | 138 (29.9) | 27 (61.4) | 11 (44) | ||
| Unknown | 20 (4.3) | 3 (6.8) | 1 (4) |
| Variable . | . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Valuea . |
|---|---|---|---|---|---|
| Sex, n (%) | Male | 449 (97) | 44 (100) | 25 (100) | .37 |
| Female | 13 (3) | 0 | 0 | ||
| Ethnicity, n (%) | White | 374 (80.9) | 16 (36.4) | 13 (52) | <.0001 |
| Hispanic | 23 (4.9) | 1 (2.3) | 3 (12) | ||
| Black or African American | 47 (10.2) | 20 (45.5) | 8 (32) | ||
| Filipino | 12 (2.6) | 6 (13.6) | 1 (4) | ||
| Other | 6 (1.3) | 1 (2.3) | 0 | ||
| Comorbid conditions, n (%) | None | 404 (87.5) | 35 (79.6) | 20 (80) | .55 |
| Diabetes | 20 (4.3) | 3 (6.8) | 1 (4) | ||
| Peptic ulcer disease | 4 (.9) | 1 (2.3) | 1 (4) | ||
| Pulmonary disease | 83 (18) | 8 (18.2) | 4 (16) | ||
| Other | 34 (7.4) | 5 (11.4) | 3 (12) | ||
| Presenting symptoms, n (%) | None | 201 (43.5) | 10 (22.7) | 8 (32) | .005 |
| Mild | 27 (5.8) | 2 (4.6) | 1 (4) | ||
| Moderate | 76 (16.5) | 2 (4.6) | 4 (16) | ||
| Severe | 138 (29.9) | 27 (61.4) | 11 (44) | ||
| Unknown | 20 (4.3) | 3 (6.8) | 1 (4) |
Abbreviations: CNS, central nervous system; DCM, disseminated coccidioidomycosis.
aChi square tests.
Results of Comparisons of Baseline Characteristics for a Sample of 531 Coccidioidomycosis Patients
| Variable . | . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Valuea . |
|---|---|---|---|---|---|
| Sex, n (%) | Male | 449 (97) | 44 (100) | 25 (100) | .37 |
| Female | 13 (3) | 0 | 0 | ||
| Ethnicity, n (%) | White | 374 (80.9) | 16 (36.4) | 13 (52) | <.0001 |
| Hispanic | 23 (4.9) | 1 (2.3) | 3 (12) | ||
| Black or African American | 47 (10.2) | 20 (45.5) | 8 (32) | ||
| Filipino | 12 (2.6) | 6 (13.6) | 1 (4) | ||
| Other | 6 (1.3) | 1 (2.3) | 0 | ||
| Comorbid conditions, n (%) | None | 404 (87.5) | 35 (79.6) | 20 (80) | .55 |
| Diabetes | 20 (4.3) | 3 (6.8) | 1 (4) | ||
| Peptic ulcer disease | 4 (.9) | 1 (2.3) | 1 (4) | ||
| Pulmonary disease | 83 (18) | 8 (18.2) | 4 (16) | ||
| Other | 34 (7.4) | 5 (11.4) | 3 (12) | ||
| Presenting symptoms, n (%) | None | 201 (43.5) | 10 (22.7) | 8 (32) | .005 |
| Mild | 27 (5.8) | 2 (4.6) | 1 (4) | ||
| Moderate | 76 (16.5) | 2 (4.6) | 4 (16) | ||
| Severe | 138 (29.9) | 27 (61.4) | 11 (44) | ||
| Unknown | 20 (4.3) | 3 (6.8) | 1 (4) |
| Variable . | . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Valuea . |
|---|---|---|---|---|---|
| Sex, n (%) | Male | 449 (97) | 44 (100) | 25 (100) | .37 |
| Female | 13 (3) | 0 | 0 | ||
| Ethnicity, n (%) | White | 374 (80.9) | 16 (36.4) | 13 (52) | <.0001 |
| Hispanic | 23 (4.9) | 1 (2.3) | 3 (12) | ||
| Black or African American | 47 (10.2) | 20 (45.5) | 8 (32) | ||
| Filipino | 12 (2.6) | 6 (13.6) | 1 (4) | ||
| Other | 6 (1.3) | 1 (2.3) | 0 | ||
| Comorbid conditions, n (%) | None | 404 (87.5) | 35 (79.6) | 20 (80) | .55 |
| Diabetes | 20 (4.3) | 3 (6.8) | 1 (4) | ||
| Peptic ulcer disease | 4 (.9) | 1 (2.3) | 1 (4) | ||
| Pulmonary disease | 83 (18) | 8 (18.2) | 4 (16) | ||
| Other | 34 (7.4) | 5 (11.4) | 3 (12) | ||
| Presenting symptoms, n (%) | None | 201 (43.5) | 10 (22.7) | 8 (32) | .005 |
| Mild | 27 (5.8) | 2 (4.6) | 1 (4) | ||
| Moderate | 76 (16.5) | 2 (4.6) | 4 (16) | ||
| Severe | 138 (29.9) | 27 (61.4) | 11 (44) | ||
| Unknown | 20 (4.3) | 3 (6.8) | 1 (4) |
Abbreviations: CNS, central nervous system; DCM, disseminated coccidioidomycosis.
aChi square tests.
| Primary Fate of Infection . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Valuea . |
|---|---|---|---|---|
| Resolution, n (%) | 75 (16.2) | 13 (29.6) | 3 (12) | <.0001 |
| Chronic nodule, n (%) | 183 (39.6) | 6 (13.6) | 5 (20) | |
| Chronic cavity, n (%) | 158 (34.2) | 4 (9.1) | 2 (8) | |
| Dissemination, n (%) | 0 | 18 (40.9) | 14 (56) | |
| Other, n (%) | 8 (4.8) | 3 (6.8) | 1 (4) | |
| Unknown, n (%) | 24 (5.2) | 0 | 0 |
| Primary Fate of Infection . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Valuea . |
|---|---|---|---|---|
| Resolution, n (%) | 75 (16.2) | 13 (29.6) | 3 (12) | <.0001 |
| Chronic nodule, n (%) | 183 (39.6) | 6 (13.6) | 5 (20) | |
| Chronic cavity, n (%) | 158 (34.2) | 4 (9.1) | 2 (8) | |
| Dissemination, n (%) | 0 | 18 (40.9) | 14 (56) | |
| Other, n (%) | 8 (4.8) | 3 (6.8) | 1 (4) | |
| Unknown, n (%) | 24 (5.2) | 0 | 0 |
Abbreviations: CNS, central nervous system; DCM, disseminated coccidioidomycosis.
aChi square tests.
| Primary Fate of Infection . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Valuea . |
|---|---|---|---|---|
| Resolution, n (%) | 75 (16.2) | 13 (29.6) | 3 (12) | <.0001 |
| Chronic nodule, n (%) | 183 (39.6) | 6 (13.6) | 5 (20) | |
| Chronic cavity, n (%) | 158 (34.2) | 4 (9.1) | 2 (8) | |
| Dissemination, n (%) | 0 | 18 (40.9) | 14 (56) | |
| Other, n (%) | 8 (4.8) | 3 (6.8) | 1 (4) | |
| Unknown, n (%) | 24 (5.2) | 0 | 0 |
| Primary Fate of Infection . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Valuea . |
|---|---|---|---|---|
| Resolution, n (%) | 75 (16.2) | 13 (29.6) | 3 (12) | <.0001 |
| Chronic nodule, n (%) | 183 (39.6) | 6 (13.6) | 5 (20) | |
| Chronic cavity, n (%) | 158 (34.2) | 4 (9.1) | 2 (8) | |
| Dissemination, n (%) | 0 | 18 (40.9) | 14 (56) | |
| Other, n (%) | 8 (4.8) | 3 (6.8) | 1 (4) | |
| Unknown, n (%) | 24 (5.2) | 0 | 0 |
Abbreviations: CNS, central nervous system; DCM, disseminated coccidioidomycosis.
aChi square tests.
Demographics
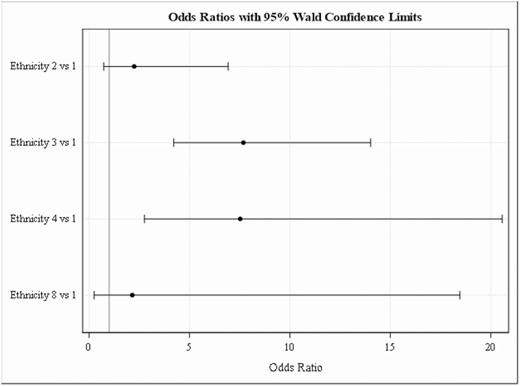
The median presenting age for the non-DCM patients was 33 years (interquartile range [IQR], 25–42), the DCM median age was 37 years (IQR, 30–48.5), and the CNS median age was 38 years (IQR, 31–47). The majority of the patients were male (Table 1)—97% in the non-DCM, 100% in the DCM, and 100% in the CNS groups (P = .37)—consistent with a military/VA database from this era. Black/African American patients were more likely to have disseminated disease (odds ratio, 7.7; 95% confidence interval, 4.2–14.1), as were those of Filipino ethnicity (odds ratio, 7.5; 95% confidence interval, 2.8–20.6; Figure 1). DCM and CNS patients were combined for an ethnicity analysis of dissemination risk, due to the small sample size. There were not statistically significant differences in Caucasian, Hispanic, and Other ethnicities (Figure 1). The majority of patients did not have comorbid medical conditions or pulmonary conditions. Diabetes was the most common preexisting condition, and there were no differences between disease categories (P = .55; Table 1).
Initial Infection
Significant differences were observed among presenting symptoms across different patient groups (P = .0045). There were more patients with no symptoms with initial infections in the non-DCM group (44%), compared to the DCM (23%) and CNS (32%) groups (Table 1). In addition, only 30% of the patients in the non-DCM group presented with severe symptoms, compared to 61% and 44% in the DCM and CNS groups, respectively (Table 1).
The pulmonary outcomes of the primary infection were statistically different across the groups (P < .0001; Table 2). There were increases in the formation of pulmonary nodules and cavities in the non-DCM group (40% and 34%, respectively), compared to DCM (14% and 9%, respectively) and CNS (20% and 8%, respectively) groups, suggesting differences in the host immune response at the time of initial exposure to Coccidioides arthroconidia.
Dissemination occurred coincident with the diagnosis of primary infection in 41% of the DCM and 56% of the CNS groups (Table 2). In general, dissemination occurred early during the course of disease, with 61% of patients disseminating within the first 2 months of infection in the DCM and 48% in the CNS groups (Table 3). The majority of patients disseminated within the first 2 months of illness onset, with 72% of those in the DCM group, and 48% in the CNS group presenting within the first 6 months of the initial coccidioidal diagnosis.
| Duration of disease at time of dissemination in months . | Non-CNS DCM, n = 44, n (%) . | CNS DCM, n = 25, n (%) . | P Valuea . |
|---|---|---|---|
| 0–2 | 27 (61.4) | 12 (48) | .38 |
| 3–5 | 5 (11.4) | 4 (16) | |
| >6 | 2 (4.6) | 3 (12) | |
| Unknown | 10 (22.7) | 6 (24) |
| Duration of disease at time of dissemination in months . | Non-CNS DCM, n = 44, n (%) . | CNS DCM, n = 25, n (%) . | P Valuea . |
|---|---|---|---|
| 0–2 | 27 (61.4) | 12 (48) | .38 |
| 3–5 | 5 (11.4) | 4 (16) | |
| >6 | 2 (4.6) | 3 (12) | |
| Unknown | 10 (22.7) | 6 (24) |
Abbreviations: CNS, central nervous system; DCM, disseminated coccidioidomycosis.
aChi square test.
| Duration of disease at time of dissemination in months . | Non-CNS DCM, n = 44, n (%) . | CNS DCM, n = 25, n (%) . | P Valuea . |
|---|---|---|---|
| 0–2 | 27 (61.4) | 12 (48) | .38 |
| 3–5 | 5 (11.4) | 4 (16) | |
| >6 | 2 (4.6) | 3 (12) | |
| Unknown | 10 (22.7) | 6 (24) |
| Duration of disease at time of dissemination in months . | Non-CNS DCM, n = 44, n (%) . | CNS DCM, n = 25, n (%) . | P Valuea . |
|---|---|---|---|
| 0–2 | 27 (61.4) | 12 (48) | .38 |
| 3–5 | 5 (11.4) | 4 (16) | |
| >6 | 2 (4.6) | 3 (12) | |
| Unknown | 10 (22.7) | 6 (24) |
Abbreviations: CNS, central nervous system; DCM, disseminated coccidioidomycosis.
aChi square test.
Laboratory Data
There were no statistically significant differences between groups in regard to coccidioidin antigen skin testing (P = .20; Table 4). There were significantly higher CF serology median titers between groups: negative in the non-DCM, 1:128 in the DCM, and 1:64 in the CNS groups (P < .0001; Table 4). There were higher percentages of leukocytosis with eosinophilia in the DCM and CNS groups (18.2% and 24%, respectively), compared to 9.7% in the non-DCM group (Table 4). However, there were no significant differences in the percentages of eosinophilia without leukocytosis between patient types: 6.93% in the non-DCM, 6.82% in the DCM, and 8% in the CNS groups (Table 4).
| Variable . | . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Value . |
|---|---|---|---|---|---|
| White blood cell count, n (%) | Normal | 242 (52.4) | 14 (31.8) | 7 (28) | .008 |
| Leukocytosis | 30 (6.5) | 6 (13.6) | 5 (20) | ||
| Leukocytosis and eosinophilia | 45 (9.7) | 8 (18.2) | 6 (24) | ||
| Eosinophilia alone | 32 (6.9) | 3 (6.8) | 2 (8) | ||
| Unknown | 113 (23.5) | 13 (29.6) | 20 (5) | ||
| Skin test, n (%) | Positive | 314 (68) | 36 (81.8) | 13 (52) | .20 |
| Negative | 80 (17.3) | 2 (4.6) | 6 (24) | ||
| Unknown | 49 (10.6) | 4 (9.1) | 4 (16) | ||
| Not performed | 19 (4.11) | 2 (4.6) | 2 (8) | ||
| Complement fixation serology, median (interquartile range) | Negative (negative to 1:8) | 1:128 (1:8 to >1:256) | 1:64 (1:4 to >1:256) | <.0001 |
| Variable . | . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Value . |
|---|---|---|---|---|---|
| White blood cell count, n (%) | Normal | 242 (52.4) | 14 (31.8) | 7 (28) | .008 |
| Leukocytosis | 30 (6.5) | 6 (13.6) | 5 (20) | ||
| Leukocytosis and eosinophilia | 45 (9.7) | 8 (18.2) | 6 (24) | ||
| Eosinophilia alone | 32 (6.9) | 3 (6.8) | 2 (8) | ||
| Unknown | 113 (23.5) | 13 (29.6) | 20 (5) | ||
| Skin test, n (%) | Positive | 314 (68) | 36 (81.8) | 13 (52) | .20 |
| Negative | 80 (17.3) | 2 (4.6) | 6 (24) | ||
| Unknown | 49 (10.6) | 4 (9.1) | 4 (16) | ||
| Not performed | 19 (4.11) | 2 (4.6) | 2 (8) | ||
| Complement fixation serology, median (interquartile range) | Negative (negative to 1:8) | 1:128 (1:8 to >1:256) | 1:64 (1:4 to >1:256) | <.0001 |
Abbreviations: CNS, central nervous system; DCM, disseminated coccidioidomycosis.
| Variable . | . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Value . |
|---|---|---|---|---|---|
| White blood cell count, n (%) | Normal | 242 (52.4) | 14 (31.8) | 7 (28) | .008 |
| Leukocytosis | 30 (6.5) | 6 (13.6) | 5 (20) | ||
| Leukocytosis and eosinophilia | 45 (9.7) | 8 (18.2) | 6 (24) | ||
| Eosinophilia alone | 32 (6.9) | 3 (6.8) | 2 (8) | ||
| Unknown | 113 (23.5) | 13 (29.6) | 20 (5) | ||
| Skin test, n (%) | Positive | 314 (68) | 36 (81.8) | 13 (52) | .20 |
| Negative | 80 (17.3) | 2 (4.6) | 6 (24) | ||
| Unknown | 49 (10.6) | 4 (9.1) | 4 (16) | ||
| Not performed | 19 (4.11) | 2 (4.6) | 2 (8) | ||
| Complement fixation serology, median (interquartile range) | Negative (negative to 1:8) | 1:128 (1:8 to >1:256) | 1:64 (1:4 to >1:256) | <.0001 |
| Variable . | . | Non-DCM . | Non-CNS DCM . | CNS DCM . | P Value . |
|---|---|---|---|---|---|
| White blood cell count, n (%) | Normal | 242 (52.4) | 14 (31.8) | 7 (28) | .008 |
| Leukocytosis | 30 (6.5) | 6 (13.6) | 5 (20) | ||
| Leukocytosis and eosinophilia | 45 (9.7) | 8 (18.2) | 6 (24) | ||
| Eosinophilia alone | 32 (6.9) | 3 (6.8) | 2 (8) | ||
| Unknown | 113 (23.5) | 13 (29.6) | 20 (5) | ||
| Skin test, n (%) | Positive | 314 (68) | 36 (81.8) | 13 (52) | .20 |
| Negative | 80 (17.3) | 2 (4.6) | 6 (24) | ||
| Unknown | 49 (10.6) | 4 (9.1) | 4 (16) | ||
| Not performed | 19 (4.11) | 2 (4.6) | 2 (8) | ||
| Complement fixation serology, median (interquartile range) | Negative (negative to 1:8) | 1:128 (1:8 to >1:256) | 1:64 (1:4 to >1:256) | <.0001 |
Abbreviations: CNS, central nervous system; DCM, disseminated coccidioidomycosis.
Mortality
All-cause mortality was 5.41% in patients within the non-DCM group, 29.55% in patients with DCM, and 96% in the CNS patients over up to 30 years of follow-up (P < .0001; Figure 2). As expected, the death rate for CNS-infected patients was much higher than for non-DCM and DCM patients. In non-DCM patients, ~1% (3/459) died due to coccidioidomycosis over the 30-year follow-up period. Of these 3 patients, 2 had cavitary lung disease, and the third had a serology greater than 1:256, suggesting the possibility of undiagnosed disseminated disease. The rates increased to 25% for DCM patients and 88% for CNS-infected patients (P < .001; Figure 3).
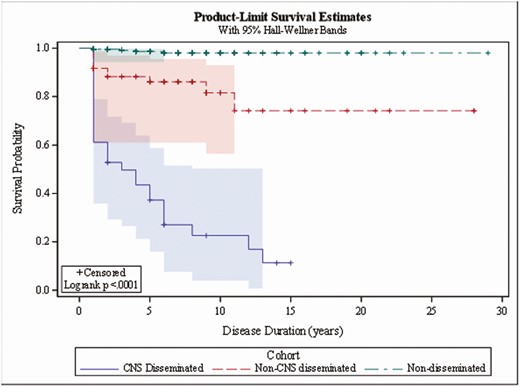
Coccidioidomycosis-associated mortality. Abbreviation: CNS, central nervous system.
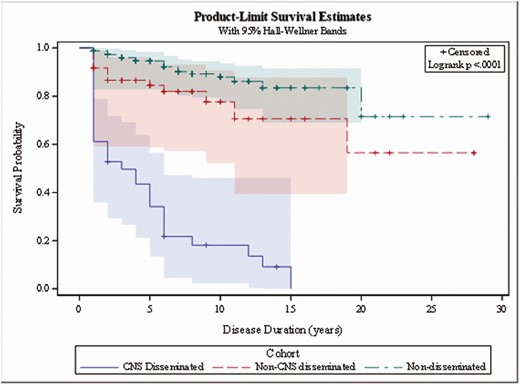
All-cause mortality. Abbreviation: CNS, central nervous system.
DISCUSSION
The VA–Armed Forces Database provides the largest coccidioidomycosis patient data repository from the pre-antifungal era. As amphotericin B deoxycholate was not approved by the Food and Drug Administration until 1966 [10], the patients included in this cohort study were not treated with conventional antifungal regimens, providing an opportunity to study the natural history of non-CNS disseminated coccidioidomycosis. Given that the study follow-up period extended until 1966, patients that were given some form of local, intravenous, systemic amphotericin B were excluded in an attempt to reduce potential confounding effects on the natural history. Beyond the size of the cohort, there was considerable follow-up time, with patients followed for over 10 years in many cases, increasing the robustness of the data set.
It has previously been reported that dissemination occurs with the initial infection, with a prior report demonstrating that 83% of dissemination occurs within the first 2 months, and the longest interval between initial infection and dissemination was 4 months [11]. However, this previous study only looked at 12 cases of dissemination, and has led to some to believe that all dissemination occurs early or with an initial infection. While our study showed that the majority of disseminated cases do in fact occur within the first 2 months, we did observe dissemination to the CNS and other sites outside of this acute early period. CNS (64%) and non-CNS (72.8%) cases exhibited dissemination within 6 months of symptom onset. When all cases reviewed were included for analysis (including those receiving topical or oral amphotericin B therapy, which is of uncertain efficacy), 22% of CNS cases and 10% of DCM cases occurred 1 year after symptom onset, suggesting that the follow-up of patients is needed for 1–2 years after initial infection. In conjunction with prior studies demonstrating that antifungal therapy does not prevent dissemination, we suggest following all patients for 1–2 years after infection to ensure dissemination does not occur [12, 13].
While the VA–Armed Forces Database is a mostly Caucasian population, ethnicity differences in dissemination rates were found. Similar to prior studies [11], we saw increased rates of dissemination in Black/African-American and Filipino patients to both the CNS and non-CNS sites (Figure 3). In addition, we saw a trend towards increased disseminated disease in the Hispanic population, but it did not reach statistical significance. Close to one-third of Black/African-American and Filipino patients in this group had evidence of dissemination. Since the large majority of this cohort were men, which has also been identified as a risk factor for DCM [14, 15], gender may also be partially responsible for the ethnic associations with DCM. Despite evidence demonstrating differences in dissemination by ethnicity, the host genomic factors responsible remain poorly understood [14]. Further investigation is required, as elucidation of the genetic differences responsible will advance our understanding of the immunopathology of the disease, and may allow for genetic screening of patients at the time of diagnosis for prognostic purposes or for counseling prior to travel to endemic regions.
The VA–Armed Forces Database is composed primarily of previously healthy patients, and thus the lack of differences in dissemination related to comorbid conditions is not surprising. As such, this study was underpowered to examine the additive or synergistic effects of comorbid conditions. This is likely confounded by the lack of immunocompromising conditions and underlying diabetes in our historical cohort, compared to more recent studies exploring genetic and environmental factors and their potential contributions to disseminated infections [15–17].
Laboratory findings demonstrated significantly higher CF titers in the DCM and the CNS groups as compared to the non-DCM groups, as expected (Table 4) [18]. However, the difference between DCM and CNS CF serology is not clinically significant. Eosinophilia has been associated with disseminated coccidioidomycosis previously [19], and the VA–Armed Forces Database has increased rates of leukocytosis both with and without eosinophilia in the DCM and CNS infection groups. However, the database is limited by not having the eosinophil percentage to correlate and expand on the role or prognostic value of eosinophilia as a risk factor or proxy of dissemination, nor of the burden of disease. While there were not statistically significant differences in the coccidioidin antigen skin testing amongst the groups, there were trends towards an increased number of patients with negative skin tests in the CNS infections, compared to those in the DCM group. This could suggest that the immune response, or lack thereof, that predisposes patients to CNS dissemination may be different than DCM.
We found large differences in the development of pulmonary nodules (large granulomas resulting from prior coccidioidal pulmonary infection) or cavities after infection in patients with different forms of the disease. Of the 194 patients that developed pulmonary nodules in our group, only 3.1% were in the DCM patients and 2.6% in the CNS patients, compared to 94.3% in nondisseminated patients. Similarly, of the 158 patients with cavities, 2.4% were in the DCM patients, 1.2% in the CNS group, and 96.3% in the nondisseminated patients. These findings suggest that an inflammatory process leading to the development of pulmonary nodules and cavities protects against dissemination, and that dissemination may be determined early in the course of disease by the lung-Coccidioides interface. It should be noted that these nodules were seen in an era prior to computed tomographic imaging, and small nodules may have been missed. Similarly, a trend of decreased responses to the coccidioidin skin test in the CNS group (Table 4) implies decreased activation of the cellular immune system [14], which suggests activation of the cellular immune system as part of an early protective process.
There were stark differences in mortality between groups. As seen previously [4], CNS infection was nearly fatal in all patients, with a mortality rate of 88% of those whose deaths were deemed secondary to CNS disease and a 96% all-cause mortality rate at the last known follow-up. Mortality due to CNS infections would have been even higher if we had excluded 4 patients whose meningitis was diagnosed prior to the initiation of the registry [4]. However, DCM had a coccidioidomycosis-associated mortality rate of 25% and a 29.55% all-cause mortality rate at the last known follow-up. Lastly, the mortality rate of non-DCM was only 0.65%, secondary to coccidioidomycosis, and exhibited a 5.4% all-cause mortality rate. For patients with DCM, this suggests a more favorable outcome, compared to those with CNS infection. Prior studies solely evaluating isolated CNS disease have shown 100% mortality in the absence of antifungal therapy, leading to the practice of lifelong treatment [20]. The natural history of DCM has not been described, and clinical practices vary regarding the duration of therapy.
The VA–Armed Forces Database provides a unique, large, and robust data set to expand the understanding of disseminated coccidioidomycosis. While there are limitations stemming from the nonrepresentative population, this study still unveils many important characteristics. Clarifying the timing of dissemination, with the majority occurring early during infection but with dissemination occurring to both the CNS and non-CNS after 1 year of infection, demonstrates the importance of follow-ups in all patients with coccidioidomycosis. The study also highlights the apparent protective inflammatory response against dissemination in patients that develop a chronic pulmonary nodule or cavity, which warrants further study to better determine this protective mechanism. The drastic differences in mortality across the groups show that DCM is not nearly as rapidly fatal as CNS infection, despite sharing many characteristics.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. M. H. and D. S. are deceased.
Financial support. This work was supported by existing department funds from the authors’ institutions.
Potential conflicts of interest. J. N. G. is Chairman of the Board and a significant stock holder of Valley Fever Solutions, a company that is developing nikkomycin Z as a treatment for coccidioidomycosis. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Forty-First Annual Meeting of the Coccidioidomycosis Study Group, San Diego, California, 5 April 1997.
54th Annual Infectious Diseases Society of America Meeting, San Diego, California, 5 October 2017.
Presented in part: Fifth International Conference on Coccidioidomycosis, Stanford, California, 24–27 August 1994.




