-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Joshua Hendrix, Lindsey Larson, Adriana M Rauseo, Sasinuch Rutjanawech, Alexander D Franklin, William G Powderly, Andrej Spec, Voriconazole Versus Itraconazole for the Initial and Step-down Treatment of Histoplasmosis: A Retrospective Cohort, Clinical Infectious Diseases, Volume 73, Issue 11, 1 December 2021, Pages e3727–e3732, https://doi.org/10.1093/cid/ciaa1555
Close - Share Icon Share
Abstract
Itraconazole is the preferred azole for histoplasmosis in the current Infectious Diseases Society of America guidelines. Voriconazole is increasingly used as treatment for histoplasmosis; it has in vitro activity against Histoplasma capsulatum and has shown success in case reports and small case series, but may have a lower barrier to resistance. No comparative studies have been published.
We constructed a single-center, retrospective cohort of adult patients diagnosed with histoplasmosis from 2002 to 2017. Individual charts were reviewed to gather clinical information, including demographics, clinical features, immune status, treatments, and mortality. Patients were categorized based on the choice of azole and use as an initial treatment or as a step-down therapy from amphotericin B. Initial therapies with other azoles were excluded. Mortality was compared using a multivariable Cox proportional hazards with Heaviside function at 42 days.
We identified 261 cases of histoplasmosis from 2002 to 2017. After excluding patients not treated with itraconazole or voriconazole, 194 patients remained. Of these, 175 (90%) patients received itraconazole and 19 (10%) received voriconazole. There were no significant demographic differences between patient populations receiving either azole as their initial azole treatment. Death at 180 days occurred in 41 patients (23.4%) in the itraconazole group and 6 patients (31.6%) in the voriconazole group. Patients on voriconazole had a statistically significant increase in mortality during the first 42 days after initiation of treatment when compared to patients receiving itraconazole (hazard ratio, 4.30; 95% confidence interval, 1.3–13.9; P = .015), when controlled for other risk factors.
Voriconazole in histoplasmosis was associated with increased mortality in the first 42 days when compared to itraconazole.
Azoles are the treatment of choice for histoplasmosis in the management of mild disease or as stepdown therapy from amphotericin B in severe or disseminated disease [1, 2]. Itraconazole is the Infectious Diseases Society of America guideline recommended choice for azole therapy based on prospective data [3], as well as significantly longer clinical experience. Data for other azoles are limited, and as a result the guidelines are cautious to recommend those azoles as first-line therapy [1]. Voriconazole has been shown to be better tolerated when compared to itraconazole, especially with long-term use [4]. Additionally, voriconazole has in vitro activity against Histoplasma capsulatum with minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) within the achievable range [5, 6]. The use of voriconazole for histoplasmosis has become increasingly common, although published data are limited to case reports [7–10] and a single retrospective case series of heterogenous groups of patients [11]. None of these studies compared differences between voriconazole and itraconazole, and they were not designed to assess the outcome differences between treatments.
Treatment of histoplasmosis with fluconazole (an azole that is structurally more similar to voriconazole than itraconazole) is associated with slower culture clearance and a higher rate of relapse [12, 13]. This is thought to be related to the emergence of resistance through a single-point mutation for the cytochrome P450 enzyme 14α-demethylase (CYP51p). This effect results in increased MICs to both fluconazole and voriconazole [14]. It is not known whether similar outcomes might be associated with voriconazole use, as this possible clinical effect has never been evaluated [2]. These same isolates did not have elevated MICs to itraconazole, posaconazole, or isavuconazole [13].
Our study aims to evaluate the mortality associated with voriconazole in the treatment of histoplasmosis, with a specific focus on mortality early in the treatment course, when compared to the standard of care with itraconazole.
METHODS
Setting
Data were collected from all patients diagnosed with an H. capsulatum infection at Barnes-Jewish Hospital in Saint Louis, Missouri, from 1 January 2002 until 31 December 2017. Barnes-Jewish Hospital is a 1368-bed tertiary care academic hospital located in an urban environment with a significant suburban and rural referral base. The study was approved by the Washington University School of Medicine Human Research Protection Office, with a waiver of informed consent.
Cohort Construction
Patients 18 years or older were included if they had 1 of the following: (1) a positive Histoplasma antigen from urine, serum, or other body fluid; (2) microbiologic isolation of H. capsulatum from any source; (3) coding for International Classification of Diseases Ninth (115.X) or Tenth Edition (B39.X) codes for Histoplasma infection; or (4) a positive Histoplasma antibody. All cases were then confirmed by 2 independent investigators to meet the criteria for proven or probable histoplasmosis by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria [15]. Cases were excluded if: (1) antigen or antibody testing was positive in the setting of another fungal infection, confirmed by culture or histopathology, that was thought to be the more likely cause; (2) the antigen was positive at a low or indeterminate level and the case was determined to be a false positive by the treating infectious diseases team at that time and/or by 2 investigators during review; (3) there was a diagnosis of presumed ocular histoplasmosis syndrome without other evidence of Histoplasma infection; (4) the patient presented with fibrosing mediastinitis, as this was thought to be sequelae of a past infection, rather than an ongoing infection needing antifungal treatment; and (5) the patient received fluconazole, posaconazole, or isavuconazole as the first azole treatment or if the patient never received azole treatment of any kind.
Patients were categorized into 2 groups based on the initial azole prescribed for treatment of histoplasmosis: itraconazole or voriconazole. Patients who were started on amphotericin B prior to starting an azole were categorized based on the first azole given as a step down from amphotericin B.
Definitions
The start of therapy was defined as the first day itraconazole or voriconazole was administered. Disseminated infection was defined as a Histoplasma infection involving extra-pulmonary sites, including blood, bone marrow, skin, liver, adrenal glands, gallbladder, extra-pulmonary lymph nodes, or the central nervous system.
Positive antibody testing was defined as a complement fixation titer of 1:32 or greater, detection of both H and M bands on immunodiffusion, or a positive M band on immunodiffusion following a documented negative M band on a previous test (seroconversion).
Data were collected through automated chart extraction and manual chart review by the authors. We collected patient characteristics, including demographics, antifungal therapies, clinical presentation, extent of infection, predisposing factors, and immunocompromised status, as well as admission status and need for intensive care.
Statistical Analysis
The statistical analysis was performed with IBM SPSS V24 (IBM, Armonk, NY). For descriptive statistics, we used a chi-square analysis for categorical variables and Mann Whitney-U tests to analyze continuous variables, due to the small cohort size and lack of normal distribution. We performed a series of univariate logistic regressions to evaluate the association of demographic factors and comorbidities with mortality in the cohort. A multivariable Cox proportional hazards model was used to further compare the mortality of the 2 treatment groups. Variables with P values less than .2 were initially included in the multivariable Cox proportional hazards model. Variables were retained based on quality of fit and clinical plausibility. Variables that violated the assumption of proportional hazards were modeled with a Heaviside function; 42 days was chosen as the point of discontinuity because of the significant difference in clearance of fungemia that is seen in the first 4–6 weeks between itraconazole and fluconazole in published trials [16]. All time-to-event analyses used the first day of azole administration as Day 0.
RESULTS
Cohort
A total of 512 patients were evaluated between 1 January 2002 and 31 December 2017. We excluded 251 patients (49.0%) from the analysis after chart review, and another 67 patients were excluded for receiving either no azole or an initial azole therapy other than itraconazole or voriconazole (Figure 1).
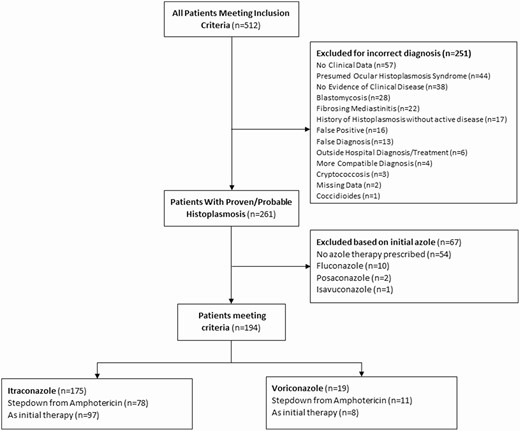
Comparison of patients treated with voriconazole and itraconazole in the management of histoplasmosis.
There were 194 patients included in the primary analysis: 175 (90.2%) patients initially treated with itraconazole and 19 (9.8%) initially treated with voriconazole.
Demographics and Presentation
Of the 194 patients included in the analysis, the median age was 48 years (interquartile range, 35–61), 112 (57.7%) were male, and 145 (74.7%) were White. The underlying conditions were diverse, with 79 (40.7%) immunocompetent patients, 29 (14.9%) with a malignancy-related diagnosis, 21 (10.8%) transplant recipients, 48 (24.7%) people living with human immunodeficiency virus (PLWH), 13 (6.7%) receiving concurrent chemotherapy, 48 (24.7%) taking prednisone, 28 (14.4%) on a biologic agent, and 40 (20.6%) taking a nonsteroidal immunosuppressive agent. These conditions did not differ amongst the 2 groups (all P values > .05; Table 1).
Comparison of Baseline Characteristics and Presentation of 194 Patients With Histoplasmosis Based on Initial Azole Therapy, 2002–2017
| . | Itraconazole, n = 175, 90.2% . | Voriconazole, n = 19, 9.8% . | Total, n = 194, 100% . | OR (95% CI) . | P value . |
|---|---|---|---|---|---|
| Race | .30 | ||||
| White | 131 (74.9%) | 14 (73.7%) | 145 (74.7%) | … | |
| African American | 35 (20.0%) | 3 (15.8%) | 38 (19.6%) | ||
| Asian/Pacific Islander | 1 (.6%) | 1 (5.3%) | 2 (1.0%) | ||
| Hispanic | 4 (2.3%) | 0 (0%) | 4 (2.1%) | ||
| Other | 4 (2.3%) | 1 (5.3%) | 5 (2.6%) | ||
| Age, median (IQR) | 47 (34.5,60.6) | 53.7 (36.1, 53.7) | 48.2 (34.5, 60.6) | .53 | |
| Age ≥ 50 | 78 (44.6%) | 10 (52.6%) | 88 (45.4%) | 1.4 (.53–3.5) | .50 |
| Male | 101 (57.7%) | 11 (57.9%) | 112 (57.7%) | 1.0 (.39–2.6) | .99 |
| Presentation | |||||
| Disseminated | 93 (53.1%) | 13 (68.4%) | 106 (54.6%) | 1.2 (.47–3.2) | .69 |
| Constitutional symptoms | 117 (66.9%) | 16 (84.2%) | 133 (68.6%) | 2.6 (.74–9.4) | .13 |
| Pulmonary symptoms | 126 (72.0%) | 11 (57.9%) | 137 (70.6%) | .53 (.20–1.4) | .21 |
| GI symptoms | 71 (40.6%) | 10 (52.6%) | 81 (41.8%) | 1.6 (.63–4.2) | .31 |
| Skin lesions | 22 (12.6%) | 4 (21.1%) | 26 (13.4%) | 1.9 (.56–6.1) | .31 |
| CNS disease | 6 (3.4%) | 1 (5.3%) | 7 (3.6%) | 1.6 (.18–13.7) | .69 |
| Amphotericin induction | 78 (44.6%) | 11 (57.9%) | 89 (45.9%) | 1.7 (.66–4.5) | .27 |
| Amphotericin duration, median (IQR) | 15 (9, 27) | 9.5 (4.3, 19.8) | 14 (8, 27) | .15 | |
| Underlying conditions | |||||
| Immunocompetent | 73 (41.7%) | 6 (31.6%) | 79 (40.7%) | .64 (.23–1.8) | .38 |
| Active malignancy | 25 (14.3%) | 4 (21.1%) | 29 (14.9%) | 1.60 (.49–5.2) | .78 |
| Transplant, solid organ | 18 (10.3%) | 2 (10.5%) | 20 (10.3%) | 1.03 (.21–4.8) | .97 |
| Transplant, bone marrow | 1 (.57%) | 0 (0%) | 1 (.52%) | 2.98 (.12–75) | .51 |
| HIV | 44 (25.1%) | 4 (21.1%) | 48 (24.7%) | .79 (.25–2.5) | .69 |
| Immunosuppressive therapy | |||||
| Chemotherapy | 10 (5.7%) | 3 (15.8%) | 13 (6.7%) | 3.1 (.77–12.4) | .095 |
| Prednisone | 41 (23.4%) | 7 (36.8%) | 48 (24.7%) | 1.9 (.70–5.2) | .20 |
| Biologic agent | 23 (13.1%) | 5 (26.3%) | 28 (14.4%) | 2.4 (.78–7.2) | .12 |
| Nonsteroid agent | 34 (19.4%) | 6 (31.6%) | 40 (20.6%) | 1.9 (.68–5.4) | .21 |
| Admission status | |||||
| Inpatient | 132 (75.4%) | 17 (89.5%) | 148 (76.3%) | 2.8 (.61–12.4) | .18 |
| ICU stay | 35 (20.0%) | 3 (15.8%) | 38 (19.6%) | .75 (.21–2.7) | .66 |
| . | Itraconazole, n = 175, 90.2% . | Voriconazole, n = 19, 9.8% . | Total, n = 194, 100% . | OR (95% CI) . | P value . |
|---|---|---|---|---|---|
| Race | .30 | ||||
| White | 131 (74.9%) | 14 (73.7%) | 145 (74.7%) | … | |
| African American | 35 (20.0%) | 3 (15.8%) | 38 (19.6%) | ||
| Asian/Pacific Islander | 1 (.6%) | 1 (5.3%) | 2 (1.0%) | ||
| Hispanic | 4 (2.3%) | 0 (0%) | 4 (2.1%) | ||
| Other | 4 (2.3%) | 1 (5.3%) | 5 (2.6%) | ||
| Age, median (IQR) | 47 (34.5,60.6) | 53.7 (36.1, 53.7) | 48.2 (34.5, 60.6) | .53 | |
| Age ≥ 50 | 78 (44.6%) | 10 (52.6%) | 88 (45.4%) | 1.4 (.53–3.5) | .50 |
| Male | 101 (57.7%) | 11 (57.9%) | 112 (57.7%) | 1.0 (.39–2.6) | .99 |
| Presentation | |||||
| Disseminated | 93 (53.1%) | 13 (68.4%) | 106 (54.6%) | 1.2 (.47–3.2) | .69 |
| Constitutional symptoms | 117 (66.9%) | 16 (84.2%) | 133 (68.6%) | 2.6 (.74–9.4) | .13 |
| Pulmonary symptoms | 126 (72.0%) | 11 (57.9%) | 137 (70.6%) | .53 (.20–1.4) | .21 |
| GI symptoms | 71 (40.6%) | 10 (52.6%) | 81 (41.8%) | 1.6 (.63–4.2) | .31 |
| Skin lesions | 22 (12.6%) | 4 (21.1%) | 26 (13.4%) | 1.9 (.56–6.1) | .31 |
| CNS disease | 6 (3.4%) | 1 (5.3%) | 7 (3.6%) | 1.6 (.18–13.7) | .69 |
| Amphotericin induction | 78 (44.6%) | 11 (57.9%) | 89 (45.9%) | 1.7 (.66–4.5) | .27 |
| Amphotericin duration, median (IQR) | 15 (9, 27) | 9.5 (4.3, 19.8) | 14 (8, 27) | .15 | |
| Underlying conditions | |||||
| Immunocompetent | 73 (41.7%) | 6 (31.6%) | 79 (40.7%) | .64 (.23–1.8) | .38 |
| Active malignancy | 25 (14.3%) | 4 (21.1%) | 29 (14.9%) | 1.60 (.49–5.2) | .78 |
| Transplant, solid organ | 18 (10.3%) | 2 (10.5%) | 20 (10.3%) | 1.03 (.21–4.8) | .97 |
| Transplant, bone marrow | 1 (.57%) | 0 (0%) | 1 (.52%) | 2.98 (.12–75) | .51 |
| HIV | 44 (25.1%) | 4 (21.1%) | 48 (24.7%) | .79 (.25–2.5) | .69 |
| Immunosuppressive therapy | |||||
| Chemotherapy | 10 (5.7%) | 3 (15.8%) | 13 (6.7%) | 3.1 (.77–12.4) | .095 |
| Prednisone | 41 (23.4%) | 7 (36.8%) | 48 (24.7%) | 1.9 (.70–5.2) | .20 |
| Biologic agent | 23 (13.1%) | 5 (26.3%) | 28 (14.4%) | 2.4 (.78–7.2) | .12 |
| Nonsteroid agent | 34 (19.4%) | 6 (31.6%) | 40 (20.6%) | 1.9 (.68–5.4) | .21 |
| Admission status | |||||
| Inpatient | 132 (75.4%) | 17 (89.5%) | 148 (76.3%) | 2.8 (.61–12.4) | .18 |
| ICU stay | 35 (20.0%) | 3 (15.8%) | 38 (19.6%) | .75 (.21–2.7) | .66 |
Data are presented as n (%) unless otherwise indicated. Constitutional symptoms consisted of arthralgias, fever, night sweats, and weight loss; GI symptoms consisted of diarrhea, dysphagia, GI upset, nausea, vomiting; and pulmonary symptoms consisted of chest pain, cough, dyspnea. Nonsteroid agents included azathioprine, cyclosporin, mycophenolate, sirolimus, and tacrolimus. Skin lesions included erythema nodosum, skin nodules, and other skin lesions.
Abbreviations: CI, confidence interval; CNS, central nervous system; GI, gastrointestinal; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; OR, odds ratio.
Comparison of Baseline Characteristics and Presentation of 194 Patients With Histoplasmosis Based on Initial Azole Therapy, 2002–2017
| . | Itraconazole, n = 175, 90.2% . | Voriconazole, n = 19, 9.8% . | Total, n = 194, 100% . | OR (95% CI) . | P value . |
|---|---|---|---|---|---|
| Race | .30 | ||||
| White | 131 (74.9%) | 14 (73.7%) | 145 (74.7%) | … | |
| African American | 35 (20.0%) | 3 (15.8%) | 38 (19.6%) | ||
| Asian/Pacific Islander | 1 (.6%) | 1 (5.3%) | 2 (1.0%) | ||
| Hispanic | 4 (2.3%) | 0 (0%) | 4 (2.1%) | ||
| Other | 4 (2.3%) | 1 (5.3%) | 5 (2.6%) | ||
| Age, median (IQR) | 47 (34.5,60.6) | 53.7 (36.1, 53.7) | 48.2 (34.5, 60.6) | .53 | |
| Age ≥ 50 | 78 (44.6%) | 10 (52.6%) | 88 (45.4%) | 1.4 (.53–3.5) | .50 |
| Male | 101 (57.7%) | 11 (57.9%) | 112 (57.7%) | 1.0 (.39–2.6) | .99 |
| Presentation | |||||
| Disseminated | 93 (53.1%) | 13 (68.4%) | 106 (54.6%) | 1.2 (.47–3.2) | .69 |
| Constitutional symptoms | 117 (66.9%) | 16 (84.2%) | 133 (68.6%) | 2.6 (.74–9.4) | .13 |
| Pulmonary symptoms | 126 (72.0%) | 11 (57.9%) | 137 (70.6%) | .53 (.20–1.4) | .21 |
| GI symptoms | 71 (40.6%) | 10 (52.6%) | 81 (41.8%) | 1.6 (.63–4.2) | .31 |
| Skin lesions | 22 (12.6%) | 4 (21.1%) | 26 (13.4%) | 1.9 (.56–6.1) | .31 |
| CNS disease | 6 (3.4%) | 1 (5.3%) | 7 (3.6%) | 1.6 (.18–13.7) | .69 |
| Amphotericin induction | 78 (44.6%) | 11 (57.9%) | 89 (45.9%) | 1.7 (.66–4.5) | .27 |
| Amphotericin duration, median (IQR) | 15 (9, 27) | 9.5 (4.3, 19.8) | 14 (8, 27) | .15 | |
| Underlying conditions | |||||
| Immunocompetent | 73 (41.7%) | 6 (31.6%) | 79 (40.7%) | .64 (.23–1.8) | .38 |
| Active malignancy | 25 (14.3%) | 4 (21.1%) | 29 (14.9%) | 1.60 (.49–5.2) | .78 |
| Transplant, solid organ | 18 (10.3%) | 2 (10.5%) | 20 (10.3%) | 1.03 (.21–4.8) | .97 |
| Transplant, bone marrow | 1 (.57%) | 0 (0%) | 1 (.52%) | 2.98 (.12–75) | .51 |
| HIV | 44 (25.1%) | 4 (21.1%) | 48 (24.7%) | .79 (.25–2.5) | .69 |
| Immunosuppressive therapy | |||||
| Chemotherapy | 10 (5.7%) | 3 (15.8%) | 13 (6.7%) | 3.1 (.77–12.4) | .095 |
| Prednisone | 41 (23.4%) | 7 (36.8%) | 48 (24.7%) | 1.9 (.70–5.2) | .20 |
| Biologic agent | 23 (13.1%) | 5 (26.3%) | 28 (14.4%) | 2.4 (.78–7.2) | .12 |
| Nonsteroid agent | 34 (19.4%) | 6 (31.6%) | 40 (20.6%) | 1.9 (.68–5.4) | .21 |
| Admission status | |||||
| Inpatient | 132 (75.4%) | 17 (89.5%) | 148 (76.3%) | 2.8 (.61–12.4) | .18 |
| ICU stay | 35 (20.0%) | 3 (15.8%) | 38 (19.6%) | .75 (.21–2.7) | .66 |
| . | Itraconazole, n = 175, 90.2% . | Voriconazole, n = 19, 9.8% . | Total, n = 194, 100% . | OR (95% CI) . | P value . |
|---|---|---|---|---|---|
| Race | .30 | ||||
| White | 131 (74.9%) | 14 (73.7%) | 145 (74.7%) | … | |
| African American | 35 (20.0%) | 3 (15.8%) | 38 (19.6%) | ||
| Asian/Pacific Islander | 1 (.6%) | 1 (5.3%) | 2 (1.0%) | ||
| Hispanic | 4 (2.3%) | 0 (0%) | 4 (2.1%) | ||
| Other | 4 (2.3%) | 1 (5.3%) | 5 (2.6%) | ||
| Age, median (IQR) | 47 (34.5,60.6) | 53.7 (36.1, 53.7) | 48.2 (34.5, 60.6) | .53 | |
| Age ≥ 50 | 78 (44.6%) | 10 (52.6%) | 88 (45.4%) | 1.4 (.53–3.5) | .50 |
| Male | 101 (57.7%) | 11 (57.9%) | 112 (57.7%) | 1.0 (.39–2.6) | .99 |
| Presentation | |||||
| Disseminated | 93 (53.1%) | 13 (68.4%) | 106 (54.6%) | 1.2 (.47–3.2) | .69 |
| Constitutional symptoms | 117 (66.9%) | 16 (84.2%) | 133 (68.6%) | 2.6 (.74–9.4) | .13 |
| Pulmonary symptoms | 126 (72.0%) | 11 (57.9%) | 137 (70.6%) | .53 (.20–1.4) | .21 |
| GI symptoms | 71 (40.6%) | 10 (52.6%) | 81 (41.8%) | 1.6 (.63–4.2) | .31 |
| Skin lesions | 22 (12.6%) | 4 (21.1%) | 26 (13.4%) | 1.9 (.56–6.1) | .31 |
| CNS disease | 6 (3.4%) | 1 (5.3%) | 7 (3.6%) | 1.6 (.18–13.7) | .69 |
| Amphotericin induction | 78 (44.6%) | 11 (57.9%) | 89 (45.9%) | 1.7 (.66–4.5) | .27 |
| Amphotericin duration, median (IQR) | 15 (9, 27) | 9.5 (4.3, 19.8) | 14 (8, 27) | .15 | |
| Underlying conditions | |||||
| Immunocompetent | 73 (41.7%) | 6 (31.6%) | 79 (40.7%) | .64 (.23–1.8) | .38 |
| Active malignancy | 25 (14.3%) | 4 (21.1%) | 29 (14.9%) | 1.60 (.49–5.2) | .78 |
| Transplant, solid organ | 18 (10.3%) | 2 (10.5%) | 20 (10.3%) | 1.03 (.21–4.8) | .97 |
| Transplant, bone marrow | 1 (.57%) | 0 (0%) | 1 (.52%) | 2.98 (.12–75) | .51 |
| HIV | 44 (25.1%) | 4 (21.1%) | 48 (24.7%) | .79 (.25–2.5) | .69 |
| Immunosuppressive therapy | |||||
| Chemotherapy | 10 (5.7%) | 3 (15.8%) | 13 (6.7%) | 3.1 (.77–12.4) | .095 |
| Prednisone | 41 (23.4%) | 7 (36.8%) | 48 (24.7%) | 1.9 (.70–5.2) | .20 |
| Biologic agent | 23 (13.1%) | 5 (26.3%) | 28 (14.4%) | 2.4 (.78–7.2) | .12 |
| Nonsteroid agent | 34 (19.4%) | 6 (31.6%) | 40 (20.6%) | 1.9 (.68–5.4) | .21 |
| Admission status | |||||
| Inpatient | 132 (75.4%) | 17 (89.5%) | 148 (76.3%) | 2.8 (.61–12.4) | .18 |
| ICU stay | 35 (20.0%) | 3 (15.8%) | 38 (19.6%) | .75 (.21–2.7) | .66 |
Data are presented as n (%) unless otherwise indicated. Constitutional symptoms consisted of arthralgias, fever, night sweats, and weight loss; GI symptoms consisted of diarrhea, dysphagia, GI upset, nausea, vomiting; and pulmonary symptoms consisted of chest pain, cough, dyspnea. Nonsteroid agents included azathioprine, cyclosporin, mycophenolate, sirolimus, and tacrolimus. Skin lesions included erythema nodosum, skin nodules, and other skin lesions.
Abbreviations: CI, confidence interval; CNS, central nervous system; GI, gastrointestinal; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; OR, odds ratio.
At the time of presentation, 106 (54.6%) patients had disseminated disease, 133 (68.6%) had constitutional symptoms, 13 (70.6%) had pulmonary symptoms, 81 (41.8%) had gastrointestinal symptoms, and 26 (13.4%) had skin lesions (Table 1).
There were 89 (45.9%) patients who started treatment with amphotericin before transitioning to azole therapy: 78 (44.6%) in the itraconazole group and 11 (57.9%) in the voriconazole group (Table 1). Patients treated with amphotericin B were not significantly more likely to be started on voriconazole or itraconazole (odds ratio [OR], 1.7; 95% confidence interval [CI], .66–4.5; P = .27). There was no mortality difference seen in patients induced with amphotericin before switching to either voriconazole or itraconazole (27.3% vs 24.4% patients, respectively; OR, 1.16; 95% CI, .28–4.83; P = .83). Similarly, there was no statistical difference in patients that required reinitiation of amphotericin within 3–90 days of starting voriconazole or itraconazole (15.8% vs 6.8% patients, respectively; OR, 1.46; 95% CI, .38–5.69; P = .58). Patients that received induction with amphotericin had no significant increase in mortality (OR, 1.16; 95% CI, .21–6.43; P = .87).
The odds of being started on voriconazole over itraconazole were not statistically different based on age, gender, or ethnicity (P > .05). The use of voriconazole was not statistically different for those diagnosed with disseminated versus localized or asymptomatic lung disease (OR, 1.2; 95% CI, .47–3.2; P = .69), was not different based on symptoms at presentation, and was not more likely to be prescribed to patients with central nervous system involvement (OR, 1.6; 95% CI, .18–13.7, P = .69; Table 1).
Immunocompetent patients were evenly distributed between the 2 groups (OR, 0.64; 95% CI, .23–1.8; P = .38). There were no differences seen for specific immunocompromising conditions: active malignancy (OR, 1.60; 95% CI, .49–5.2; P = .78), living with human immunodeficiency virus (OR, 0.79; 95% CI, .25–2.5; P = .69), or transplant status (solid organ and stem cell transplant; OR, 0.97; 95% CI, .21–4.5; P = .97). The choice of azole was not statistically different based on concomitant immunosuppressive therapy (Table 1).
Among those prescribed itraconazole as the first azole, 37 (19%) were switched to another azole therapy: 13 (7%) to fluconazole, 17 (9%) to voriconazole, 4 (2%) to posaconazole, and 3 (2%) to isavuconazole. Amongst those prescribed voriconazole, 9 (47%) were switched to another therapy: 8 (42%) to itraconazole and 1 (5%) to posaconazole.
Mortality
There were 47 (24.2%) patients who died within 180 days after initiation of azole therapy. Of those who died, 41 (23.4%) patients were in the itraconazole group and 6 (31.6%) were in the voriconazole group. The survival curves for the primary analysis violated the assumption of proportional hazards, with a discontinuity point at approximately 42 days. When controlling for disseminated disease, our survival analysis showed a statistically significant association of voriconazole with an increase in mortality early in the course of treatment (0 to 42 days; HR, 4.3; 95% CI, 1.3–13.9; P = .015) that was not seen later in the course of therapy (43 to 180 days; HR, 0; 95% CI, 0 to >99.0; P = .89; Table 2 and Figure 2). Disseminated disease was an independent predictor of mortality (HR, 3.1; 95% CI, 1.1–8.4; P = .026). Other variables were not significant in the multivariable model, and are thus not reported. The years of therapy starts were randomly distributed, as ascertained by a direct visualization using a histogram. When year of diagnosis was included in a multivariable analysis, it had no significant effect on the point estimate or the CI of the primary outcomes (voriconazole vs itraconazole).
Comparison of 180-Day Mortality for Patients Treated With Voriconazole and Itraconazole, 2002–2017
| . | Hazard Ratio . | ||
|---|---|---|---|
| Azole choice . | aHR . | 95% CI . | P value . |
| Voriconazole, 0–42 days | 4.3 | 1.3–13 | .015 |
| Voriconazole, 43–180 days | 0 | 0 to >99 | .90 |
| . | Hazard Ratio . | ||
|---|---|---|---|
| Azole choice . | aHR . | 95% CI . | P value . |
| Voriconazole, 0–42 days | 4.3 | 1.3–13 | .015 |
| Voriconazole, 43–180 days | 0 | 0 to >99 | .90 |
The aHR was adjusted for disseminated disease status of patients with histoplasmosis.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval.
Comparison of 180-Day Mortality for Patients Treated With Voriconazole and Itraconazole, 2002–2017
| . | Hazard Ratio . | ||
|---|---|---|---|
| Azole choice . | aHR . | 95% CI . | P value . |
| Voriconazole, 0–42 days | 4.3 | 1.3–13 | .015 |
| Voriconazole, 43–180 days | 0 | 0 to >99 | .90 |
| . | Hazard Ratio . | ||
|---|---|---|---|
| Azole choice . | aHR . | 95% CI . | P value . |
| Voriconazole, 0–42 days | 4.3 | 1.3–13 | .015 |
| Voriconazole, 43–180 days | 0 | 0 to >99 | .90 |
The aHR was adjusted for disseminated disease status of patients with histoplasmosis.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval.
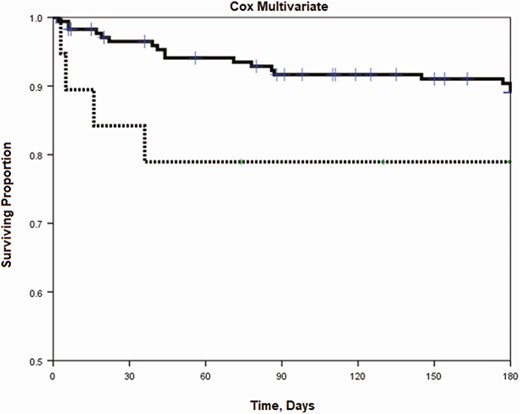
Multivariate Cox regression of patients with histoplasmosis treated with itraconazole or voriconazole, 2002–2017. Analysis shows a statistically significant difference in mortality early (0 to 42 days; aHR, 4.3; 95% CI, 1.3–13.9; P = .015) compared to later (43 to 180 days; aHR, 0; 95% CI, 0 to >99.0; P = .90), when adjusted for disseminated status. Itraconazole is shown as a solid line and voriconazole is shown as a dashed line. Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval.
DISCUSSION
Itraconazole is a broad-spectrum triazole with antifungal activity against many medically important fungi, and is the preferred azole for treatment of histoplasmosis [1] and other dimorphic fungal infections [17, 18]. However, due to its widely variable absorption and poor tolerability [19], other, newer-generation azoles, such as voriconazole, have been used in clinical practice in an attempt to overcome these limitations. In our cohort, we found a difference in mortality in the first 42 days. The mortality rate for patients receiving voriconazole as their initial azole treatment was 4.3 times higher in the first 42 days, suggesting that voriconazole might be inferior to itraconazole. The mortality appeared similar between the 2 groups after 42 days; however, there were so few events that occurred between days 42 and 180 that our cohort was not powered to find even a large difference.
Although some previously published clinical data suggest success when using voriconazole in patients with histoplasmosis, these data are sparse and uncontrolled. Prior literature comes from patients receiving voriconazole as salvage therapy for endemic mycoses, not as a first-line treatment, and is limited to case reports and small case series, where the data appear largely encouraging [7–11, 20, 21]. To our knowledge, prior to our study, there have been no other clinical studies comparing outcomes between itraconazole and voriconazole when used to treat histoplasmosis or any other endemic mycosis.
Though there are little data to compare the in vivo efficacy of voriconazole to itraconazole in histoplasmosis, its in vitro activity has been well studied [22]. Though the MICs and MFCs to itraconazole and voriconazole have suggested similar fungistatic effects against H. capsulatum, the MICs for voriconazole have been confirmed to have a much wider range than itraconazole [22], with a higher MIC90 of 0.25 ug/mL, compared to 0.06 ug/mL with itraconazole [6, 13]. Both azoles have been shown to have a fungicidal effect over H. capsulatum, though itraconazole has a lower MFC50 (2 ug/mL) compared to voriconazole (8 ug/mL) [6].
Voriconazole is derived from and is structurally related to fluconazole, but exhibits greater potency, a broader spectrum of activity, and lower MICs to Histoplasma when compared to fluconazole [13, 23, 24]. In PLWH, fluconazole has been found to be less effective than itraconazole for treatment of histoplasmosis, resulting in slower blood culture clearance, particularly in the first 4 weeks [25]; a higher rates of relapse [16]; and concerns for developing resistance [13]. After exposure to fluconazole, one-third of clinical isolates in PLWH show reduced susceptibility to fluconazole [16, 26], and increases of up to 70% in MICs have been reported in PLWH [13]. This exposure is presumed to induce a point mutation in the cytochrome P450-dependant enzymes 14α-demethylase (CYP51p) and 3-ketosteroids reductase via the Y136F mutation [27], which results in reduced susceptibility to fluconazole and a 4-fold increase in voriconazole MICs [12, 13].
In our study, we found that that the early time period (first 6 weeks) was a critical period where the outcomes diverged, similar to data in PLWH treated with fluconazole [25]. Although there is no evidence that voriconazole can cause the Y136F mutation, in PLWH fluconazole does simultaneously increase MICs, and voriconazole is fluconazole’s structural analogue, which suggests that exposure to voriconazole could induce resistance by similar mechanisms [13]. The Y136F mutation does not confer increased MICs to itraconazole, posaconazole, or isavuconazole [12, 13]. This could be a potential cause of treatment failure and difference in mortality, as seen in our study.
Other newer-generation azoles, including posaconazole and isavuconazole, have also been poorly studied for treatment of histoplasmosis. Posaconazole is more structurally similar to itraconazole [28], and it differs from voriconazole in that cross resistance has not been observed after exposure to fluconazole [13]. Isavuconazole is structurally similar to fluconazole and voriconazole, but exhibits a higher barrier to resistance and is not affected by the Y136F mutation that resulted in reduced sensitivity to both fluconazole and voriconazole [13]. As a result, MICs for isavuconazole remained unchanged in relapsed isolates exposed to fluconazole [12]. Though each azole has shown some clinical efficacy against H. capsulatum [26, 29], only posaconazole has been directly compared against itraconazole. As in our study, the research on posaconazole was retrospective and included relatively few patients to make any direct conclusions [29]. As a result, itraconazole remains the mainstay of treatment and is considered the standard of care in the clinical practice guidelines for the treatment of histoplasmosis [30].
Our study is limited by a small population size, especially in the voriconazole group. Additionally, this study is limited as a retrospective analysis of a cohort from a single tertiary academic center in the United States, and our results may not be generalizable to other populations. There is also a chance for confounding by indication; however, we attempted to control for this, and there was no pattern detected as to a group of patients that preferentially received voriconazole.
This analysis provides important information for physicians and practitioners regarding a medication that is largely unstudied but still used in histoplasmosis. In our series, patients with histoplasmosis experience better outcomes when their initial azole therapy is itraconazole, at least in the first 42 days. Further research is needed to assess additional factors impacting patient outcomes when treated with voriconazole, both as initial and secondary treatment, and ultimately determine when it is appropriate to treat histoplasmosis with this choice of azole. Until more is known about outcomes when voriconazole is used to treat histoplasmosis, other azoles should be used preferentially.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Financial support. This work was supported by the Washington University Institute of Clinical and Translational Sciences (grant number KL2T002346) from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Potential conflicts of interest. W. G. P. reports grants and personal fees from Merck Labs, outside the submitted work. A. S. reports grants from Astellas, grants and personal fees from Mayne, and personal fees from Scynexis, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




